Abstract
Background:
The aim of this study was to compare the clinical outcomes between patients with preoperative cholangitis and noncholangitis patients to determine whether the preoperative cholangitis would be able to serve as an independent predictive factor on hilar cholangiocarcinoma (HCC) outcomes.
Methods:
A systematic literature search for reported preoperative cholangitis in patients with hilar cholangiocarcinoma was performed in 4 databases: PubMed, Web of Science, Embase, and the Cochrane Library, published from 1979 to 2017.
Results:
In total, the initial search identified 1228 articles. Of these studies only 9 studies met the inclusion criteria and were included in this analysis. Differences between preoperative cholangitis existing and noncholangitis patients were observed in terms of mortality (RR = 2.29; 95% CI = 1.48–3.52; P = .0002), overall morbidity (RR = 1.15;95% CI = 1.00–1.32; P = .04), Liver failure (RR = 1.15;95% CI = 1.00–1.32; P = .04), Infection (RR = 1.52;95% CI = 1.16–2.00; P = .003), sepsis (RR = 2.40;95% CI = 1.25–4.5; P = .008).
Conclusions:
The results lend support to the notion that in hilar cholangiocarcinoma patients, the existence of preoperative cholangitis is statistically associated with the higher postoperative mortality and morbidity. Also that it increases the risk of liver failure and infection. therefore, it is very important to properly control the preoperative cholangitis before surgery.
Keywords: hilar cholangiocarcinoma, morbidity, mortality, preoperative cholangitis, prognosis
1. Introduction
Hilar cholangiocarcinoma (HCC), also labeled as Klatskin tumor, was firstly reported by Altemeier et al[1] in 1957. It is a cholangiocarcinoma that occurs between the opening of the cystic duct and the secondary branches of the right and left hepatic ducts. According to the Bismuth-Corlette system HCC can be divided into 4 types: tumors for type I infiltrate the common hepatic duct, tumors for type II invade the hilus, tumors for type IIIA/B affect the right or left hepatic duct, and tumors for type IV symbolize both right and left hepatic ducts and the subsegments have been invaded,[2] which preoperative assessment aid us with evaluating local tumor spread and determining the extent of resection for HCC.[3] Studies have revealed that complete resection of HCC with histologically negative margins provides a better possibility for long-time survival postoperatively.[4,5]
Radical resection (R0 resection) appears to be the best approach to achieve higher long-term survival rate for patients with HCC.[6] It was reported that when the radical removal rate was 19% to 75%, the 5-year survival rate reached 10% to 44%.[7–9] Surgical radical resection should include hemihepatic, caudate resection, hepatic portal lymph node dissection, and vascular resection if vascular system was also involved.[10,11]
Diagnosis of preoperative cholangitis has traditionally been made by following the criteria: Temperature: body temperature is higher than 38°C. Liver function: abnormalities in liver function test results and exception of jaundice. Symptoms: the upper right abdominal pain in the presence of a positive bile culture.[12,13] It has been reported that the existence of preoperative cholangitis in patients with HCC is closely related to the incidence of postoperative complications such as liver failure, infection, sepsis, and persistent biliary anastomotic leakage.[14] It is even reported that preoperative cholangitis affected the postoperative survival of patients with HCC.[15] However, it has not been studied whether preoperative cholangitis will affect the prognosis of patients with HCC after radical resection.
The aim of this study is to determine whether preoperative cholangitis will affect the mortality, morbidity, liver failure, infection, sepsis, and survival of patients with HCC after radical resection.
2. Methods
2.1. Literature research
A comprehensive literature search was performed using PubMed, EMBASE, the Cochrane Library and the Web of Science. The keywords and key phrases used for search include: “hilar bile duct neoplasms or hilar bile duct carcinoma or Klatskin tumor or perihilar cholangiocarcinoma or hilar cholangiocarcinoma” and “cholangitis or angiocholitis or choledochitis.” According to the criteria of evaluation and exclusion, all titles and abstracts, full texts if needed, were reviewed. The differences are revealed by consensus. The papers include cross reference to find further relevant research. We also searched for the references contained in the original studies by hand to identify studies that were missing in the initial search. All procedures were approved by the ethics committee for human experiments of the First Hospital of Lanzhou University.
2.2. Study selection criteria
Whether the published studies included preoperative cholangitis and postoperative hilar cholangiocarcinoma related research. Those studies that have no enough data to extract, or unrelated cancers studies (for example, distal bile duct cancer, gallbladder cancer, pancreatic cancer), or HCC studies without operation information were excluded.
2.3. Data extraction
Data extraction was performed independently by 2 researchers (YW and WF), with the discrepancies resolved by the consensus of these 2 researchers (any differences on a contradictory research are solved through full discussion). Information includes authors, years of publication, countries, number of patients, average age range, gender, and postoperative outcomes. The main results were postoperative complications, including mortality, morbidity, infection, and liver failure.
2.4. Statistical analysis
The software Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014) was used to do data analysis. The risk ratio (RR) for each trial was calculated from the number of evaluable patients. Also the RRs with their 2-sided 95 % confidence intervals (CIs) were used for dichotomous outcomes as the confirmatory effect size estimate and test criterion. The fixed-effect model was applied. The hypothesis tests were based on the 95 % CIs, and the P values were used for illustration. Funnel plots were also constructed to look for potential publication bias. We used the χ2 test to evaluate heterogeneity between trials and the I2 statistic to assess the extent of the inconsistency, wherein an I2 test >50% suggests significant heterogeneity. Statistical heterogeneity was assessed using an I2 test and was categorized into low (<50%), moderate (51%–75%), or high (>75%) groups according to predefined criteria.
3. Results
The initial search identified 1228 articles based on the search keywords and phrases. Around 9 retrospective cohort studies were eligible to be included in the study and the data were extracted for this systematic review and meta-analysis. Search procedure and the results are displayed in Figure 1. Of these data, the study reported by Michio et al[16] studying 118 patients with advanced carcinoma (the gallbladder and the proximal bile duct cancers) involved the hepatic hilus. Table 1 provides the detailed information about these 9 studies[14,16–23] that were included in the systematic review and meta-analysis. No randomized control trial was included, the quality of the studies included in the meta-analysis was assessed by the NOS scale. Overall, an average medium quality (5 out of 9 stars) was achieved in all studies (range 5–6). Table 2 illustrated the effect of preoperative cholangitis on patients with hilar cholangiocarcinoma.
Figure 1.
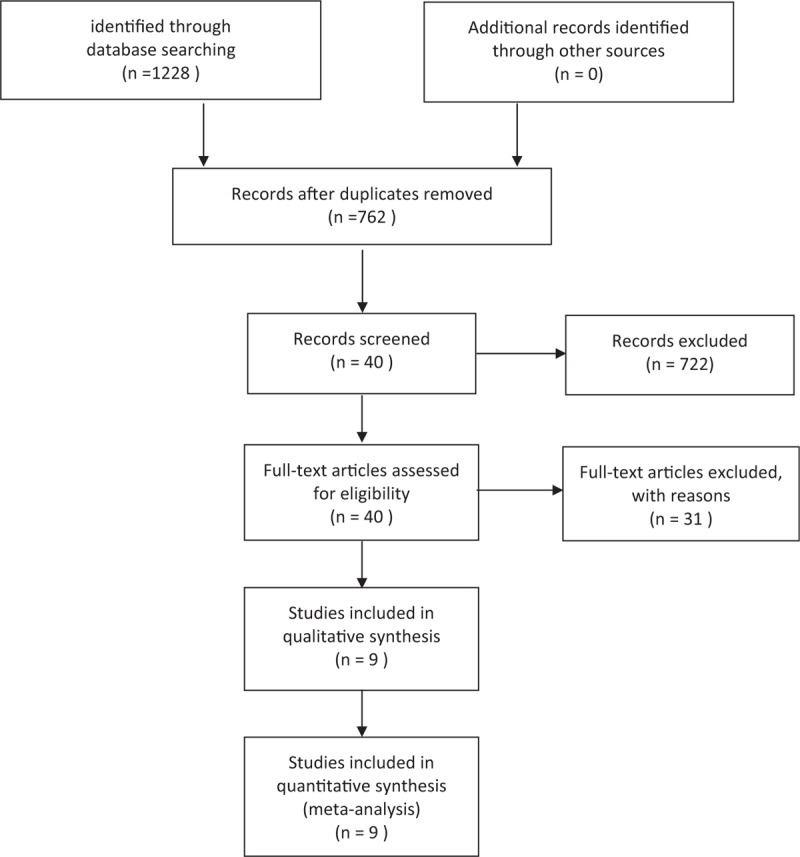
Search flow diagram.
Table 1.
Characteristics of included studies.
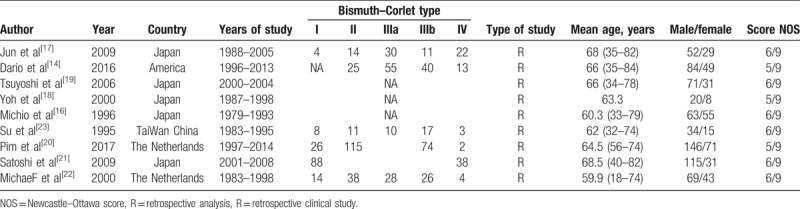
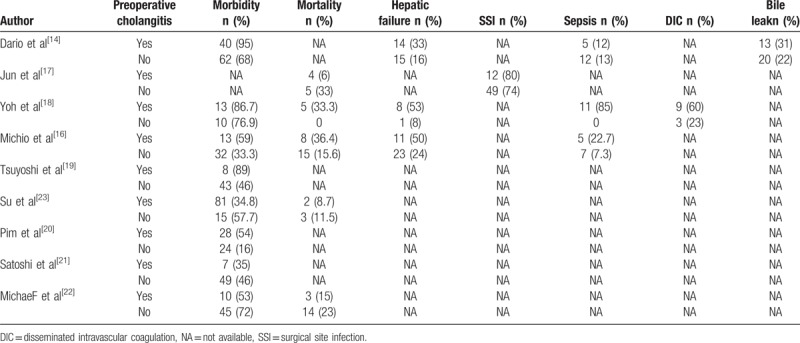
Table 2.
The characteristics of preoperative cholangitis-related outcomes of included studies.
3.1. Primary outcomes: morbidity and mortality
Postoperative morbidity was identified in 7 studies[14,16,18,19,21–23] (n = 638 patients) in total. The RR and 95% CI for each study and the pooled RR are shown in Figure 2. In https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5671862/figure/F2/the fixed effects model (RR = 1.15; 95% CI = 1.00–1.32), heterogeneity testing revealed I2 = 79% and revealed a significant difference in the incidence of overall complications in favor of the no-cholangitis (P = .04).
Figure 2.
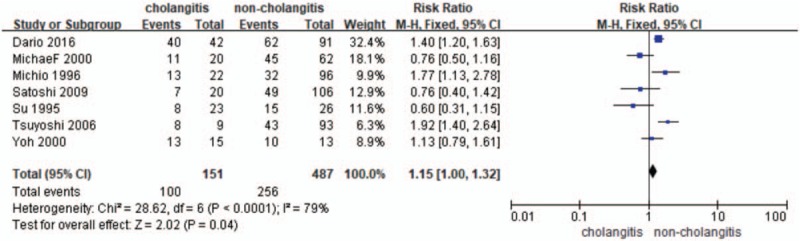
Forest plot for morbidity (cholangitis vs noncholangitis).
Six out of the 7 studies[14,16–18,22,23] provided the data (n = 491 patients) on the incidence of mortality. The RR and 95% CI for each study and the pooled RR are shown in Figure 3. The overall summary estimated RR was 2.29 (95% CI: 1.48–3.52; P = .0002). Heterogeneity testing revealed I2 = 60% and the P value for heterogeneity is .06, when analyzed using a fixed-effect model.
Figure 3.
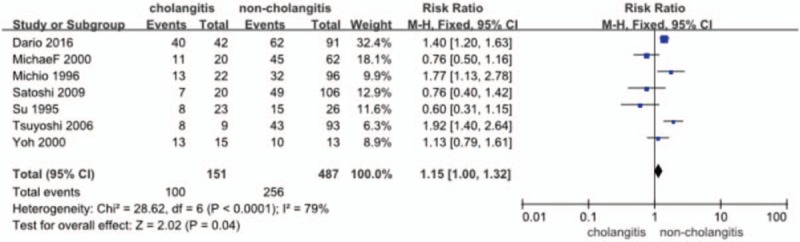
Forest plot for mortality (cholangitis vs noncholangitis).
3.2. Secondary outcomes: the incidence of hepatic failure, infection and sepsis
3.2.1. Hepatic failure
Data were extracted from 7 studies[14,16,18,19,21–23] (n = 638 patients) on the incidence of hepatic failure. The RR and 95% CI for each study and the pooled RR are shown in Figure 4. The fixed effects model (RR = 1.15; 95% CI = 1.00–1.32) showed a significant difference in the incidence of hepatic failure, in favor of the no-cholangitis group (P = .04). Heterogeneity testing revealed I2 = 79%.
Figure 4.
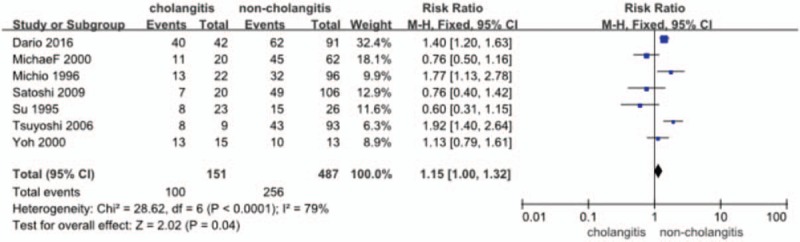
Forest plot for the incidence of hepatic failure (cholangitis vs noncholangitis).
3.2.2. Infection
Four studies[14,16–18] provided the data (n = 360 patients) on the incidence of infection. Of them, one study reported by Sakata et al[17] compared surgical site infection (SSI) between preoperative cholangitis and noncholangitis patients. The RR and 95% CI for each study and the pooled RR are shown in Figure 5. The fixed effects model (RR = 1.52; 95% CI = 1.16–2.00) showed a significant difference in the incidence of infection, in favor of the no-cholangitis group (P = .003). Heterogeneity testing revealed I2 = 74%.
Figure 5.
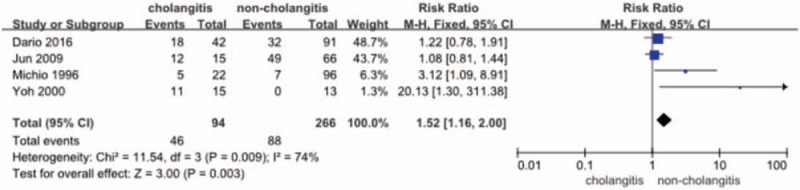
Forest plot for the incidence of infection (cholangitis vs noncholangitis).
3.2.3. Sepsis
Three studies[14,16,18] provided the data (n = 279 patients) on the incidence of sepsis. The RR and 95% CI for each study and the pooled RR are shown in Figure 6. The fixed effects model (RR = 2.40; 95% CI = 1.25–4.59) showed a significant difference in the incidence of sepsis in favor of the no-cholangitis group (P = .008). Heterogeneity testing revealed I2 = 79%.
Figure 6.
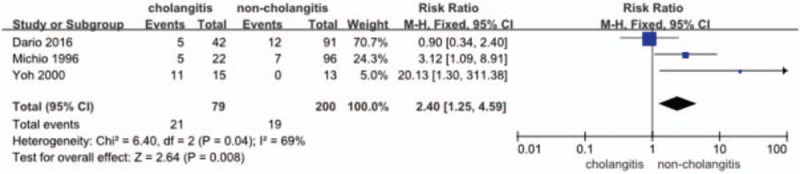
Forest plot for the incidence of sepsis (cholangitis vs noncholangitis).
3.2.4. Publication bias
The funnel plot (Fig. 7) showed no evidence of noticeable asymmetry. Egger test similarly showed no publication bias (Egger t value = −1.37 P = .229).
Figure 7.
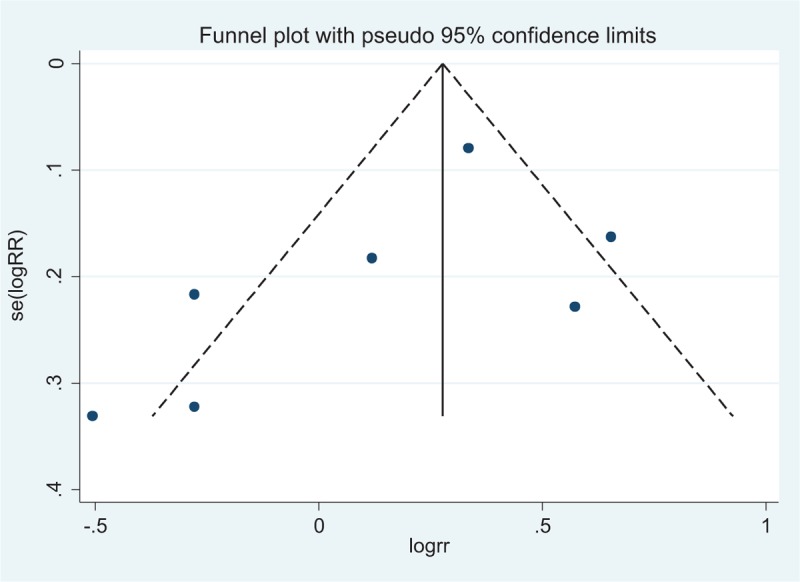
Funnel plot for publication bias (postoperative morbidity between cholangitis and noncholangitis).
4. Discussion
Radical resection is standard of care and is the only method of long-term survival for patients with HCC.[6] Surgical resection of hilar cholangiocarcinoma often requires hemi hepatectomy and complete caudate lobectomy in order to achieve R0 resection.[24] Some surgeons advocate that biliary drainage should be performed before surgery.[25] The biliary drainage method mainly includes percutaneous transhepatic biliary drainage (PTBD), endoscopic nasobiliary drainage (ENBD), and endoscopic biliary stenting (EBS). But most of these operations will induce cholangitis. Doctors have made some effort to avoid cholangitis, but the effect is not satisfactory. It is not clear whether preoperative cholangitis will lead to poor prognosis of patients with hilar cholangiocarcinoma after radical surgery. We found that (primary sclerosing cholangitis) PSC patients had significantly higher overall survival and disease-free survival compared with non-PSC patients.[26] However, some studies showed that preoperative cholangitis considered as an independent predictor of postoperative morbidity,[19,27] was associated with worse short-term outcomes such as postoperative hospitalization, in-hospital mortality, and postoperative infectious complications for patients with hilar cholangiocarcinoma after radical resection.[28,29] Therefore, more study needed to be done to draw a clearer conclusion.
The present study demonstrated that by controlling the incidence of preoperative cholangitis, postoperative morbidity and mortality reduced, and also improved long-term patient prognosis.[30] Therefore, sufficient management of preoperative cholangitis is highly recommended for HCC patients who has cholangitis. As such, the current study is important because the data demonstrated that through careful management of preoperative cholangitis, the margin of long-term survival without increasing postoperative morbidity can be achieved. No recommendations have been reached regarding to the most appropriate drainage method.[31] Tang et al[32] showed that PTBD should be used as the initial method of biliary drainage in type III or IV patients to reduce the incidence of procedure-related cholangitis, pancreatitis, and to improve the rates of palliative relief of cholestasis. For patients who had major hepatectomy, ENBD was recommended for biliary drainage to save the liver function due to its more sufficient potency and less preoperative cholangitis compared to endoscopic retrograde biliary drainage (ERBD).[33,34] Complete preoperative drainage of the FLR (future liver remnant) segments corelates with lower postoperative mortality in patients with an FLR volume below 50%. By contrast, there is lack of evidence to support preoperative biliary drainage in the presence of an FLR volume above 50%. For these patients, the risk of cholangitis and associated mortality developing after drainage seems to outweigh the questionable benefit of biliary decompression.[35]
In this meta-analysis, for the first time we extracted all qualified published data comparing the complications associated with preoperative cholangitis in patients with hilar cholangiocarcinoma and pooled them together. The primary outcome showed that preoperative cholangitis is closely associated with higher risk of morbidity and mortality in patients with hilar cholangiocarcinoma, compared to that of noncholangitis. Seven studies including 638 patients with hilar cholangiocarcinoma provide postoperative morbidity data. The postoperative overall morbidity was 66.23% (100/151) of patients in preoperative cholangitis group compared to 52.57% (256/487) in the noncholangitis group. Six studies including 491 patients with hilar cholangiocarcinoma provide postoperative mortality data. The postoperative mortality was 24.09% (33/137) in patients with preoperative cholangitis compared to the rate of 11.58% (41/354) in the noncholangitis group.
The second outcome demonstrated that the incidence of hepatic failure, infection, and sepsis were significantly higher in the preoperative cholangitis group than those in the noncholangitis group. Because the lack of sufficient studies to describe the overall survival, it is not possible to make Forest plot. In univariate analysis, preoperative cholangitis patients had significantly reduced overall survival (5-year estimate 29.9%) compared to noncholangitis patients (40.5%) (P = .009).[36] And cholangitis was associated with a significant decrease in both disease-free and overall survival.[30]
For the first time, we show here an independent and strong association of preoperative cholangitis with an increased risk of death and postoperative complications, such as liver failure, infection, sepsis, and persistent biliary anastomotic leakage, and a poor prognosis from R0 resection of hilar cholangiocarcinoma. That preoperative cholangitis frequently results in postoperative complications were shown in several studies, nevertheless, these previous studies failed to find a direct link between preoperative cholangitis and considerable risk of main complications or deaths after R0 resection, indicating that the exact effect of cholangitis on post-resection prognosis, in the light of these evidence, was poorly defined and difficult to evaluate.
This meta-analysis still has limitations. First, the included studies are retrospective and some of them with a limited sample size. Second, due to the paucity of data, we were not able to compare overall survival in patients with cholangitis versus no-cholangitis patients, and we were also unable to perform a subgroup analysis based on the type of malignancy, the method of surgery. Third, with the advances in technology, the result should also be affected in the different study period of the included studies (3 of these[16,22,23] were published before 2000).
The advantage of this meta-analysis was the use of the high-quality methodology of statistical analysis, which incorporated many patients associated with this study. The new test is included in this study, adding the latest published data, and this study still the first systematic analysis assessing the preoperative cholangitis-related complications for patients with HCC.
In conclusion, evidence was provided in this systematic review and meta-analysis that higher overall morbidity, mortality, and other complications were concerned with preoperative cholangitis. Additionally, further randomized control trials should be performed to confirm our conclusions. We confirm that preoperative cholangitis directly affects the outcomes after radical resection in patients with hilar cholangiocarcinoma, so, effective strategies should be carried out to reduce the risk of preoperative cholangitis and improve the prognosis of patients with HCC.
Author contributions
Wenbo Meng, Wence Zhou and Xun Li: study concept and design, study supervision, critical revision of the manuscript and funding obtaining.
Wengkang Fu: data collection, extraction, statistical analysis and interpretation; critical revision of the intellectual content of the manuscript.
Yudong Wang: study concept and design; data collection, extraction, statistical analysis and interpretation; manuscript drafting; critical revision of the intellectual content of the manuscript.
Zengwei Tang: critical revision of the intellectual content of the manuscript.
Conceptualization: Yudong Wang, Wence Zhou, Xun Li.
Data curation: Yudong Wang, Wenkang Fu.
Formal analysis: Yudong Wang, Wenkang Fu, Zengwei Tang.
Funding acquisition: Wenbo Meng.
Investigation: Yudong Wang, Wenkang Fu, Zengwei Tang.
Methodology: Yudong Wang, Wenkang Fu.
Resources: Yudong Wang, Wenkang Fu.
Software: Yudong Wang, Wenkang Fu.
Supervision: Wenbo Meng, Wence Zhou, Xun Li.
Writing – original draft: Yudong Wang.
Writing – review & editing: Yudong Wang, Wenkang Fu, Zengwei Tang, Wenbo Meng, Wence Zhou, Xun Li.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, HCC = hilar cholangiocarcinoma, OS = overall survival, RR = relative risk, SSI = surgical site infection.
Fundings: The study was funded by West Light Foundation of The Chinese Academy of Science [2015 (90)]. The funding sources had no role in the design conduct of the study, collection, analysis, management and interpretation of the data, or preparation, review, or approval of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Altemeier WA, Gall EA, Zinninger MM, et al. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg 1957;75:450. [DOI] [PubMed] [Google Scholar]
- [2].Baton O, Azoulay D, Adam DV, et al. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and long term outcomes. J Am Coll Surg 2007;204:250–60. [DOI] [PubMed] [Google Scholar]
- [3].Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Byrnes V, Afdhal N. Cholangiocarcinoma of the hepatic hilum (Klatskin tumor). Curr Treat Options Gastroenterol 2002;5:87–94. [DOI] [PubMed] [Google Scholar]
- [5].Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg 2014;399:693–705. [DOI] [PubMed] [Google Scholar]
- [6].Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol 2007;13:1505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Poruk KE, Pawlik TM, Weiss MJ. Perioperative management of hilar cholangiocarcinoma. J Gastrointest Surgn 2015;19:1889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu HJ, Mao H, Shrestha A, et al. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: a single-institution experience in China. World J Gastroenterol 2016;22:2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ebata T, Kosuge T, Hirano S, et al. Proposal to modify the International Union Against Cancer staging system for perihilar cholangiocarcinomas. Br J Surg 2014;101:79–88. [DOI] [PubMed] [Google Scholar]
- [10].Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg 2013;83:268–74. [DOI] [PubMed] [Google Scholar]
- [11].Hemming AW, Mekeel K, Khanna A, et al. Portal vein resection in management of hilar cholangiocarcinoma. J Am Coll Surg 2011;212:604–13. [DOI] [PubMed] [Google Scholar]
- [12].Nomura T, Shirai Y, Hatakeyama K, et al. Impact of bactibilia on the development of postoperative abdominal septic complications in patients with malignant biliary obstruction. Int Surg 1999;84:204–8. [PubMed] [Google Scholar]
- [13].Nomura T, Shirai Y, Hatakeyama K, et al. Bacteribilia and cholangitis after percutaneous transhepatic biliary drainage for malignant biliary obstruction. Dig Dis Sci 1999;44:542–6. [DOI] [PubMed] [Google Scholar]
- [14].Ribero D, Zimmitti G, Aloia TA, et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg 2016;223:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okuno Masataka, Ebata Tomoki, Yokoyama Yukihiro, et al. Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. J Gastroenterol 2016;51:153–61. [DOI] [PubMed] [Google Scholar]
- [16].Kanai Michio, Nimura Yuji, Kamiya Junichi, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery 1996;119:498–504. [DOI] [PubMed] [Google Scholar]
- [17].Sakata J, Shirai Y, Tsuchiya Y, et al. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbecks Arch Surg 2009;394:1065–72. [DOI] [PubMed] [Google Scholar]
- [18].Yoh H, Takao S, Shinchi H, et al. Management of preoperative cholangitis improves the hepatic failure after hepatectomy for hilar cholangiocarcinoma. Nippon Shokaki Geka Gakkai Zasshi 2000;33:44–52. [Google Scholar]
- [19].Sano T, Shimada K, Sakamoto Y, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg 2006;244:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Olthof PB, Wiggers JK, Oberkampf BG, et al. Postoperative liver failure risk score: identifying patients with resectable perihilar cholangiocarcinoma who may benefit from portal vein embolization. J Am Coll Surg 2017;225:387. [DOI] [PubMed] [Google Scholar]
- [21].Hirano S, Kondo S, Tanaka E, et al. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci 2010;17:455. [DOI] [PubMed] [Google Scholar]
- [22].Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma—a single center experience. Surgery 2000;127:395–404. [DOI] [PubMed] [Google Scholar]
- [23].Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg 1996;223:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Min SC, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672–9. [DOI] [PubMed] [Google Scholar]
- [25].Ratti F, Cipriani F, Ferla F, et al. Hilar cholangiocarcinoma: preoperative liver optimization with multidisciplinary approach. Toward a better outcome. World J Surg 2013;37:1388–96. [DOI] [PubMed] [Google Scholar]
- [26].Lehrke HD, Heimbach JK, Wu TT, et al. Prognostic significance of the histologic response of perihilar cholangiocarcinoma to preoperative neoadjuvant chemoradiation in liver explants. Am J Surg Pathol 2016;40:510–8. [DOI] [PubMed] [Google Scholar]
- [27].Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kitahata Y, Kawai M, Tani M, et al. Preoperative cholangitis during biliary drainage increases the incidence of postoperative severe complications after pancreaticoduodenectomy. Am J Surg 2014;208:1–0. [DOI] [PubMed] [Google Scholar]
- [29].Liu F, Li Y, Wei Y, et al. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 2011;56:663–72. [DOI] [PubMed] [Google Scholar]
- [30].Dumitrascu T, Chirita D, Ionescu M, et al. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg 2013;17:913–24. [DOI] [PubMed] [Google Scholar]
- [31].Forsmark CE, Diniz AL, Zhu AX, et al. Consensus conference on hilar cholangiocarcinoma. HPB 2015;17:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tang Z, Yang Y, Meng W, et al. Best option for preoperative biliary drainage in Klatskin tumor: a systematic review and meta-analysis. Medicine 2017;96:8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kawashima H, Itoh A, Ohno E, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg 2013;257:121–7. [DOI] [PubMed] [Google Scholar]
- [34].Kawakami H, Kondo S, Kuwatani M, et al. Preoperative biliary drainage for hilar cholangiocarcinoma: which stent should be selected? J Hepatobiliary Pancreat Sci 2011;18:630–5. [DOI] [PubMed] [Google Scholar]
- [35].Wiggers JK, Koerkamp BG, Cieslak KP, et al. Development of a preoperative risk score for postoperative mortality after liver resection for presumed perihilar cholangiocarcinoma. HPB 2016;18:863–4. [Google Scholar]
- [36].Okuno M, Ebata T, Yokoyama Y, et al. Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. J Gastroenterol 2016;51:153–61. [DOI] [PubMed] [Google Scholar]


