Supplemental Digital Content is available in the text
Keywords: cryptogenic stroke, device closure, medical therapy, patent foramen ovale
Abstract
Background:
It was under debate whether cryptogenic stroke patients benefited from patent foramen ovale (PFO) closure. We sought to determine secondary prevention strategy in these patients.
Methods:
Scientific databases were searched for randomized controlled trials enrolling cryptogenic stroke patients with PFO who underwent PFO closure or medical therapy. The random-effect model was used to analyze the outcomes.
Results:
We identified 6 trials enrolling 3630 participants in this meta-analysis. When compared with medical therapy, PFO closure reduced risks of recurrent stroke (risk ratio [RR] 0.52, 95% confidence interval [CI] 0.29–0.93) and composite of stroke and transient ischemic attack (TIA) (RR 0.60, 95% CI 0.46–0.80). And no differences in all-cause death (RR 0.80, 95% CI 0.37–1.72) and cardiovascular death (RR 1.47, 95% CI 0.36–5.94) between 2 groups were observed. The risks of major bleeding (RR 0.96, 95% CI 0.47–1.96) and any serious adverse event (RR 1.03, 95% CI 0.92–1.16) did not differ between 2 groups. Yet, PFO closure increased risk of atrial fibrillation (RR 4.25, 95% CI 2.10–8.60).
Conclusion:
PFO closure, as compared with medical therapy, was associated with decreased risk of recurrent stroke and increased risk of atrial fibrillation in cryptogenic stroke patients with PFO.
1. Introduction
Stroke was the third-leading death cause among adults and the main factor of long-term functional impairment and disability.[1] Approximately 25% to 40% of ischemic strokes had no identifiable cause, which were classified as cryptogenic strokes.[2,3] A part of cryptogenic strokes might be the results of paradoxical embolism which arose from venous embolus reaching systemic circulation due to right-to-left shunt in congenital heart disease patients. Patent foramen ovale (PFO), observed in 14.9% to 27% of populations,[4,5] provided anatomic substrate for paradoxical embolism. Numerous observational studies indicated that cryptogenic stroke was associated with PFO[6,7] and this relationship was more convincingly demonstrated in young patients.[8] This implied PFO closure might be beneficial to secondary prevention of cryptogenic stroke. But not all studies were in favor of this association. The Northern Manhattan Study[5] showed PFO was not associated with the increased stroke risk in males and females. In addition, PFO was not the significant and independent predictor of stroke among normal subjects older than 45 years.[9]
Therefore, physicians have shown great interest in the role of PFO closure in secondary prevention of cryptogenic stroke. Observational studies[10,11] and meta-analysis of observational studies[12] demonstrated PFO closure significantly lowered stroke recurrence rate when compared with medical therapy. However, 3 randomized controlled trials[13–15] which were published in 2012 and 2013 indicated PFO closure was not superior to medical therapy in cryptogenic stroke patients. But there were some limitations in these trials. In CLOSURE I trial,[13] the number of enrolled patients was lower than expected, and these patients were treated with off-label device. Meanwhile, periprocedural complication rate was relatively high. And the magnitude of effect estimates of RESPECT trial[14] and PC trial[15] was low. These drawbacks limited physicians’ interpretation of the results into clinical practice. Two meta-analyses[16,17] of these 3 trials suggested PFO closure was more appropriate than medical therapy in secondary prevention of cryptogenic stroke. But the results of these 2 meta-analyses were confused because results of enrolled trials were negative while the meta-analysis demonstrated positive result. Thus, 2014 AHA/ASA guideline[18] and 2016 AAN guideline[19] recommended existing evidence was not enough to prove that cryptogenic stroke patients could benefit from PFO closure. Recently, CLOSE trial,[20] Gore REDUCE trial,[21] long-term result of RESPECT trial,[22] and DEFENSE-PFO trial[23] have been published, suggesting stroke recurrence rate of patients undergoing device closure was lower than that of participants accepting medical treatment.
Therefore, the secondary prevention strategy in cryptogenic stroke patients with PFO was still controversial. The aim of this meta-analysis was to compare outcomes of PFO closure and medical therapy in these patients, offering physicians a more comprehensive picture of management strategies in these patients.
2. Methods
2.1. Search strategy and eligibility criteria
Two investigators (YM and DL) independently searched PubMed, Embase, and Cochrane Library for randomized controlled trials enrolling cryptogenic stroke patients with PFO undergoing device closure or medical therapy as a management strategy, which were published before May 18, 2017 and restricted to English. Notably, medical therapy included antiplatelet therapy, anticoagulation therapy, or both.[18,19] Our search strategy in PubMed incorporated the Cochrane Highly Sensitive Search Strategy for identification of randomized controlled trials.[24] The main search terms were “patent foramen ovale,” “cryptogenic stroke,” “device closure,” and “medical therapy.” The details of the search strategies were listed in the Supplementary Materials. Two authors (YM and DL), respectively, performed the screening of titles and abstracts, reviewed full texts of articles if needed, and determined their eligibility. We also searched the reference lists of the original articles identified for full text review to find other eligible studies. Divergences were resolved by discussion.
2.2. Data extraction and quality assessment
Two independent reviewers (YM and FQ) extracted data from included studies. Data extracted from studies included study characteristics, patient characteristics, details regarding closure and medical therapy groups, and outcome measures. Outcomes of interest for this meta-analysis were classified as procedure-related or device-related endpoints, efficacy endpoints, and safety endpoints. Procedure-related or device-related endpoints included implantation success rate, effective closure rate, and procedure-related or device-related complication rate. Efficacy endpoints included recurrent stroke, transient ischemic attack (TIA), a composite of stroke and TIA, all-cause death, and cardiovascular death. Safety endpoints included atrial fibrillation, major bleeding, and any serious adverse event. Specifically, major bleeding was defined as a reduction in the hemoglobin level of at least 20 g/L, transfusion of at least 2 units of packed red cells or bleeding occurred at a critical site.[25] And any serious adverse event was defined as the adverse event that resulted in permanent impairment of a body function or permanent damage to a body structure, prolongation of hospitalization, life-threatening events, or death.[13–15,20–22]
Quality of studies was assessed through the Cochrane Collaboration Tool. More specific, sequence generation for randomization, allocation concealment, masking of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias were evaluated in detail.
2.3. Statistical analysis
Intention-to-treat meta-analysis was performed by using Review Manager 5.3 and Stata 12.0. We did not perform per-protocol and as-treated meta-analyses. Because some trials[15,20,22] did not incorporate the data of per-protocol and as-treated analyses. Many investigators[24] and we thought bias would occur if we used data of intention-to-treat analyses of these trials in per-protocol and as-treated meta-analyses like some authors did.[16]
The categorical variables were reported as the counts and percentages and continuous variables were presented as mean and standard deviation or median and interquartile range, as appropriate. Outcome data were extracted as risk ratios (RRs) and 95% confidence intervals (CIs) for device closure versus medical therapy among cryptogenic stroke patients with PFO. We reported unadjusted RRs since adjusted RR was presented in only 1 trial[13] and 4 trials[14,15,20–23] did not report the adjusted RRs. RRs for each outcome were calculated using the random-effect model.[24] Given the heterogeneity in the study design and included populations and variability in the definition of medical therapy, a random-effect model rather than a fixed-effect model was considered more appropriate. The Cochran Q test and I2 test were performed to assess the heterogeneity of the summary effects. If the P-value of Cochran Q test was <.10 and I2 was >50%, heterogeneity was considered to exist. Publication bias and bias associated with small study effects were assessed with funnel plot, Begg test and Egger test, respectively. Whether effect sizes were distributed symmetrically or not was judged visually. In addition, if P-values of Begg test and Egger test were <.10, and publication bias was considered to exist.
2.4. Sensitivity analysis
Jackknife sensitivity analyses were performed for each outcome of interest to verify the robustness of the results and the impact of each single study on the summary estimate of the effect. Pooled estimates were recalculated multiple times by using a random-effect model, each time with removal of a single study from the baseline group.
3. Results
3.1. Study selection
The results of study selection process were shown in Supplementary Figure 1. After the screening, we identified 7 articles that fulfilled the inclusion criteria. The short-term result of RESPECT trial was reported in 2013[14] and long-term result was shown in 2017.[22] Kaplan–Meier curve of this study published in 2013[14] continued to diverge, suggesting that postulated benefit might need more time to become obvious. Moreover, investigators were able to make a more thorough evaluation of effects of intervention on patients with extension of follow-up duration.[26] Therefore, we excluded the article published[14] in 2013. In the end, we enrolled 6 randomized controlled trials[13,15,20–23] in this meta-analysis.
3.2. Baseline characteristics
Baseline characteristics of the included patients were shown in Supplementary Table 1. A total of 3560 participants were enrolled in 6 trials and the range of mean duration of follow-up was 2 to 5.9 years. The patients of closure groups underwent antiplatelet or anticoagulation therapy after the device implantation, including aspirin, clopidogrel, and warfarin. But dose and treatment duration were different in each study. The drug regimens of medical therapy groups were different, too. But all the drug regimens were in accord with the guideline recommendations.[18,19] Enrolled participants were predominantly men and with stroke risk factors of hypertension, diabetes, hyperlipemia, and smoking. Within each trial, baseline characteristics were similar between the closure and medical therapy groups.
3.3. Procedure-related or device-related endpoints
The procedure success rate was 97% (95% CI 95.3–99.2%) in patients undergoing device implantation. After 6 to 12 months of follow-up, effective closure was documented in 85.9% of patients (95% CI 74.5–97.3%). During the whole follow-up period, procedure-related or device-related complication rate was 3.7% (95% CI 2.3–5.1%).
3.4. Efficacy endpoints
Compared with medical therapy, closure reduced risk of recurrent stroke by 48% (RR 0.52, 95% CI 0.29–0.93) (Fig. 1). There was no difference in TIA between closure and medical therapy groups (RR 0.76, 95% CI 0.52–1.12) (Fig. 2). In addition, closure was associated with a 40% lower risk of composite of stroke and TIA (RR 0.60, 95% CI 0.46–0.80) relative to medical therapy (Fig. 3). However, there were no significant differences in all-cause death (RR 0.80, 95% CI 0.37–1.72) (Supplementary Figure 2) and cardiovascular death (RR 1.47, 95% CI 0.36–5.94) (Supplementary Figure 3) between closure and medical therapy groups.
Figure 1.
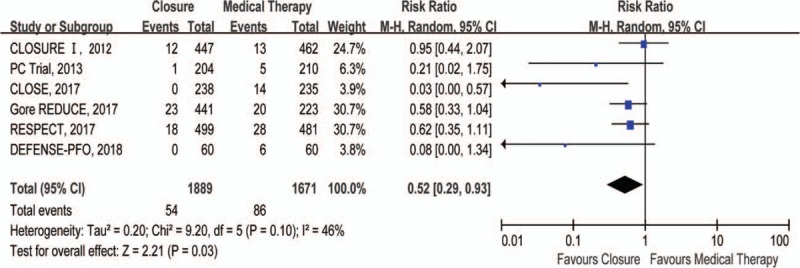
Forest plot with individual and summary estimates of the risk ratio (RR) and 95% confidence interval (CI) of recurrent stroke.
Figure 2.
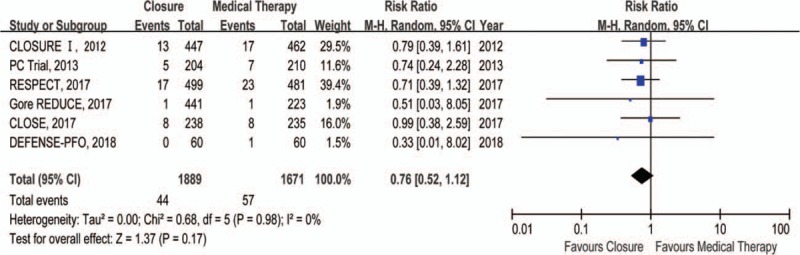
Forest plot with individual and summary estimates of the risk ratio (RR) and 95% confidence interval (CI) of transient ischemic attack (TIA). CI = confidence interval.
Figure 3.
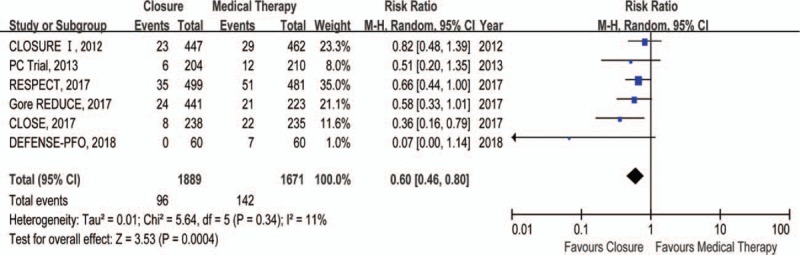
Forest plot with individual and summary estimates of the risk ratio (RR) and 95% confidence interval (CI) of composite of stroke and transient ischemic attack (TIA).
3.5. Safety endpoints
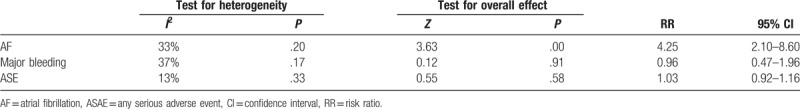
As compared with medical therapy, closure significantly increased risk of atrial fibrillation (RR 4.25, 95% CI 2.10–8.60) (Table 1 and Supplementary Figure 4). However, no significant differences were observed in major bleeding (RR 0.96, 95% CI 0.47–1.96) (Table 1 and Supplementary Figure 5) and any serious adverse event (RR 1.03, 95% CI 0.92–1.16) (Table 1 and Supplementary Figure 6) between closure and medical therapy groups.
Table 1.
Summary of data comparing closure with medical therapy in the intention-to-treat populations.
3.6. Sensitivity analyses
Jackknife sensitivity analyses (Supplementary Table 2) demonstrated 3 studies significantly affected pooled RRs of recurrent stroke. After removal of PC trial,[15] Gore REDUCE trial,[21] or RESPECT trial,[22] there was no difference in recurrent stroke between closure and medical therapy groups.
3.7. Risk of bias
All the trials included in this meta-analysis were randomized, open-label trials. Method of randomization was adequately described (computer generated or automated telephone system) in all trials and allocation concealment was promised. All the trials were open-label trials which might impact the results, but the extent of impact was unknown. Therefore, risk of bias of all the trials in binding of participants and personnel was unclear. Masking of outcome assessors was described in all trials and losses to follow-up were reported in all trials. All trials were free of selective outcome reporting. The risks of bias across all studies were summarized in Supplementary Figure 7.
Effect sizes reported in studies were distributed symmetrically (Supplementary Figure 8), and there was no significant bias from small studies (Begg test P = .81; Egger test P = .56).
4. Discussion
Our study indicated that procedure success rate was high in the closure group and effective closure could be achieved in most patients with a low procedure-related or device-related complication rate. When compared with medical therapy, PFO closure significantly reduced the risks of recurrent stroke and composite of stroke and TIA, but there was no difference in TIA between 2 groups. And no significant differences in all-cause death and cardiovascular death between closure and medical therapy groups were observed. The risks of major bleeding and any serious adverse event did not differ significantly between study groups. Yet, PFO closure was associated with increased risk of atrial fibrillation.
All the studies included in this meta-analysis were designed to compare the role of PFO closure and medical therapy in secondary prevention of cryptogenic stroke and it is impossible to hide the fact that participants were receiving device implantation or medical therapy. Thus, the design of double-blind trial was hard to carry out. Design of open-label trial might introduce bias to studies, but as shown in Supplementary Figure 7, the risks of bias in other aspects were low. Of note, randomization was conducted in the subgroups in which patients were allocated in the light of contraindications to oral anticoagulant or closure device in the CLOSE trial.[20] After verifying the absence of interaction between treatment effect and treatment group investigators performed the analyses with combined data of patients who accepted the same treatment in different subgroups. Hence, the design and statistical analyses of CLOSE trial were reliable with relatively low bias. Therefore, all included studies were of high quality and the results of this meta-analysis based on these trials would be helpful to guide the clinical work.
Although medical treatments that patients received differed between studies, these treatments were consisted with recommendations of guidelines[18,19] and were approved by the steering committees. This was similar with clinic practice because physicians had to adjust the dosage or switch the medication according to the patient's risks of thrombosis or bleeding. So, it is appropriate to combine these studies where types and doses of medications were different.
If effective PFO closure could not be achieved and residual shunts existed, it would be possible that embolus traversed from venous system and into systemic circulation. It might mask the real efficacy of PFO closure in the secondary prevention of cryptogenic stroke. Meta-analysis of proportions showed that the procedure success rate and effective closure rate were 97% and 85.9%, respectively. Our results were consistent with previous study data,[27] suggesting that the conditions of closure procedures in the included studies approached to clinic practice and the internal and external validity of this meta-analysis was high.
Our analysis demonstrated that PFO closure was associated with significant improvement in recurrent stroke and composite of stroke and TIA, which was similar with results of some previous observational studies,[10,11,28] randomized controlled trials[21,22] and meta-analyses.[12,16] However, results of some studies were contradictory to ours. Windecker et al[10] reported that PFO closure could not prevent stroke recurrence (RR 0.36, 95% CI 0.08–1.74). This might be because patients treated with PFO closure, as compared with medical therapy group, had suffered from more than one stroke event before they were enrolled in this study. And they were more prone to stroke. Investigators of CLOSURE I[13] did not observe sufficient evidence to support the use of closure device in patients with PFO for secondary prevention of cryptogenic stroke. This could be explained by the utilization of the STARFlex device. Thrombi on the device surface were observed in approximately 3.6% of patients of the STARFlex group though they accepted adequate antiplatelet treatment.[29] But no thrombus was observed in participants undergoing implantation of other types of device.[29] It was possible for patients in STARFlex group to suffer from stroke when thrombus detached from device surface, which might dilute the actual beneficial effect of device closure. The short-term results of the RESPECT trial[14] did not show beneficial effect of closure for stroke prevention, but the rate of stroke in device closure versus medical therapy appeared to separate after follow-up of 2.1 years and continued to diverge. And the long-term results of RESPECT trial[22] confirmed this trend, demonstrating that PFO closure was effective for secondary prevention of cryptogenic stroke.
Atrial fibrillation was common in cryptogenic stroke patients.[30] Most included studies[13,20–23] showed the rates of atrial fibrillation in the closure groups were higher than those in the medical therapy groups and majority of these cases in the closure groups occurred within periprocedural periods, implying catheter was associated with the increased risk of atrial fibrillation. Notably, atrial fibrillation was transient in most patients and could be terminated with or without pharmacologic cardioversion. And few atrial fibrillation patients suffered from stroke in most included studies.[13,15,20,22] Perhaps, atrial arrhythmias might be transient without adverse effect if treated properly. Furthermore, the rates of atrial fibrillation after the periprocedural period did not differ between 2 groups.[22] This might be because extra electrocardiograph monitoring contributed to higher diagnosis rates in closure groups during periprocedural periods and the rates of atrial fibrillation in the medical therapy groups were underestimated. Thus, it was confusing for us to evaluate the real effects of atrial fibrillation in the trials.
Different management strategies did not make difference in the occurrence of major bleeding and any serious adverse event. In some ways, the safety of closure was comparable with that of medical therapy. Although the included trials[13,15,20–23] did not reported the occurrence time of major bleeding events, the observational study[30] showed most major bleeding events occurred during the period of anticoagulation therapy. This suggested drug was major risk factor of major bleeding and appropriate anticoagulation or antiplatelet therapies with shorten duration might prevent major bleeding events. There was no difference in adverse event rate, but great variability was observed in types of adverse events between groups. Procedure-related and device-related complications were observed only in closure group and STARFlex device significantly increased risk of adverse events.[20] Thus, it is helpful in the prevention of this kind of adverse events to choose safer device and improve the implantation procedures. Most adverse events in medical therapy groups were related to the medicine; hence, rational use of the medicine might reduce the incidence of adverse events.
4.1. Study limitation
Our study had some limitations inherent to the included studies and to meta-analysis. Firstly, the trials differed meaningfully in study devices, medical regimens, and durations of follow-up. However, it can be argued that it extends the generalizability of our findings to a wider range of population. Secondly, this meta-analysis lacked specified individual data to conduct meta-regression or subgroup analyses to explore the source of heterogeneity. In addition, even a small change in the rate of event might lead to a dramatic change in the results because of relatively low event rates.
5. Conclusion
The PFO closure, as compared with medical therapy, reduced risks of stroke and the composite of stroke and TIA in patients with PFO after cryptogenic stroke. But PFO closure did not lower the mortality risk. They showed comparable safety in terms of major bleeding and any serious adverse event. However, PFO closure was associated with increased risk of atrial fibrillation.
Author contributions
Conceptualization: Shenghua Zhou, Qiming Liu.
Data curation: Yingxu Ma, Dongping Li, Fan Bai, Fen Qin, Jiayi Li.
Formal analysis: Yingxu Ma, Dongping Li.
Investigation: Jiayi Li, Yixi Li, Na Liu.
Methodology: Dongping Li, Yixi Li, Na Liu.
Software: Yingxu Ma, Dongping Li, Hui Xie.
Supervision: Shenghua Zhou, Qiming Liu.
Writing – original draft: Yingxu Ma, Dongping Li.
Writing – review & editing: Shenghua Zhou, Qiming Liu.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, PFO = patent foramen ovale, RRs = risk ratios, TIA = transient ischemic attack.
YM and DL are first co-authors and contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Gaist D, Goldstein LB, Cea S, et al. Statins and the risk of intracerebral hemorrhage in patients with previous ischemic stroke or transient ischemic attack. Stroke 2017;48:3245–51. [DOI] [PubMed] [Google Scholar]
- [2].O’Gara PT, Messe SR, Tuzcu EM, et al. Percutaneous device closure of patent foramen ovale for secondary stroke prevention: a call for completion of randomized clinical trials: a science advisory from the American Heart Association/American Stroke Association and the American College of Cardiology Foundation. Circulation 2009;119:2743–7. [DOI] [PubMed] [Google Scholar]
- [3].Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989;25:382–90. [DOI] [PubMed] [Google Scholar]
- [4].Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20. [DOI] [PubMed] [Google Scholar]
- [5].Di Tullio MR, Sacco RL, Sciacca RR, et al. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007;49:797–802. [DOI] [PubMed] [Google Scholar]
- [6].Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA study. Atrial Septal Aneurysm. Stroke 2002;33:706–11. [DOI] [PubMed] [Google Scholar]
- [7].Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation 2002;105:2625–31. [DOI] [PubMed] [Google Scholar]
- [8].Handke M, Harloff A, Olschewski M, et al. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007;357:2262–8. [DOI] [PubMed] [Google Scholar]
- [9].Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006;47:440–5. [DOI] [PubMed] [Google Scholar]
- [10].Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004;44:750–8. [DOI] [PubMed] [Google Scholar]
- [11].Wahl A, Juni P, Mono ML, et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation 2012;125:803–12. [DOI] [PubMed] [Google Scholar]
- [12].Agarwal S, Bajaj NS, Kumbhani DJ, et al. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv 2012;5:777–89. [DOI] [PubMed] [Google Scholar]
- [13].Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991–9. [DOI] [PubMed] [Google Scholar]
- [14].Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092–100. [DOI] [PubMed] [Google Scholar]
- [15].Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083–91. [DOI] [PubMed] [Google Scholar]
- [16].Khan AR, Bin AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013;6:1316–23. [DOI] [PubMed] [Google Scholar]
- [17].Kent DM, Dahabreh IJ, Ruthazer R, et al. Device closure of patent foramen ovale after stroke: pooled analysis of completed randomized trials. J Am Coll Cardiol 2016;67:907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- [19].Messe SR, Gronseth G, Kent DM, et al. Practice advisory: Recurrent stroke with patent foramen ovale (update of practice parameter): Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2016;87:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mas J, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 2017;377:1011–21. [DOI] [PubMed] [Google Scholar]
- [21].Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017;377:1033–42. [DOI] [PubMed] [Google Scholar]
- [22].Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 2017;377:1022–32. [DOI] [PubMed] [Google Scholar]
- [23].Lee PH, Song JK, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: The DEFENSE-PFO trial. J Am Coll Cardiol 2018;71:2335–42. [DOI] [PubMed] [Google Scholar]
- [24].Pursnani S, Korley F, Gopaul R, et al. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv 2012;5:476–90. [DOI] [PubMed] [Google Scholar]
- [25].Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–3694. [DOI] [PubMed] [Google Scholar]
- [26].Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–3962. [DOI] [PubMed] [Google Scholar]
- [27].Thaman R, Faganello G, Gimeno JR, et al. Efficacy of percutaneous closure of patent foramen ovale: comparison among three commonly used occluders. Heart 2011;97:394–9. [DOI] [PubMed] [Google Scholar]
- [28].Schuchlenz HW, Weihs W, Berghold A, et al. Secondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovale. Int J Cardiol 2005;101:77–82. [DOI] [PubMed] [Google Scholar]
- [29].Taaffe M, Fischer E, Baranowski A, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder). Am J Cardiol 2008;101:1353–8. [DOI] [PubMed] [Google Scholar]
- [30].Gaillard N, Deltour S, Vilotijevic B, et al. Detection of paroxysmal atrial fibrillation with transtelephonic EKG in TIA or stroke patients. Neurology 2010;74:1666–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


