Abstract
Background:
The aims of the present systematic review and meta-analysis were to evaluate published, randomized controlled trials that investigate the effects on whole-body vibration (WBV) training on total, femoral neck, and lumbar spine bone mineral density (BMD) in postmenopausal women, and identify the potential moderating factors explaining the adaptations to such training.
Methods:
From a search of electronic databases (PubMed, Web of Science, and Cochrane) up until September 2017, a total 10 studies with 14 WBV groups met the inclusion criteria. Three different authors tabulated, independently, the selected indices in identical predetermined forms. The methodological quality of all studies was evaluated according to the modified PEDro scale. For each trial, differences within arms were calculated as mean differences (MDs) and their 95% confidence intervals between pre- and postintervention values. The effects on bone mass between exercise and control groups were also expressed as MDs. Both analyses were performed in the total sample and in a specific class of postmenopausal women younger than 65 years of age (excluding older women).
Results:
The BMD of 462 postmenopausal women who performed WBV or control protocol was evaluated. Significant pre-post improvements in BMD of the lumbar spine were identified following WBV protocols (P = .03). Significant differences in femoral neck BMD (P = .03) were also found between intervention and control groups when analyzing studies that included postmenopausal women younger than 65 years.
Conclusions:
WBV is an effective method to improve lumbar spine BMD in postmenopausal and older women and to enhance femoral neck BMD in postmenopausal women younger than 65 years.
Keywords: bone mass, exercise, perimenopause, whole-body vibration training, women
1. Introduction
One of the major risk factors associated with fragility fractures is low bone mineral density (BMD)[1] that can, ultimately, result in a higher predisposition for osteoporosis.[2] There has been an increased research interest in populations who suffer from an accelerated loss of bone mass, particularly older adults (men and women age ≥65 yr[3] and postmenopausal women). Menopause is characterized by hormonal changes, which include a decline in estrogen levels, that play an important role in bone remodeling in females.[4]
Although pharmaceutical treatments are used to increase bone mass,[5] physical exercise has been shown to be an effective treatment.[6] It is known that a mechanical stimulus is necessary to maintain bone health.[7] In this regard, different training programs, such as resistance and multicomponent trainings, have shown increases in BMD of the femoral neck and lumbar spine in postmenopausal[8,9] and older women.[9,10] In addition, whole-body vibration (WBV) training has been used as an alternative exercise intervention and has shown to also increase bone density via mechanical load.[11] WBV involves standing on an oscillating plate that generates vertical acceleration, which transmits high-frequency mechanical stimuli to sensory receptors throughout the body.[12] The vibration training requires a greater response from the muscle and bone tissues to absorb and dampen the energy caused by oscillatory actions. It has been shown that WBV can produce osteogenic effects counteracting age-related alterations in bone mass.[13,14] Furthermore, the training program on a vibratory platform has its added benefits with a shortened duration of treatment and lower perceived exertion.[15]
Numerous studies have examined the effect of WBV on bone mass in postmenopausal women,[16–30] but the findings are somehow contradicting. Iwamoto et al[29] observed that 12 months of WBV (intensity of 20 Hz, frequency once per week, and exercise duration of 4 min) plus alendronate had a significant improvement on BMD in the lumbar spine in postmenopausal, osteoporotic women. In contrast, Slatkovska et al[31] showed that WBV (frequency of 90 or 30 Hz, with a peak of acceleration of 0.3 g) did not increase BMD of the calcaneal after 12 months. Also, Rubin et al[23] found no changes in bone mineral content (BMC) of the spine, hip, and distal radius in postmenopausal women following WBV (frequency of 30 Hz and magnitude of 0.2 g).
It remains controversial as to whether WBV has a positive effect on bone mass and structure in women. Thus, the aims of this systematic review and meta-analysis were to evaluate published, randomized controlled trials (RCTs) that investigated the effects of WBV on total, femoral neck, and lumbar spine BMD in postmenopausal women and identify the potential moderating factors explaining the adaptations to such training.
2. Methods
2.1. Study design
The present research followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.[32] Ethical approval and informed patient consent were not required, as this research was a systematic review and had no direct patient contact or influence on patient care. Eligibility criteria were predetermined by the authors. Only RCTs studies were considered for inclusion in the present review. Three different authors (EM-C, JAR-A, and DJR-C) tabulated independently, the selected indices in identical predetermined forms. Any discrepancies in methodology, retrieval of articles, and statistical analysis were resolved by the consensus of all authors.
2.2. Literature search and data collection
Searches were conducted in PubMed, Web of Science, and Cochrane up until September 2017. The following keyword combinations were used: “women” OR “older adults” OR “elderly” AND “whole body vibration” OR “WBV” AND “bone mineral density” OR “bone mass” OR “BMD” OR “bone mineral content” OR “BMC”. Figure 1 shows a flow diagram of the results from the entire search process.
Figure 1.
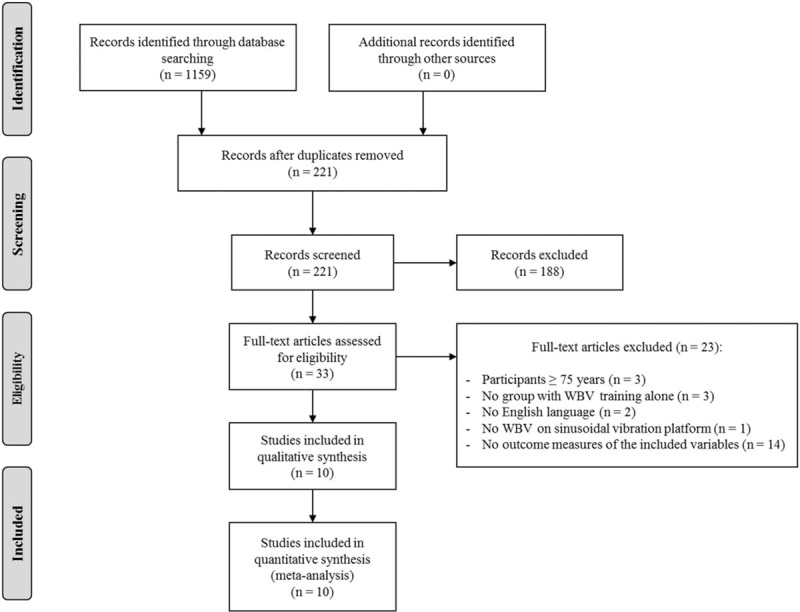
Flow diagram of the process of study selection. WBV = whole-body vibration.
2.3. Selection criteria
Only clinical, WBV, RCTs published in the English language were included. The following inclusion criteria had to be met: participants were postmenopausal (the definition of the postmenopausal period was the years following the year when menstruation ceased) and/or older women (women older than 65 yr); 1 group of the study performed WBV; total, femoral neck, or lumbar spine BMD were one of the outcome measures; and dal-energy x-ray absorptiometry was used to measure the different variables. Studies were excluded if participants were ≥75 years; they did not use WBV on sinusoidal vibration platforms; there was no control group; participants were not standing on the platform (ie, sitting or lying position); and participants were taking medical treatments which might have influenced bone mass.
2.4. Quality assessment
The methodological quality of all RCTs studies were evaluated according to the modified PEDro scale using the following criteria: eligibility criteria were specified; participants were randomly allocated to groups (in a crossover study, participants were randomly allocated to treatment groups); allocation was concealed; the groups were similar at baseline with regards to the most important prognostic indicators; all participants were blinded to the interventions; all therapists who administered the therapy were blinded; there was blinding of all assessors who measured at least 1 key outcome; measures of at least 1 key outcome were obtained from >85% of the participants initially allocated to groups; all participants for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least 1 key outcome were analyzed by “intention to treat”; the results of between-group statistical comparisons were reported for at least 1 key outcome; and the study provided both point measures and measures of variability for at least 1 key outcome.
2.5. Statistical methods
The meta-analysis and statistical analyses were performed using Review Manager software (RevMan 5.2; Cochrane Collaboration, Oxford, UK) and Comprehensive Meta-analysis software (Version 2; Biostat, Englewood, NJ). For each trial, differences within arms were calculated as mean differences (MDs) and their 95% confidence intervals between pre- and postintervention values. The effects on bone mass between exercise and control groups were also expressed as MDs. Both analyses were performed in the total sample and in a specific class of postmenopausal women younger than 65 years of age (excluding older women).
Statistical heterogeneity was assessed using the Cochran chi-square, I2 statistics. I2 values of 30% and 60% represented a moderate level of heterogeneity. A P value <.1 for the chi-square was defined as indicating the presence of heterogeneity.
Potential moderating factors were evaluated by subgroup analysis, comparing trials grouped by dichotomous or continuous variables that could potentially influencing bone mass. Median values of continuous variables were used as cut-off values for grouping trials. Changes in potential moderating factors were expressed and analyzed as post-minus preintervention values. Publication bias was evaluated using the estimating funnel plot asymmetry test. A P value ≤.05 was considered statistically significant.
3. Results
3.1. Characteristics of included studies
In the initial literature search, 1159 titles and relevant abstracts were found. Among them, there were 938 duplicates leaving 221 articles. A total of 188 studies were excluded based on abstract/title screening. Full texts were retrieved for the remaining 33 articles, of which only 10 RCTs were included in the qualitative synthesis based on the inclusion criteria (ie, these were only 10 articles that contained BMD outcomes that could be compared with at least 1 other study). Figure 1 shows the flow diagram of the study selection process.
Table 1 summarizes the main characteristics and properties of the 10 studies in this review.[16–25] The RCTs included in this systematic review were published between the years 2004 and 2017, and the total number of postmenopausal women was 462 (ranging from 22[17] to 96[25] participants). Some of these studies included >1 intervention group and control group (ie, parallel group design). The mean age of the participants was from 53.4[17] to 68.9[16] years. Regarding the sample population, the studies comprised in this systematic review showed postmenopausal women in 3 different conditions (no disease, osteopenic, or osteoporotic); 1 study included women with osteopenia and osteoporosis [18] and another presented only osteoporotic volunteers.[22]
Table 1.
Main characteristics ∗ of included studies in the meta-analysis.

3.2. Characteristics of the interventions
The characteristics of the different WBV interventions are present in Table 2. The intensity of the protocols varied from 12.5[16] to 50 Hz[21] and the amplitude from 1.5[17] to 12 mm.[25] The duration of the different interventions ranged from 12[19,22] to 52 weeks of training[23] with a weekly frequency of 2[16] to 7 sessions.[23] Total session length varied from 90[21] to 1800 seconds.[24] The values for acceleration (g) ranged from 0.2[23] to 20.12 m/s2[21]; thus, the intensity of the training was different among the studies.
Table 2.
Characteristics of whole-body vibration training intervention and bone mass assessment of studies included in the meta-analysis.

3.3. Main effects analysis
When all studies and respective WBV groups were examined, there was no significant pre-post effect on total (P = .96; MD = 0.0; Fig. 2A) and femoral neck BMD (P = .44; MD = 0.01; Fig. 2B). However, there was a significant pre-post improvement in BMD of the lumbar spine (P = .03; MD = 0.02; Fig. 2C). When comparing WBV with control groups, no significant differences were observed in total (P = .74; MD = 0.01; Fig. 3A), femoral neck (P = .28; MD = 0.02; Fig. 3B) and lumbar spine BMD (P = .46; MD = 0.02; Fig. 3C).
Figure 2.
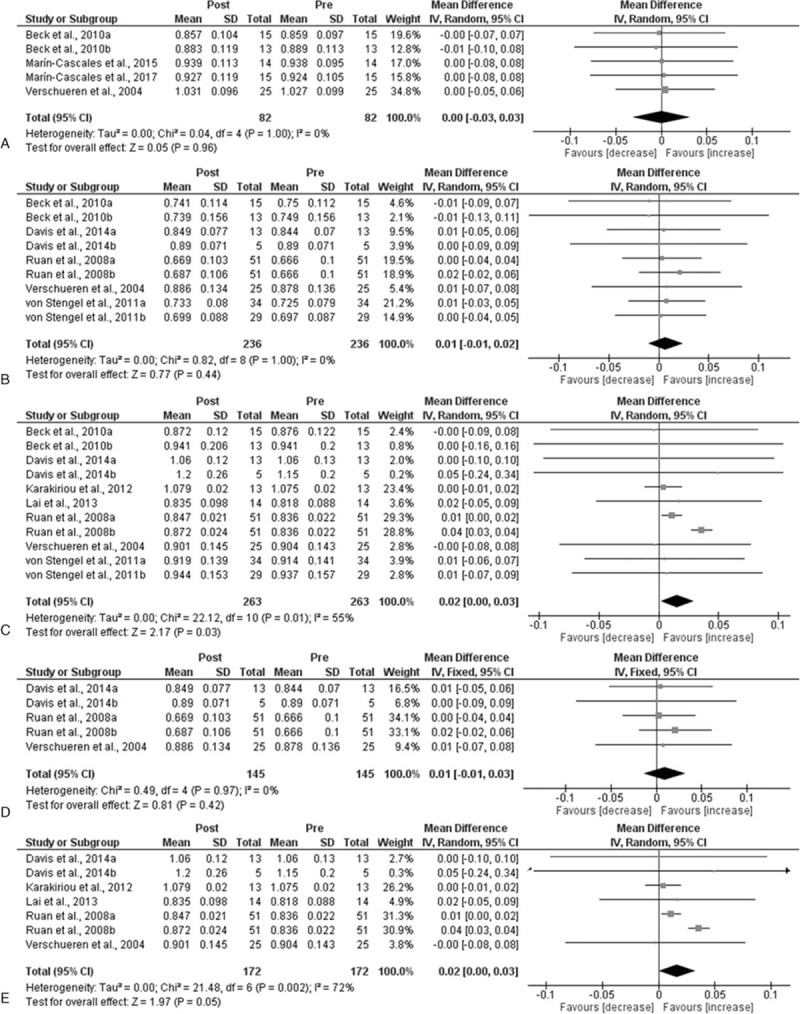
Mean difference (MD) in bone mineral density (BMD) between post- and preintervention. Squares represent the MDa for each trial. Diamonds represent the pooled MD across trials. (A) Total BMD; (B) femoral neck; (C) low back; (D) Femoral neck in postmenopausal women younger than 65 years; and (E) low back in postmenopausal women yonger than 65 years. CI = confidence interval, SD = standard deviation.
Figure 3.
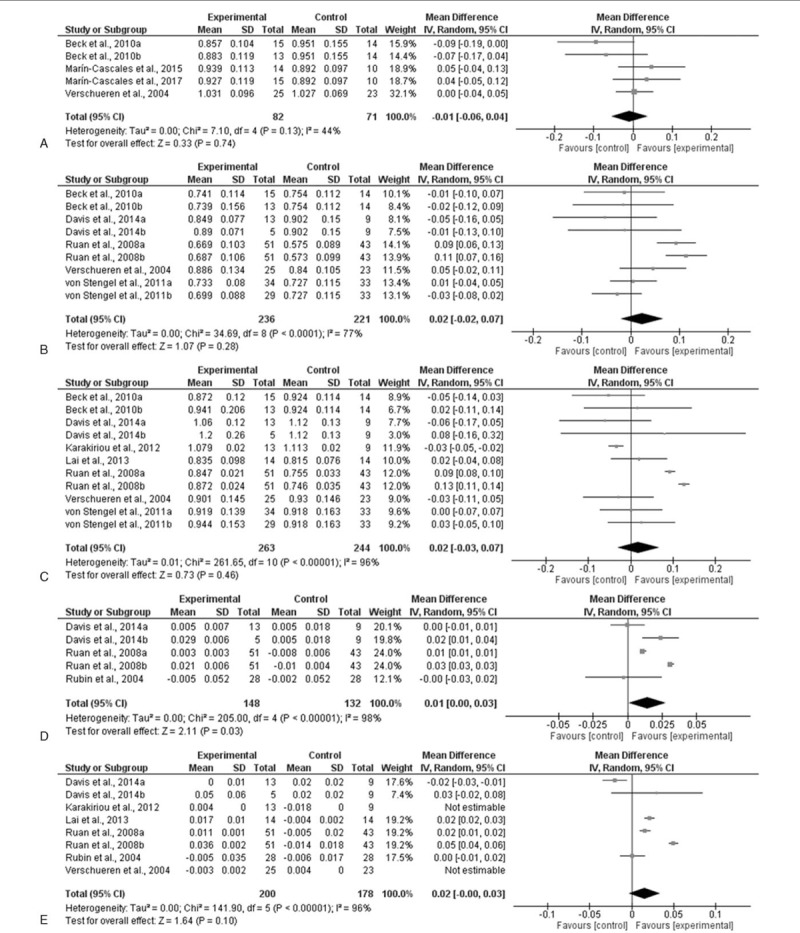
Mean difference (MD) in postintervention bone mineral density (BMD) between whole-body vibration (WBV)-trained and control postmenopausal women. Squares represent the MDa for each trial. Diamonds represent the pooled MD across trials. (A) Total BMD; (B) femoral neck; (C) low back; (D) femoral neck in postmenopausal women younger than 65 years; and (E) low back in postmenopausal women younger than 65 years. CI = confidence interval, SD = standard deviation.
3.4. Subgroup analysis
Interestingly, when analyzing studies that included postmenopausal women younger than 65 years no significant pre-post change in femoral neck BMD was found (P = .42; MD = 0.01; Fig. 2D). However, there was a significant increase in BMD of the lumbar spine (P = .05; MD = 0.02; Fig. 2E) following WBV between pre and postintervention. In addition, significant differences in femoral neck BMD (P = .03; MD = 0.01; Fig. 3D) were also observed between WBV and control groups. Nevertheless, no statistical significance was found in lumbar spine BMD (P = .10; MD = 0.02; Fig. 3E) when comparing WBV with control groups.
Subgroup analysis assessing potential moderating factors for BMD of the femoral neck and lumbar spine are presented in Tables 3 and 4 , respectively.
Table 3.
Subgroup analyses assessing potential moderating factors for femoral neck bone mineral density in postmenopausal women and postmenopausal women younger than 65 years.
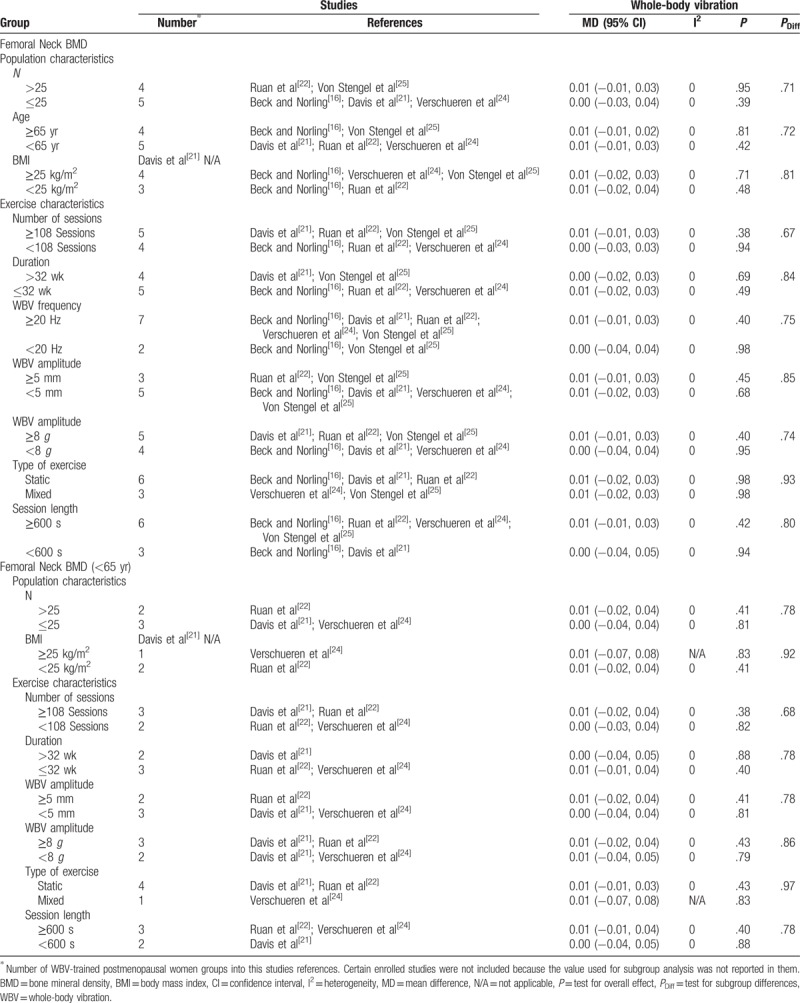
Table 4.
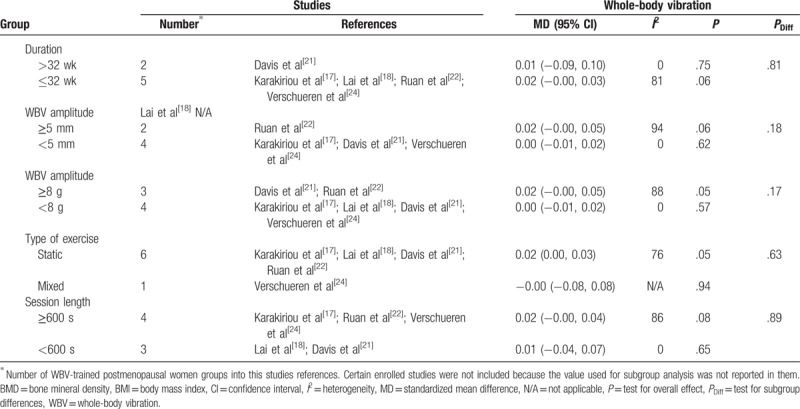
Subgroup analyses assessing potential moderating factors for low back bone mineral density in postmenopausal women and postmenopausal women younger than 65 years.
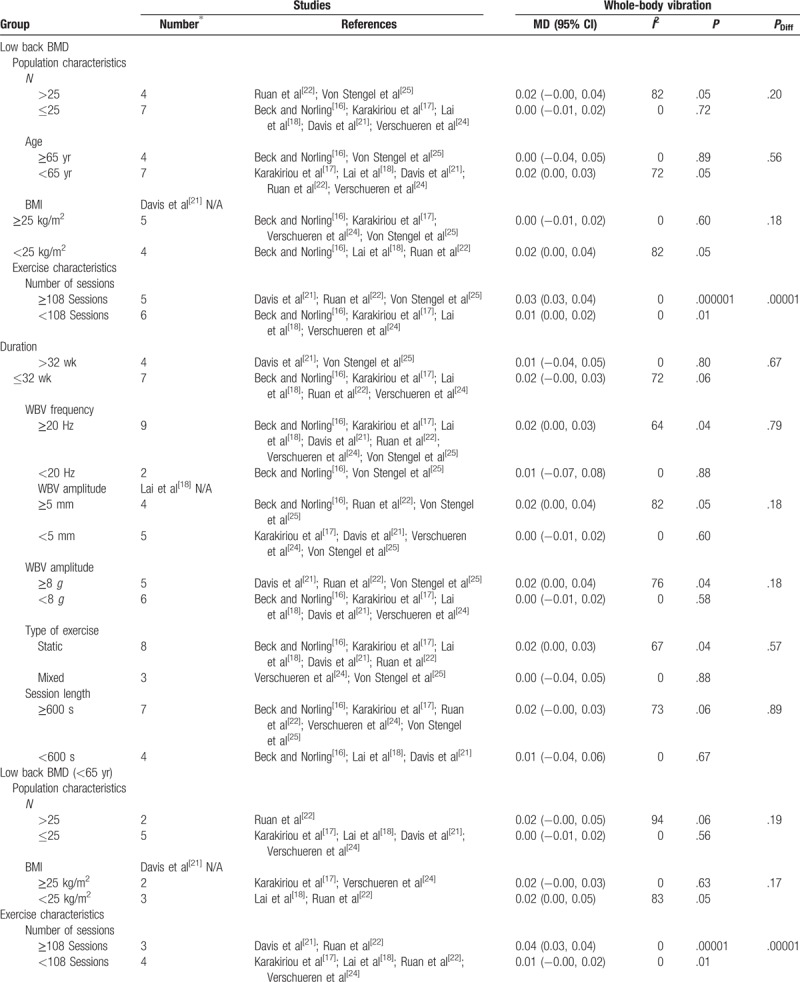
Table 4 (Continued).
Subgroup analyses assessing potential moderating factors for low back bone mineral density in postmenopausal women and postmenopausal women younger than 65 years.
Concerning population and exercise characteristics, from all the studies that had femoral neck as an outcome variable, no significant differences were observed between subgroups (Table 3): the number of the participants, age, BMI, number of sessions, duration, frequency, amplitude, type of exercise, and sessions length were not factors in femoral neck BMD changes with WBV in postmenopausal women.
As for the number of participants (n), studies with >25 participants presented significant training effect on BMD of the lumbar spine (P = .05; MD = 0.02; Table 4 ). For participants younger than 65 years old and with a BMI younger than 25 kg/m2, WBV was effective in reducing bone loss at the lumbar spine (P = .05; MD = 0.02; Table 4 ). Nevertheless, no significant differences were obtained between subgroups (Table 4 ).
Regarding the total number of sessions, a significant pre-post effect on lumbar spine BMD was observed for more[21,22,25] and less[16–18,22,24] than 108 sessions (P = .000001; MD = 0.03; and P = .01; MD = 0.01, respectively). Furthermore, significant differences were obtained between subgroups (P = .00001) with 108 or more sessions presenting a higher MD (Table 4 ). When including postmenopausal women younger than 65 years the results are similar (≥108 sessions: P = .00001; MD = 0.04; <108 sessions: P = .01; MD = 0.01; Table 4 ). Again, there were statistical differences between subgroups in women younger than 65 years (P = .00001) with 108 or more sessions presenting a higher MD (Table 4 ).
With respect to WBV frequency, training at 20 Hz or more induced a significant pre-post effect on lumbar spine BMD (P = .04; MD = 0.02; Table 4 ). However, no statistical significance was found between groups that trained with higher and lower frequencies (P = .79; Table 4 ).
There was a significant pre-post WBV effect when the training sessions consisted of amplitudes heavier than 5 mm (P = .05; MD = 0.02) or 8 g (P = 0.04; MD = 0.02) on bone mass at the lumbar spine. However, no statistical group differences were observed between groups that used higher and lower amplitudes (Table 4 ).
Finally, in relation to the type of exercise used, no significant differences were found between subgroups. However, when WBV was based on static exercises, a significant effect on BMD lumbar spine was observed (P = .04; MD = 0.02; Table 4 ).
4. Discussion
The main purpose of this meta-analysis was to evaluate published, RCTs that investigated the effects of WBV training on bone mass in postmenopausal women. The present meta-analysis showed that 3 to 13 months of WBV had no overall pre-post effect on total or femoral neck BMD in postmenopausal women. However, it was determined that this training method is effective in improving lumbar spine BMD in postmenopausal women. Furthermore, the present meta-analysis showed significant differences in femoral neck BMD between the intervention and control groups when analyzing the studies that also included postmenopausal women younger than 65 years.
The exercise characteristics of the WBV appear to explain some of the contrasting findings in the literature. This meta-analysis observed that there were significant pre-post adaptations in lumbar spine BMD, independent of the total number of WBV sessions. However, performing 108 or more sessions showed greater MD compared to <108, with significant differences between subgroups being found in lumbar spine BMD. It is worth noting that the cumulative dose (ie, the total time in which the subjects stand on the vibration platform) is positively related with improved bone mass and that it seems to be more important than the duration of intervention.[18,33] Hence, the number of training sessions per week is more relevant to attain significant improvements on BMD. Interestingly, the results indicated that the magnitude of the increments on bone mass after WBV were independent of the frequency and amplitude of WBV as no significant differences were observed between these subgroups. However, WBV generated improvements on lumbar spine BMD with frequencies >20 Hz and amplitudes >5 mm or 8 g. This is in accordance with previous research that indicated the use of frequency <20 Hz does not provide sufficient training stimulus.[34,35] Moreover, it suggests that the mechanical signals of high frequency and lower amplitudes are needed to effectively transfer the energy to the spine and hip, and thus recommending the employment of frequencies >20 Hz.[36] In relation to the duration of intervention and sessions length, no significant differences were obtained on bone mass. Nevertheless, a tendency for significance was identified (P = .06) for longer length sessions (≥600 seconds). This is in line with other studies that obtained statistical improvements with extended length sessions.[22,24] The subgroup analysis concerning the type of exercise showed no significant differences between groups. Nonetheless, studies that included WBV with static exercises produced positive effects on lower back BMD in comparison with dynamic/mixed training protocols, which showed no changes. This agrees with previous research that observed no changes in lumbar spine BMD following 24 weeks of WBV using static and dynamic knee-extensors exercises.[24] However, Von Stengel et al[25] demonstrated increased in lumbar spine BMD following 12 months of WBV using dynamic squat exercises in postmenopausal women. Thus, it remains unclear whether the type of exercise (static, dynamic or mixed) affects bone mass differently in WBV and further investigations are needed to identify what type of exercise is most effective in improving bone health in this population.
The improvement on bone mass observed with WBV may have depended on a variety of factors that could have interacted with one another, such as loading frequency, magnitude, and rest periods.[37] The varying methodological differences (vibration protocols) among the included studies may explain the controversial results between them. There are several factors, such as elderly,[38] sex, muscle dystrophy,[39,40] and neurological disorders,[41] which are determinants of increased risk for osteoporosis. It is assumed that the vibration training may produce microtrauma to the bone tissue which is then repaired by the osteoblast action,[42] increasing bone density after physical stress. Furthermore, WBV has demonstrated improvements in growth hormone and testosterone levels at the lumbar and hip regions in men and women.[43–45] Several studies have found increases in BMD after WBV,[18,22] although others have not shown any improvement following WBV.[19,21] Ruan et al[22] observed that BMD of the lumbar spine increased by 4.3% and that femoral neck BMD improved by 3.2% following 6 months of WBV (10 min duration, 5 times per week, frequency of 30 Hz and amplitude of 5 mm). The authors observed a significant decrease in BMD in the control group by 1.9% at the lumbar spine and 1.7% at the femoral neck. Karakiriou et al[17] observed no change in BMD of the lumbar spine in the vibration treatment group but a decrease in the control group, suggesting that WBV may have helped maintain BMD. In contrast, Davis et al[21] analyzed bone density in a group of postmenopausal women (62.2 ± 6.0 yr), who were randomly assigned to 3 groups low intensity (2 mm; 30–35 Hz); high-intensity (4 mm; 40–50 Hz); and control group. They showed no changes in BMD in any of the groups following WBV.[21]
There are some explanations for the discrepancy in the literature regarding the benefits of WBV on BMD. It has been suggested that mechanotransduction varies at different regions of the body due to the nonlinear musculoskeletal system, as well as due to the different body positions used during vibration training.[36,46] Therefore, this may explain the differences between the training effect on the femoral neck and lumbar spine based on the amount of stimuli that the region receives. The discrepancy in the literature may also be due to varying sample sizes among the included studies.[47]
In a prior meta-analysis on WBV by Oliveira et al,[48] a significant effect on lumbar spine BMD with WBV was found when compared with a no intervention group, which is in line with our findings. Nevertheless, to the best of the authors’ knowledge, the present meta-analytical review is the first focusing on BMD adaptations in postmenopausal that takes into account age. The current meta-analysis includes a subgroup of postmenopausal women under the age of 65 years. Age and menopause transition are determinant factors to BMD loss. Therefore, it is essential to examine individuals who start this transition well before 65 years of age and how bone loss evolves in these individuals over time.
There are some limitations to the present meta-analysis that should be addressed: the low number of RCTs reviewed due to the few publications in the literature that focused on the effect of WBV intervention on bone mass in postmenopausal women; the authors of the studies used a wide age range when defining menopausal women, which included older women; and the high heterogeneity between studies with regards to WBV protocols.
5. Conclusion
This meta-analysis demonstrated that WBV is a potential nonpharmacological intervention for improving bone mass in postmenopausal and older women, particularly on lumbar spine, which was shown the most sensitive area. In addition, significant differences were found between intervention and control groups in BMD of the femoral neck in postmenopausal women younger than 65 years. WBV is a safe and effective method and may be used in addition to other training methods to minimize BMD reduction in postmenopausal women. However further studies are still needed to define the optimal protocol in this population. Based on the results obtained in the present meta-analysis WBV training improves bone mass in the lumbar spine in postmenopausal women, particularly in women younger than 65 years of age with a BMI <25 kg/m2. When prescribing this type of protocol, professionals should take into consideration the following guidelines: the volume of work should be ≥108 total sessions, the vibration frequency should be ≥20 Hz, and the amplitude of the oscillation should be ≥5 mm/8 g.
Author contributions
Conceptualization: Elena Marín-Cascales, Pedro E. Alcaraz, Jacobo Á. Rubio-Arias.
Data curation: Elena Marín-Cascales, Domingo J. Ramos-Campo, Alejandro Martinez-Rodriguez, Jacobo Á. Rubio-Arias.
Formal analysis: Elena Marín-Cascales, Alejandro Martinez-Rodriguez, Jacobo Á. Rubio-Arias.
Investigation: Pedro E. Alcaraz, Jacobo Á. Rubio-Arias.
Methodology: Elena Marín-Cascales, Domingo J. Ramos-Campo, Alejandro Martinez-Rodriguez, Jacobo Á. Rubio-Arias.
Project administration: Elena Marín-Cascales, Pedro E. Alcaraz, Jacobo Á. Rubio-Arias.
Supervision: Pedro E. Alcaraz, Linda H. Chung, Jacobo Á. Rubio-Arias.
Validation: Pedro E. Alcaraz, Jacobo Á. Rubio-Arias.
Visualization: Pedro E. Alcaraz, Jacobo Á. Rubio-Arias.
Writing – original draft: Elena Marín-Cascales.
Writing – review and editing: Elena Marín-Cascales, Linda H. Chung, Jacobo Á. Rubio-Arias.
Footnotes
Abbreviations: BMC = bone mineral content, BMD = bone mineral density, DXA = dual-energy x-ray absorptiometry, MD = mean difference, RCT = randomized controlled trial, WBV = whole-body vibration.
The authors have no conflicts of interest to disclose.
References
- [1].Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 2000;11:669–74. [DOI] [PubMed] [Google Scholar]
- [2].Gregg EW, Kriska AM, Salamone LM, et al. The epidemiology of quantitative ultrasound: a review of the relationships with bone mass, osteoporosis and fracture risk. Osteoporos Int 1997;7:89–99. [DOI] [PubMed] [Google Scholar]
- [3].Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1435–45. [DOI] [PubMed] [Google Scholar]
- [4].Christenson ES, Jiang X, Kagan R, et al. Osteoporosis management in post-menopausal women. Minerva Ginecol 2012;64:181–94. [PubMed] [Google Scholar]
- [5].World Health Organization. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- [6].ACSM. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 1998;30:975–91. [DOI] [PubMed] [Google Scholar]
- [7].Frost HM. Wolff's Law and bone's structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod 1994;64:175–88. [DOI] [PubMed] [Google Scholar]
- [8].Mosti MP, Kaehler N, Stunes AK, et al. Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J Strength Cond Res 2013;27:2879–86. [DOI] [PubMed] [Google Scholar]
- [9].Marin-Cascales E, Alcaraz PE, Ramos-Campo DJ, et al. Effects of multicomponent training on lean and bone mass in postmenopausal and older women: a systematic review. Menopause 2018;25:346–56. [DOI] [PubMed] [Google Scholar]
- [10].Marques EA, Mota J, Machado L, et al. Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcif Tissue Int 2011;88:117–29. [DOI] [PubMed] [Google Scholar]
- [11].Prioreschi A, Oosthuyse T, Avidon I, et al. Whole body vibration increases hip bone mineral density in road cyclists. Int J Sports Med 2012;33:593–9. [DOI] [PubMed] [Google Scholar]
- [12].Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact 2010;10:193–8. [PubMed] [Google Scholar]
- [13].Liu PY, Brummel-Smith K, Ilich JZ. Aerobic exercise and whole-body vibration in offsetting bone loss in older adults. J Aging Res 2011;2011:379674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Totosy de Zepetnek JO, Giangregorio LM, Craven BC. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J Rehabil Res Dev 2009;46:529–42. [DOI] [PubMed] [Google Scholar]
- [15].Gomez-Cabello A, Ara I, Gonzalez-Aguero A, et al. Effects of training on bone mass in older adults: a systematic review. Sports Med 2012;42:301–25. [DOI] [PubMed] [Google Scholar]
- [16].Beck BR, Norling TL. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil 2010;89:997–1009. [DOI] [PubMed] [Google Scholar]
- [17].Karakiriou SK, Douda HT, Smilios IG, et al. Effects of vibration and exercise training on bone mineral density and muscle strength in post-menopausal women. Eur J Sport Sci 2012;12:81–8. [Google Scholar]
- [18].Lai CL, Tseng SY, Chen CN, et al. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging 2013;8:1603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marin-Cascales E, Rubio-Arias JA, Romero-Arenas S, et al. Effect of 12 weeks of whole-body vibration versus multi-component training in post-menopausal women. Rejuvenation Res 2015;18:508–16. [DOI] [PubMed] [Google Scholar]
- [20].Marin-Cascales E, Alcaraz PE, Rubio-Arias JA. Effects of 24 weeks of whole body vibration versus multicomponent training onmuscle strength and body composition in postmenopausal women: a randomized controlled trial. Rejuvenation Res 2017;20:193–201. [DOI] [PubMed] [Google Scholar]
- [21].Davis R, Rowe J, Nichols D, et al. Effects of two intensities of whole body vibration on fall related risk factors in postmenopausal women. J Womens Health Issues Care 2014;3:2. [Google Scholar]
- [22].Ruan XY, Jin FY, Liu YL, et al. Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin Med J (Engl) 2008;121:1155–8. [PubMed] [Google Scholar]
- [23].Rubin C, Recker R, Cullen D, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 2004;19:343–51. [DOI] [PubMed] [Google Scholar]
- [24].Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 2004;19:352–9. [DOI] [PubMed] [Google Scholar]
- [25].Von Stengel S, Kemmler W, Bebenek M, et al. Effects of whole-body vibration training on different devices on bone mineral density. Med Sci Sports Exerc 2011;43:1071–9. [DOI] [PubMed] [Google Scholar]
- [26].Liphardt AM, Schipilow J, Hanley DA, et al. Bone quality in osteopenic postmenopausal women is not improved after 12 months of whole-body vibration training. Osteoporos Int 2015;26:911–20. [DOI] [PubMed] [Google Scholar]
- [27].Slatkovska L, Alibhai SM, Beyene J, et al. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med 2011;155:668–79. W205. [DOI] [PubMed] [Google Scholar]
- [28].Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord 2006;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iwamoto J, Takeda T, Sato Y, et al. Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in post-menopausal osteoporotic women treated with alendronate. Aging Clin Exp Res 2005;17:157–63. [DOI] [PubMed] [Google Scholar]
- [30].Russo CR, Lauretani F, Bandinelli S, et al. High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil 2003;84:1854–7. [DOI] [PubMed] [Google Scholar]
- [31].Slatkovska L, Beyene J, Alibhai SM, et al. Effect of whole-body vibration on calcaneal quantitative ultrasound measurements in postmenopausal women: a randomized controlled trial. Calcif Tissue Int 2014;95:547–56. [DOI] [PubMed] [Google Scholar]
- [32].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fratini A, Bonci T, Bull AM. Whole body vibration treatments in postmenopausal women can improve bone mineral density: results of a stimulus focussed meta-analysis. PLoS One 2016;11:e0166774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kleinöder H, Mester J. Sicherheit und leistungsoptimierung im vibrationstraining1. BISP Jahrbuch 2003. 253–8. [Google Scholar]
- [35].Yue Z, Mester J. A modal analysis of resonance during the whole-body vibration. Stud ApplMath 2004;112:293–314. [Google Scholar]
- [36].Rubin C, Pope M, Fritton JC, et al. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine (Phila Pa 1976) 2003;28:2621–7. [DOI] [PubMed] [Google Scholar]
- [37].Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev 2003;31:45–50. [DOI] [PubMed] [Google Scholar]
- [38].Duque G, Troen BR. Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc 2008;56:935–41. [DOI] [PubMed] [Google Scholar]
- [39].Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 2000;20:71–4. [PubMed] [Google Scholar]
- [40].Apkon S, Coll J. Use of weekly alendronate to treat osteoporosis in boys with muscular dystrophy. Am J Phys Med Rehabil 2008;87:139–43. [DOI] [PubMed] [Google Scholar]
- [41].Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006;29:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Burr DB, Martin RB, Schaffler MB, et al. Bone remodeling in response to in vivo fatigue microdamage. J Biomech 1985;18:189–200. [DOI] [PubMed] [Google Scholar]
- [43].Murphy S, Khaw KT, Cassidy A, et al. Sex hormones and bone mineral density in elderly men. Bone Miner 1993;20:133–40. [DOI] [PubMed] [Google Scholar]
- [44].Kelly PJ, Pocock NA, Sambrook PN, et al. Dietary calcium, sex hormones, and bone mineral density in men. BMJ 1990;300:1361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 1997;12:1833–43. [DOI] [PubMed] [Google Scholar]
- [46].Kiiski J, Heinonen A, Jarvinen TL, et al. Transmission of vertical whole body vibration to the human body. J Bone Miner Res 2008;23:1318–25. [DOI] [PubMed] [Google Scholar]
- [47].Slatkovska L, Alibhai SM, Beyene J, et al. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int 2010;21:1969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Oliveira LC, Oliveira RG, Pires-Oliveira DA. Effects of whole body vibration on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Osteoporos Int 2016;27:2913–33. [DOI] [PubMed] [Google Scholar]


