Abstract
Background:
To assess the effect of dexmedetomidine added to ropivaccaine on the onset and duration of sensory block, as well as postoperative analgesia during caudal anesthesia in patients undergoing hemorrhoidectomy.
Methods:
Fifty adult patients scheduled for hemorrhoidectomy were divided into 2 groups. The group R received caudal anesthesia using 18 mL 0.3% ropivacaine plus 2 mL normal saline. The group RD received 18 mL 0.3% ropivacaine plus 2 mL 1 μg/kg dexmedetomidine. Heart rate, mean blood pressure, onset time and duration of sensory block, and duration of analgesia were observed.
Results:
The onset time of sensory block was shortened (9.2 ± 1.3 vs 7.2 ± 1.2), and the duration of sensory block (3.0 ± 0.7 vs 3.8 ± 0.8) and duration of analgesia (3.9 ± 0.7 vs 5.3 ± 0.8) were prolonged in group RD compared with group R (P < .05). The heart rate and the mean blood pressure were also lower in the group RD compared with group R at each observation time points, except the baseline (P < .05). No bradycardia or hypotension was reported.
Conclusion:
Dexmedetomidine as an adjuvant to ropivacaine prolonged the duration of caudal block and improved postoperative analgesia without significant side effects in adult patients undergoing hemorrhoidectomy.
Keywords: caudal anesthesia, dexmedetomidine, postoperative analgesia, ropivacaine
1. Introduction
Hemorrhoidectomy is performed for patients with severe hemorrhoid diseases; however, hemorrhoidectomy is generally associated with intense and prevalent pain.
Successful treatment of perioperative acute pain could facilitate early rehabilitation and improve patient comfort.
Single-shot caudal block could be as an ideal perioperative pain management strategy in patient undergoing hemorrhoidectomy, because of the ease of performing, the reliable analgesia and the relaxation of anal sphincter it provides.[1] However, the duration of analgesia provided by this strategy is limited by the short duration of action of the local anesthetic.[2] Various adjuvants to local anesthetics have been investigated to improve the quality of block and duration of analgesia, including fentanyl,[3] morphine,[4] ketamine,[5] midazolam,[6] and magnesium.[7] Each of these adjuvants has side effects specific to the type and dose of adjuvant used. For instance, behavioral changes have been noted with the use of caudal ketamine, opioids are associated with risk of respiratory depression, and the neurotoxicity of midazolam is still controversy. Therefore, an ideal adjuvant that provides maximal analgesia with minimal side effects for caudal block is still a matter of contention.
Dexmedetomidine (DEX) is a highly selective α2-adrenoceptor agonist, possesses sedative, analgesic, anxiolytic, and anti-inflammatory properties.[8–10] When administered in combination with local anesthetics in the epidural space, it has been shown to reduce postoperative analgesic requirements[11] and have a significantly analgesic effect.[12] The advantage of DEX compared to other adjuvants makes it an attractive choice for further study.
Therefore, we designed a prospective, double-blinded, randomized study to assess the efficacy of DEX as an adjuvant to ropivacaine for caudal block in patients undergoing hemorrhoidectomy.
2. Methods
The study was approved by the Hospital Ethics Committee of the First People's Hospital of Lianyungang. After obtaining written information consent from patients, 50 patients aged 18 to 60 years with American Society of Anesthesiologists grades I–II and underwent hemorrhoidectomy were enrolled. Exclusion criteria included a history of allergy to any local anesthetics, body mass index >40 kg/m2, chronic use of analgesics, contraindication caudal block (e.g., coagulation defects, infection at puncture site, and preexisting neurological or spinal disease), and history of severe cardiac, respiratory, hepatic, or renal disease.
Patients were randomly divided into 2 groups by using a computer-generated random number table. The group allocation was concealed in sealed opaque envelopes, which were opened just before performance of the block.
Group R received caudal block with 18 mL 0.3% ropivacaine plus 2 mL normal saline. Group RD received caudal block with 18 mL 0.3% ropivacaine plus 2 mL 1.0 μg/kg DEX dissolved in normal saline. The drug solutions were prepared by an anesthesiologist who was not involved in the study.
After arriving at the operation room, all the patients received standard examinations including electrocardiogram, pulse oxygen saturation (SpO2), and noninvasive blood pressure (BP) monitoring. A 20-G cannula was inserted into the dorsum of the patient's hand, Ringer lactate was infused at a rate of 4 to 6 mL/min. The patient was in the left lateral position. With a sterile technique, the caudal space was identified using standard landmarks, a 22-G needle was inserted into the caudal epidural space. After negative aspiration for blood or cerebrospinal fluid, and confirmation of the caudal epidural space by modified swoosh test, then the study drugs was injected into the caudal.
Patient demographic information including age, height, weight, gender, block performance time, and surgery time were recorded.
Patients’ heart rate (HR), noninvasive BP, and oxygen saturation (SpO2) were recorded at baseline, after administration of the block, and then every 5 minutes until the end of the surgery.
Perioperative hypotension (systolic BP < 20% of baseline) and bradycardia (HR < 50 beats/min) were recorded.
Sensory block was assessed by pinprick sensation using a 3-point scale (0–2, 0 = normal sensation, 1 = decreased pain sensation to pinprick, 2 = loss of pain sensation to pinprick) every 2 minutes for 20 minutes.
The onset time of sensory block (time since administration of block until loss of pain sensation) was recorded.
Duration of sensory block (time interval between administration of the block and complete resolution of anesthesia) was recorded.
Duration of analgesia (time interval between onset of block to the first complaint of pain [visual analog scale score > 4 or first rescue analgesic]) was recorded. Intravenous parecoxib sodium 40 mg was administered as a rescue analgesic.
2.1. Statistical analysis
The sample size was calculated on the basis of our pilot study, and a power analysis was performed using the onset time of sensory block. We projected a mean onset time of sensory block value of 9.1 minutes and standard deviation (SD) 1.8 minutes in 10 patients who received caudal block with ropivacaine. We calculated that 21 patients was required for each group to detect significant between-group differences of 20%, with an α = 0.05, 2-tailed, and β = 0.1. Taking into account potential dropouts in both groups, we decided to enroll 50 patients.
Statistical analyses were performed using SPSS 16.0 for windows (SPSS 16, Chicago, IL). Continuous numerical data were expressed as mean and SD. Categorical data were expressed as frequencies and percentages. Normally distributed numerical data between groups were analyzed using the Student t test. Categorical variables were compared using the Fisher exact test or the Pearson Chi-squared test as applicable. All tests were 2-tailed. P < .05 was considered statistically significant.
3. Results
Fifty patients were enrolled in this study. Two patients refused caudal anesthesia, and 2 patients did not meet the inclusion criteria. Therefore, 23 patients were randomized in each group in this study.
Patients in both groups were comparable with respect to demographic characteristic data and block performance time, as well as surgery time (P > .05) (Table 1).
Table 1.
Comparison of demographics, block performance time, and surgery time between the 2 groups.

Compared with group R, the onset time of sensory block in group RD was decreased. The duration of sensory block was significantly prolonged in group RD. Similarly, the duration of analgesia was also prolonged in group RD as compared to group R (P < .05) (Table 2).
Table 2.
Onset time and duration of block and postoperative analgesia.

Compared with group R, HR, and mean BP were lower in group RD at each observation time points (P < .05), except at baseline (Figs. 1 and 2). However, none of the patients developed hypotension or bradycardia.
Figure 1.
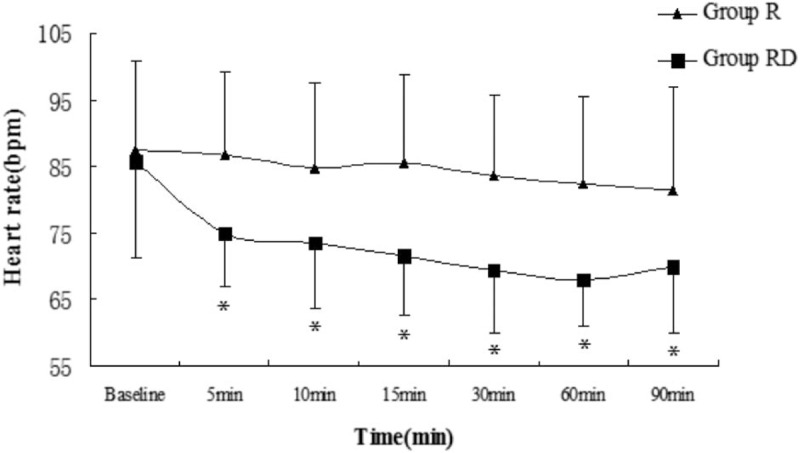
Heart rate (mean value) in both groups at observation time points. Bar indicates standard deviation. ∗P < .05. R = ropivacaine, RD = ropivacaine with dexmedetomidine.
Figure 2.
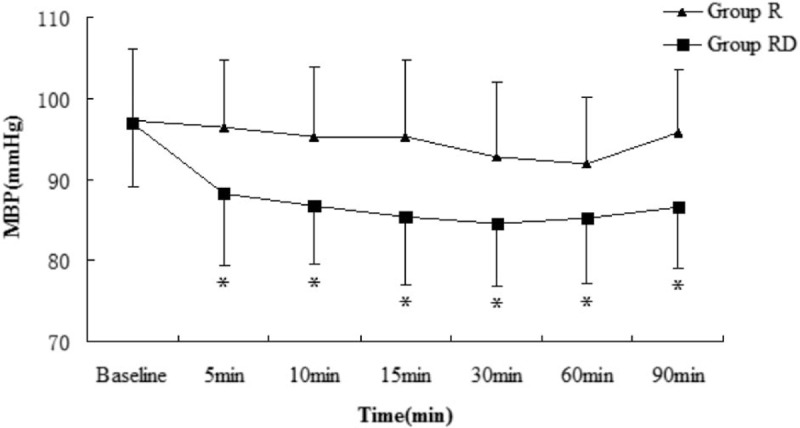
Mean blood pressure (MBP; mean value) in both groups at observation time points. Bar indicates standard deviation. ∗P < .05. R = ropivacaine, RD = ropivacaine with dexmedetomidine.
4. Discussion
In this prospective study, our results showed that the addition of DEX to ropivacaine for caudal block significantly reduced the onset time of sensory block, prolonged the duration of sensory block, as well as postoperative analgesia in patients undergoing hemorrhoidectomy.
Perioperative pain in hemorrhoid surgery is especially intense and prevalent, which may have a negatively affect on postoperative recovery quality. Single-shot caudal block is an acceptable and popular method of providing intra- and postoperative analgesia for hemorrhoidectomy. However, the short duration of action of local anesthetics is one major limitation of this technique.
The DEX is a highly selective α2-adrenergic agonist with sedative and analgesic properties, which has been found as an ideal adjuvant to local anesthetics to relieve postoperative pain and reduce postoperative opioid consumption in many surgical settings.[13] Makhni et al reported that DEX as an intrathecal adjuvant with ropivacaine for spinal anesthesia was better than MgSO4 in providing early onset of sensory and motor block as well as in providing postoperative analgesia.[14] Park et al reported that DEX as an epidural adjuvant had a greater analgesic and local anesthetic-sparing effect, compared to fentanyl, in the early postoperative period in children undergoing major orthopedic lower extremity surgery.[12] In addition, it has been reported that addition of DEX to local anesthetics for brachial plexus block shortens sensory and motor block onset time and extends sensory block, motor block, and analgesia duration.[15] Furthermore, adding DEX to caudal local anesthetics has been recently reported to prolong postoperative analgesia. Kamal et al reported that addition of DEX to caudal ropivacaine significantly prolongs analgesia in children undergoing lower abdominal surgeries.[16] Goyal et al reported that DEX as adjuvant to bupivacaine increases duration of caudal analgesia and improves hemodynamic stability in children undergoing infraumbilical surgeries.[17] However, studies about DEX added to local anesthetics for caudal anesthesia in adult patients are fewer than that in pediatric patients. In our study, we found that adding DEX to ropivacaine for caudal block shortened the onset time of sensory block, prolonged the duration of sensory block and postoperative analgesia. Our results were similar to the study conducted by Neerja et al, they concluded that the addition of DEX to ropivacaine-lidocaine shortened the onset time and prolonged the duration of supraclavicular brachial plexus block, as well as improved postoperative analgesia in patients undergoing upper limb surgeries.[18]
The spinal mechanism may be mainly responsible for the analgesia effect of DEX when it was added to ropivacaine for caudal block. By inhibition of substance P release in the nociceptive pathway at the level of dorsal root neuron and by the activation of α2-adrenoceptors in the locus coeruleus.[19,20]
Bradycardia and hypotension are considered to be the most significant side effects of DEX. Esmaoglu et al reported that the HR, systolic arterial BP and diastolic arterial BP were lower when added DEX to levobupivacaine for axillary brachial plexus block, they also found that the addition of 100 μg DEX to 0.5% levobupivacaine caused bradycardia (HR < 50) in 7 of 30 patients.[21] Neerja et al conducted a study using 1 μg/kg DEX along with 0.75% ropivacaine and 1% lidocaine for supraclavicular brachial plexus block in patients undergoing upper limb surgeries; they found that HR and mean arterial pressure were lower in the DEX group compared with the control group at observation time points.[18] In our study, we found that HR and mean BP were also lower in the DEX group at each observation time points, except at the baseline. However, there was no bradycardia or hypotension recorded in our study.
4.1. Limitations
One limitation of our study is that we only used one concentration of DEX and we also did not detect the plasma levels of DEX. However, we used 1 μg/kg DEX for caudal block; this has been safely used for caudal anesthesia and analgesia.[22] An additional limitation of our study is that we did not assess the sedation scoring which is one of the commonest side effects of α2-adrenergic agonists. Further clinical studies are needed to resolve these problems.
In conclusion, we conclude that the addition of 1 μg/kg DEX to 0.3% ropivacaine for caudal block shortens the onset time of sensory block, prolongs the duration of sensory block and postoperative analgesia. Although decreased HR and mean BP were noted in the DEX group, there was no significant adverse such as bradycardia or hypotension noted.
Author contributions
Conceptualization: Deming Xu, Mingyu Xiu.
Data curation: Deming Xu, Mingyu Xiu, Xiaobao Zhang, Pin Zhu, Liang Tian, Jiying Feng.
Formal analysis: Deming Xu, Mingyu Xiu.
Investigation: Yong Wu.
Methodology: Xiaobao Zhang, Pin Zhu.
Supervision: Zhibin Zhao.
Writing – original draft: Deming Xu, Mingyu Xiu.
Writing – review & editing: Hengfei Luan.
Footnotes
Abbreviations: BP = blood pressure, DEX = dexmedetomidine, DR = dexmedetomidine with ropivacaine, HR = heart rate, R = ropivacaine, SpO2 = oxygen saturation.
DX and MX contributed equally to this work.
This work was funded by the Hansoh Foundation of Lianyungang (QN150203).
The authors have no conflicts of interest to disclose.
References
- [1].Jöhr M, Berger TM. Caudal blocks. Paediatr Anesth 2012;22:44–50. [DOI] [PubMed] [Google Scholar]
- [2].Silvani P, Camporesi A, Agostino MR, et al. Caudal anesthesia in pediatrics: an update. Minerva Anestesiol 2006;72:453–9. [PubMed] [Google Scholar]
- [3].Farooq N, Singh RB, Sarkar A, et al. To evaluate the efficacy of fentanyl and dexmedetomidine as adjuvant to ropivacaine in brachial plexus block: a double-blind, prospective, randomized study. Anesth Essays Res 2017;11:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].El Sherif FA, Mohamed SA, Kamal SM. The effect of morphine added to bupivacaine in ultrasound guided transversus abdominis plane (TAP) block for postoperative analgesia following lower abdominal cancer surgery, a randomized controlled study. J Clin Anesth 2017;39:4–9. [DOI] [PubMed] [Google Scholar]
- [5].Othman AH, El-Rahman AM, El Sherif F. Efficacy and safety of ketamine added to local anesthetic in modified pectoral block for management of postoperative pain in patients undergoing modified radical mastectomy. Pain Physician 2016;19:485–94. [PubMed] [Google Scholar]
- [6].Nahravani M, Tekye SM, Alipour M, et al. Analgesia following arthroscopy - a comparison of intra-articular bupivacaine and/or midazolam and or fentanyl. Arch Bone Jt Surg 2017;5:28–31. [PMC free article] [PubMed] [Google Scholar]
- [7].Sun J, Feng X, Zhu Q, et al. Analgesic effect of perineural magnesium sulphate for sciatic nerve block for diabetic toe amputation: A randomized trial. PLoS One 2017;12:e0176589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999;54:146–65. [DOI] [PubMed] [Google Scholar]
- [9].Ergenoglu P, Akin S, Bali C, et al. Effect of low dose dexmedetomidine premedication on propofol consumption in geriatric end stage renal disease patients. Braz J Anesthesiol 2015;65:326–32. [DOI] [PubMed] [Google Scholar]
- [10].Tan F, Gan X, Deng Y, et al. Intraoperative dexmedetomidine attenuates postoperative systemic inflammatory response syndrome in patients who underwent percutaneous nephrolithotomy: a retrospective cohort study. Ther Clin Risk Manag 2018;14:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zeng X, Jiang J, Yang L, et al. Epidural dexmedetomidine reduces the requirement of propofol during total intravenous anaesthesia and improves analgesia after surgery in patients undergoing open thoracic surgery. Sci Rep 2017;7:3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park SJ, Shin S, Kim SH, et al. Comparison of dexmedetomidine and fentanyl as an adjuvant to ropivacaine for postoperative epidural analgesia in pediatric orthopedic surgery. Yonsei Med J 2017;58:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med 2010;83:11–25. [PMC free article] [PubMed] [Google Scholar]
- [14].Makhni R, Attri JP, Jain P, et al. Comparison of dexmedetomidine and magnesium sulfate as adjuvants with ropivacaine for spinal anesthesia in infraumbilical surgeries and postoperative analgesia. Anesth Essays Res 2017;11:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koraki E, Stachtari C, Kapsokalyvas I, et al. Dexmedetomidine as an adjuvant to 0.5% ropivacaine in ultrasound-guided axillary brachial plexus block. J Clin Pharm Ther 2018;43:348–52. [DOI] [PubMed] [Google Scholar]
- [16].Kamal M, Mohammed S, Meena S, et al. Efficacy of dexmedetomidine as an adjuvant to ropivacaine in pediatric caudal epidural block. Saudi J Anaesth 2016;10:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goyal V, Kubre J, Radhakrishnan K. Dexmedetomidine as an adjuvant to bupivacaine in caudal analgesia in children. Anesth Essays Res 2016;10:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Neerja B, Dinesh K, Sardana, et al. The analgesic efficacy of dexmedetomidine as an adjunct to local anesthetics in supraclavicular brachial plexus block: a randomized controlled trial. Anesth Analg 2015;121:1655–60. [DOI] [PubMed] [Google Scholar]
- [19].Gabriel JS, Gordin V. Alpha 2 agonists in regional anesthesia and analgesia. Curr Opin Anaesthesiol 2001;14:751–3. [DOI] [PubMed] [Google Scholar]
- [20].Guo TZ, Jiang JY, Buttermann AE, et al. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology 1996;84:873–81. [DOI] [PubMed] [Google Scholar]
- [21].Esmaoglu A, Yegenoglu F, Akin A, et al. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg 2010;111:1548–51. [DOI] [PubMed] [Google Scholar]
- [22].Trifa M, Tumin D, Tobias JD. Dexmedetomidine as an adjunct for caudal anaesthesia and analgesia in children: a review. Minerva Anestesiol 2018;84:836–47. [DOI] [PubMed] [Google Scholar]


