Supplemental Digital Content is available in the text
Keywords: acute myeloid leukemia, hematologic toxicities, hypomethylating agents, meta-analysis, myelodysplastic syndromes, systematic review
Abstract
Background:
Hypomethylating agents (HMAs) are believed to have reliable efficacy in treating myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Meanwhile, the adverse events of HMAs have become an increasing concern. There is, however, no systematic meta-analysis available to evaluate overall hematologic toxicities for HMAs. In this meta-analysis, we aim to determine the risk of hematologic toxicities in patients treated with HMAs.
Methods:
Relevant studies were identified from PubMed, Embase, Cochrane Library, and the Clinical Trials. gov databases incepted to February 2018. All phase II and III trials meeting the inclusion criteria included adequate safety data. We calculated the relative risk (RR) of high-grade hematologic toxicities (HTEs) with corresponding 95% CI using Review Manager. The incidences of HTEs were also evaluated by R. Heterogeneity was calculated and reported mainly via I2 analyses.
Results:
A total of 2337 MDS or AML patients from 14 studies were identified in this meta-analysis. The overall incidences of high-grade hematologic toxicities in patients who received HMAs were: 27% of the patients with anemia, 45% with neutropenia, 38% with thrombocytopenia, and 25% with febrile neutropenia, respectively. There was a significantly increased RR of neutropenia and thrombocytopenia using HMAs, in comparison with conventional care regimens (CCR) based on the drug type (decitabine vs azacitidine).
Conclusions:
We conclude that the use of HMAs are associated with an increased risk of neutropenia and thrombocytopenia in MDS or AML patients, and our results also demonstrate that HMAs exposure does not significantly increase the risk of high-grade anemia, leukopenia, or febrile neutropenia compared with CCR.
1. Introduction
Myelodysplastic syndromes (MDS) manifest themselves with characteristic clonal hematopoietic stem cell disorders, dyshaematopoiesis of one or more lineage blood cells, ineffective hematopoiesis, and high risk of progression to acute myeloid leukemia (AML).[1] AML is a group of malignant clonal diseases originating from hematopoietic stem cells, leading to a large number of immature hematopoietic cells proliferating and accumulating in the bone marrow and peripheral blood.[2] Although allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is the only curative treatment for high risk MDS and AML,[3,4] it is expensive and difficult to find an appropriate match, and many patients are not eligible for Allo-HSCT. Therefore, there is an urgent need to develop an effective therapeutic approach for these patients who are ineligible for transplantation.
MDS and AML are diseases of disordered differentiation, in which abnormal DNA methylation plays a critical role in their pathogenesis.[5–8] With the breakthrough of molecular biology research on the characteristics and pathogenesis of MDS and AML, hypomethylating agents (HMAs) have become a hot spot for the treatment of MDS and AML. In fact, 2 representative HMAs, azacitidine and decitabine, have been approved by the United States Food and Drug Administration (FDA) for treating MDS and AML. Surprisingly, several previous clinical trials have shown that the efficacy of demethylation therapy, azacitidine and decitabine, is superior to conventional care regimens (CCR).[9–15]
Although the efficacy of HMAs has been recognized, their clinical application is largely limited by the inherent cytotoxicity. In clinical practice, bone marrow suppression is the most common adverse reaction in demethylation therapy, and it is also the main reason for the dose-reduction or discontinuations of therapeutic regimen. Kantarjian found that serious adverse events were experienced by 69% of decitabine patients, and 43 out of 89 patients (48%) received no or only minimal (and possibly ineffective) therapy due to myelosuppression-related side effects.[9] Likely, another study showed that febrile neutropenia was noted in 25% of patients receiving decitabine compared with 7% of patients receiving best supportive care (BSC), and this result was similar to Kantarjian study.[11] Additionally, other studies also found that discontinuations of the treatment before the completion of the study in the HMAs group compared with CCR group were mostly related to myelosuppression, particularly during early treatment.[10,12,13] High concentrations of decitabine inhibit DNA synthesis and induce cell death, resulting in cytotoxic effect. However, low-dose decitabine has the demethylation effect rather than cytotoxic effect,[16,17] which makes it feasible to reduce myelosuppression by decreasing the dosage. In the meantime, the maximal demethylation effect can be achieved through shortening the interval between cycles and prolonging the duration of treatment.
The previous meta-analysis about HMAs mainly focused on the efficacy of drugs, while the studies on drug adverse events were few and not detailed enough. For instance, one meta-analysis involving 7 trials (only 1 randomized controlled trial, RCT) with small sample sizes has evaluated the incidence of developing hematologic toxicity effects (HTEs) with the use of decitabine,[18] however, it did not mention the relative risk (RR) analysis of HMAs. In addition, the other 2 meta-analysis analyzed HTEs as secondary outcomes, but the quality of the included studies was relatively low or too few RCTs included.[19,20] After referring to we found that quite a few RCTs had inconsistent conclusions and their conclusions were not conducive to the use of clinicians. For example, Fenaux research showed that grade 3/4 neutropenia and thrombocytopenia were both more common with azacitidine than with CCR.[10] However, another study demonstrated that azacytidine was generally well-tolerated, and grade 3/4 neutropenia and thrombocytopenia were similar in patients receiving azacytidine and CCR.[13] Up to now, the risk of using HTEs as an index for HMAs is uncertain and clinicians lack the clinical evidence to select specific HMAs. Therefore, the purpose of the current study is to fully assess the incidence and RR of the HTEs associated with HMAs by performing a meta-analysis.
2. Materials and methods
2.1. Data sources
An independent literature search and review of relevant articles were conducted from inception to February 2018 using PubMed, Embase, Cochrane Library, and the Clinical Trials. gov databases. Key words included hypomethylating agents, azacitidine, decitabine, myelodysplastic syndrome, and acute myeloid leukopenia. Additional relevant abstracts were also included from the proceedings of American Society of Hematology, the American Society of Clinical Oncology, and the European Hematology Association. Only the latest updated report was chosen for meta-analysis. Trials were reviewed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA).[21] The detailed search strategies were listed in Supplementary Table 1.
2.2. Study inclusion criteria
Publications that meet the following inclusion criteria were included: Phase II and III trials of adults with morphologically proved diagnosis of AML or MDS, and without previous Allo-HSCT, for RR analysis, data were extracted from RCT, and participants were randomized to treatment with either HMAs (azacitidine or decitabine) or conventional care regimens BSC, low-dose cytarabine (LDAC), or intensive chemotherapy (IC) in a setting of first-line treatment, for incidence analysis, trials that individuals were randomized to HMA monotherapy were included, and sample size and safety events were both available for high grade HTEs. Trial data were used only once in the analysis from the most recent publication.
2.3. Data extraction, clinical end points
Two investigators (JW and CG) independently read and extracted data with a piloted extraction form. Any disagreement between the 2 investigators was resolved by consensus with other co-authors after reviewing of the full text. The following data were extracted from each study: the first author's name, year of publication, phase of trials, underlying disease, population size, median age, French-American-British (FAB) classification, bone marrow (BM) blast count, cytogenetic risk categories, treatment and dosing regimens, median treatment duration, number of patients available for analysis, and adverse events of interest. The main analysis of the following coprimary endpoints included: neutropenia, leukopenia, thrombocytopenia, and anemia and febrile neutropenia. Count data for all HTEs were defined and recorded according to the common toxicity criteria of adverse events (CTCAE) version 2.0, 3.0 or 4.0 (http://ctep.cancergov/reporting/ctc_archive.html), which had been widely used in clinical trials.
2.4. Assessment of bias risk
We used the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias of each enrolled RCT (for RR analysis).[22] Criteria for evaluation was made separately according to random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other source of bias.
2.5. Statistical analysis
Data analyses were carried out using Review Manager (version 5.3; the Nordic Cochrane Centre, Copenhagen, Denmark) and R (version 3.4.2; Comprehensive R Archive Network, TUNA Team, Tsinghua University, Beijing, China). We calculated the RR of high-grade (grade 3–4) hematologic toxicities with corresponding 95% confidence interval (CI). For incidence analysis, the number of HTEs was extracted from the single-arm and selected randomized clinical trials. For the calculation of RR of HTEs, data were extracted from RCT, comparing the HTEs in participants assigned to HMAs versus controls in each trial. Random effects model was calculated for all analyses, and subgroup analyses were performed to explore the source of heterogeneity, as described by DerSimonian and Laird,[23] which consider within-study and between-study variation. The Cochrane Q statistic (χ2) was used to estimate the heterogeneity and the I2 test was used to quantify the inconsistency.[24] A two-tailed P < .05 was considered statistically significant in all statistical tests.
2.6. Ethics
All the analyses were based on previous published studies. Therefore, ethical approval is not necessary for systematic review and meta-analysis.
3. Results
3.1. Literature search results
We searched a total of 713 potentially relevant articles using the initial search strategy, and detailed selection process was shown in the Flow Diagram. After reviewing of the titles and abstracts, 689 studies were judged as ineligible for inclusion criteria and were therefore excluded. A total of 23 studies were reviewed in full text, of which 9 studies were excluded due to the lack of HTEs data[17,25–32] (Flow Diagram), the remaining 14 trials were identified and included in this meta-analysis, including 8 Phase II trials (n = 468) and 6 Phase III trials (n = 1869).[9–15,33–39]
3.2. Publication characteristics
The yielded 14 studies included 2337 MDS or AML patients meeting our inclusion criteria in this meta-analysis, of which 1421 patients were treated with either azacitidine (n = 616) or decitabine (n = 805), and 916 patients were treated with CCR, including BSC (n = 393), LDAC (n = 439), and IC (n = 84). Currently, decitabine and azacitidine are the only widely used demethylation drugs in clinical practice. In this meta-analysis, 9 studies used decitabine and 5 studies evaluated azacitidine. The HTEs of included trials were assessed according to the National Cancer Institute's CTCAE criteria (http://ctep.cancergov/reporting/ctc_archive.html, version 2.0, 3.0 or 4.0). Nine studies assessed MDS treatment, of which 5 studies used decitabine and 4 studies used azacitidine. Five studies assessed AML treatment, of which 4 studies used decitabine and 1 study used azacitidine. The baselines characteristics of included studies were summarized in Table 1 .
Table 1.
Baseline characteristics of the trials included in the meta-analysis.
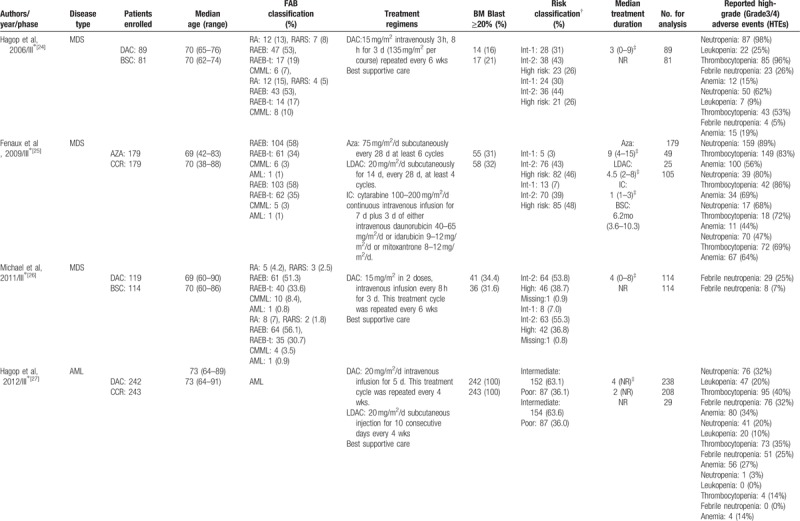
Table 1 (Continued).
Baseline characteristics of the trials included in the meta-analysis.
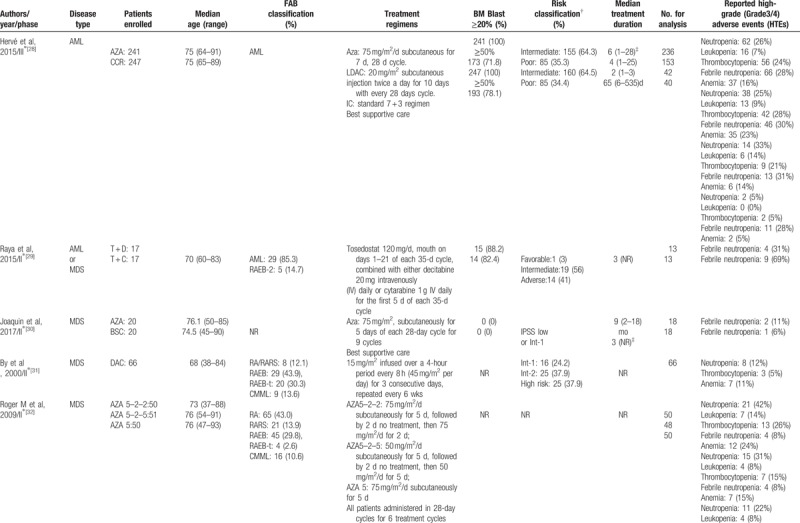
Table 1 (Continued).
Baseline characteristics of the trials included in the meta-analysis.
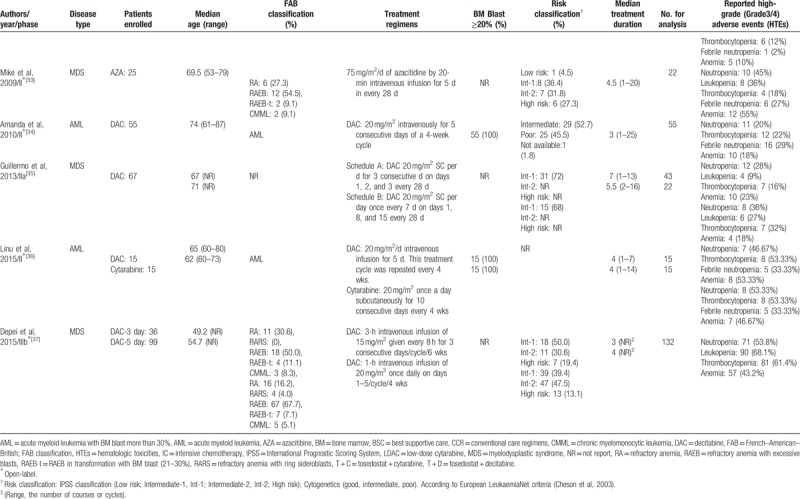
3.3. Risk of bias
Bias analysis was shown in Figs. 1 and 2. For RR analysis, all 7 trials were open-labeled RCT.[9–15] One study was not performed adequately in random sequence generation,[15] and 3 studies were not performed adequately in allocation concealment.[11,14,15] The adequacy of blinding of participants and personnel (performance bias) was evaluated by a description of blind methods for researchers and participants in the study, and the adequacy of outcome assessment blinding was judged by whether efficacy of the treatment was assessed by a reviewer who did not know which group the patient belongs to. Three studies performed blinding of participants and personnel.[10,12,13] In one study, treatment response was assessed by a third person who was a specialist in related fields.[9] Randomization, follow-up, and safety analysis about HTEs were well designed and conducted. Thus, attrition bias and reporting bias were unlikely to exist. In 2 studies, too few patients were enrolled to substantiate their results.[14,15]
Figure 1.
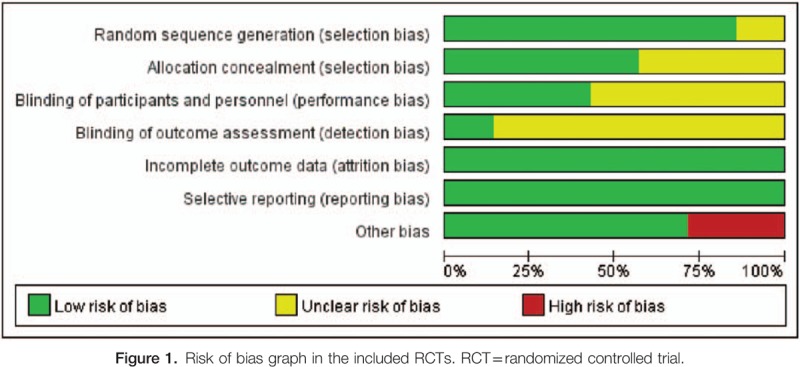
Risk of bias graph in the included RCTs. RCT = randomized controlled trial.
Figure 2.
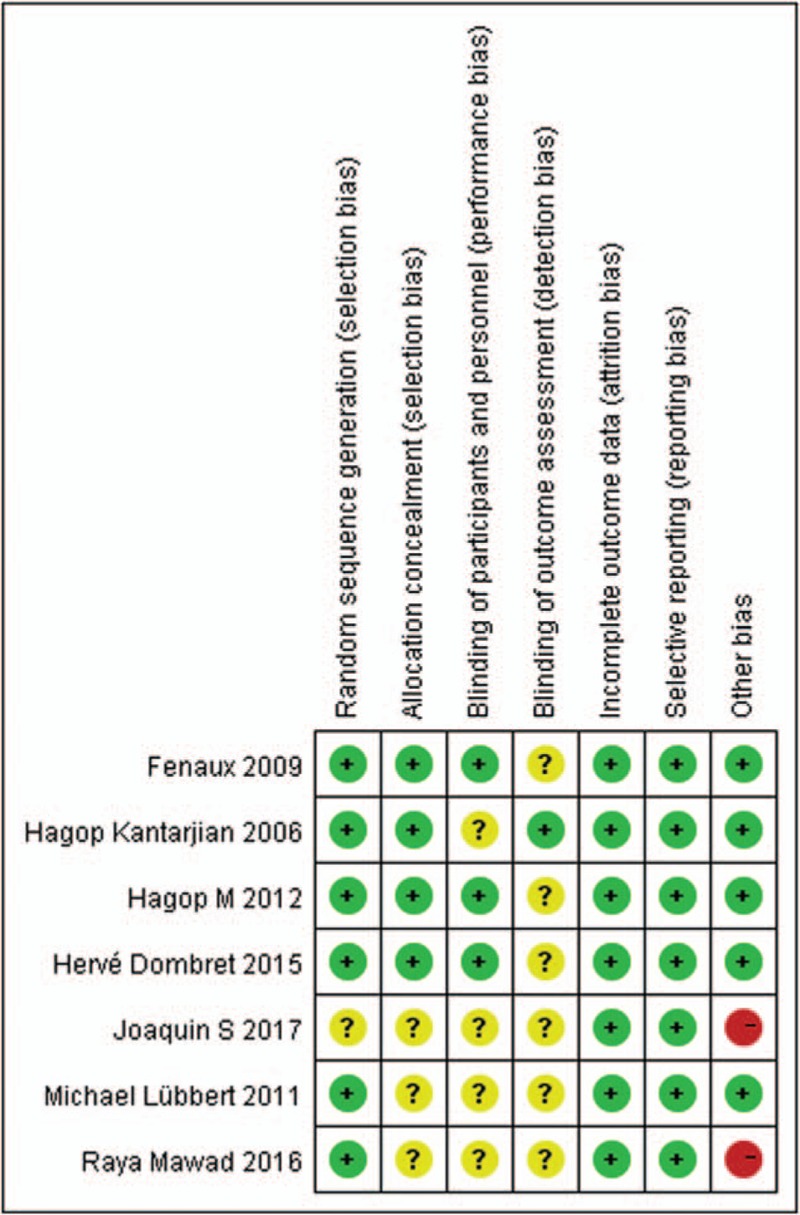
Summary of the risk of bias in the included RCTs. RCT = randomized controlled trial.
3.4. Incidence of high-grade hematologic toxicities
For the incidence of HTEs analysis, only monotherapy treatment with decitabine or azacitidine was considered, excluding the arms combined with chemotherapy and/or other treatments with potential hematologic toxicity. A total of 1377 patients from 13 studies who received HMAs were included for the analysis,[9–13,15,33–39]and the random-effect model was applied. The summary including incidences of high-grade anemia, neutropenia, thrombocytopenia, and febrile neutropenia in patients who received HMAs was presented in Supplementary Figure 1, with an RR value of 0.27 (95% CI 0.18–0.38) for anemia, 0.45 (95% CI 0.30–0.61) for neutropenia, 0.38 (95% CI 0.23–0.57) for thrombocytopenia, and 0.25 (95% CI 0.19–0.31) for febrile neutropenia. Further exploratory analysis was performed to assess the incidence of high-grade hematologic toxicities based on specific HMAs (decitabine vs azacitidine). There was no statistically significance in the incidence of anemia, neutropenia, thrombocytopenia, or febrile neutropenia between these subgroups (Supplementary Figure 2).
3.5. RR of high-grade neutropenia
High-grade neutropenia was calculated in 4 involved studies, which contained patients assigned to the HMA group versus control group (Fig. 3). The pooled analysis showed that the administration of HMAs significantly increased the risk of developing high grade neutropenia. The RR of high-grade neutropenia was 1.41 (95% CI 1.18–1.69; P < .001, 4 studies, 1474 pts). However, there was heterogeneity in RR of high-grade neutropenia (I2 = 66%, P < .05) analysis across studies.
Figure 3.
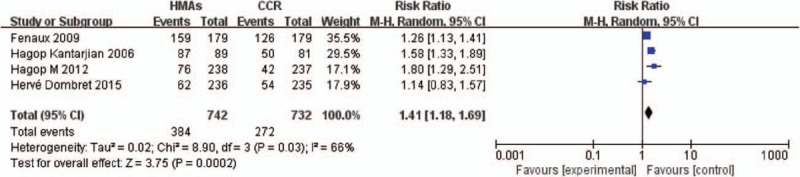
Forest plot of RR of high-grade neutropenia associated with HMAs versus CCR. CCR = conventional care regimens, HMAs = hypomethylating agents, RR = relative risks.
3.6. RR of high-grade thrombocytopenia
For the RR calculation of high-grade thrombocytopenia, 4 trials with patients who received HMAs versus CCR were used for analysis (Fig. 4). Administration of HMAs increased the risk of developing high-grade thrombocytopenia. The RR of high-grade thrombocytopenia was 1.28 (95% CI 1.01–1.62; P < .05, 4 studies, 1474 pts). By I2 statistics, substantial heterogeneity tested was observed (I2 = 81%, P < .05).
Figure 4.
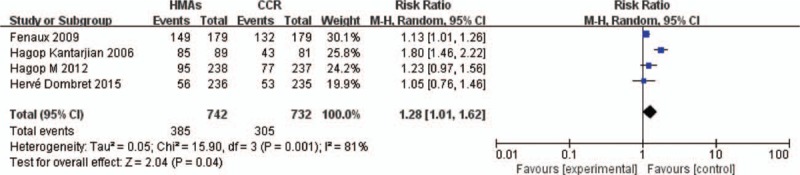
Forest plot of RR of high-grade thrombocytopenia associated with HMAs vs CCR. CCR = conventional care regimens, HMAs = hypomethylating agents, RR = relative risks.
3.7. RR of high-grade anemia, leukopenia, and febrile neutropenia
The RR calculation of anemia, leukopenia, and febrile neutropenia was shown in Fig. 5, and 7 randomized trials with patients received HMAs versus CCR were available in this meta-analysis. The RR of high-grade anemia, leukopenia, and febrile neutropenia were 0.98 (95% CI 0.76–1.25; P > .05, 4 studies, 1474 pts), 1.76 (95% CI 0.85–3.64; P > .05, 3 studies, 1116 pts), and 1.56 (95% CI 0.86–2.75; P > .05, 6 studies, 1406 pts), respectively, suggesting that HMAs did not significantly increase the risk of developing any outcomes. There was significant heterogeneity among included studies (I2 = 56%, P > .05), (I2 = 75%, P < .05), (I2 = 81%, P < .001).
Figure 5.
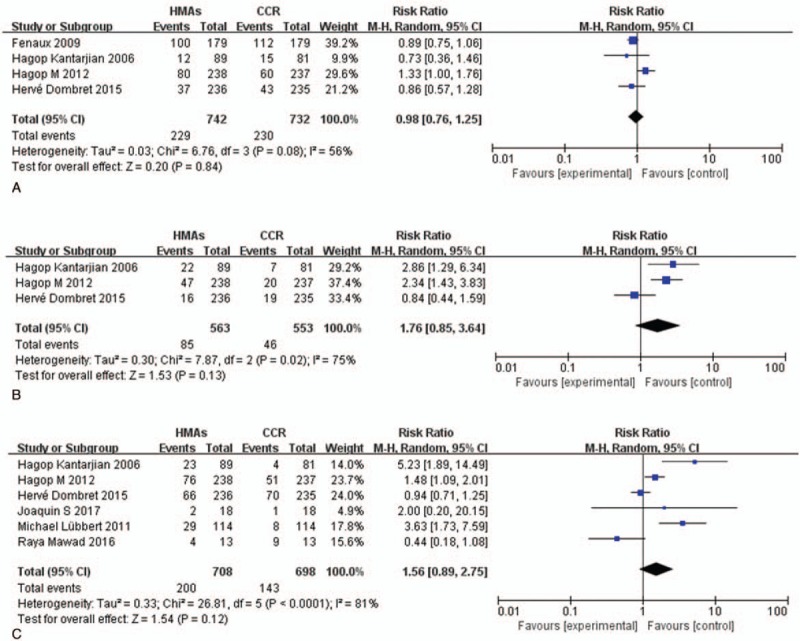
RR of high-grade HTEs in patients associated with HMAs versus CCR. (A) Forest plot of RR of high-grade anemia. (B) Forest plot of RR of high-grade leukopenia. (C) Forest plot of RR of high-grade febrile neutropenia. CCR = conventional care regimens, HMAs = hypomethylating agents, HTEs = hematologic toxicity effects, RR = relative risks.
3.8. RR of hematologic toxicities by specific HMAs
To investigate the relationship between hematologic toxicities and different HMAs, we performed subgroup analyses based on the drugs used. For high-grade neutropenia, both decitabine treatment (RR = 1.63, 95% CI: 1.40–1.90, P < .001) and azacitidine treatment (RR = 1.25, 95% CI: 1.13–1.38, P < .001) significantly increased the risk compared with CCR (Fig. 6A), and there was statistical significance between decitabine and azacitidine (P < .05). As for the RR of high-grade thrombocytopenia (Fig. 6B), both decitabine group (RR = 1.49, 95% CI: 1.01–2.21, P < .05) and azacitidine group (RR = 1.15, 95% CI: 1.04–1.27, P < .05) increased the risk of thrombocytopenia compared with CCR; however, no statistical significance was observed among decitabine and azacitidine (P > .05). There was no statistical significance in association of anemia (RR = 1.07, 95% CI: 0.61–1.89, P > .05) or febrile neutropenia (RR = 0.89, 95% CI: 0.76–1.04, P > .05) in groups treated with HMAs compared with CCR (Fig. 7).
Figure 6.
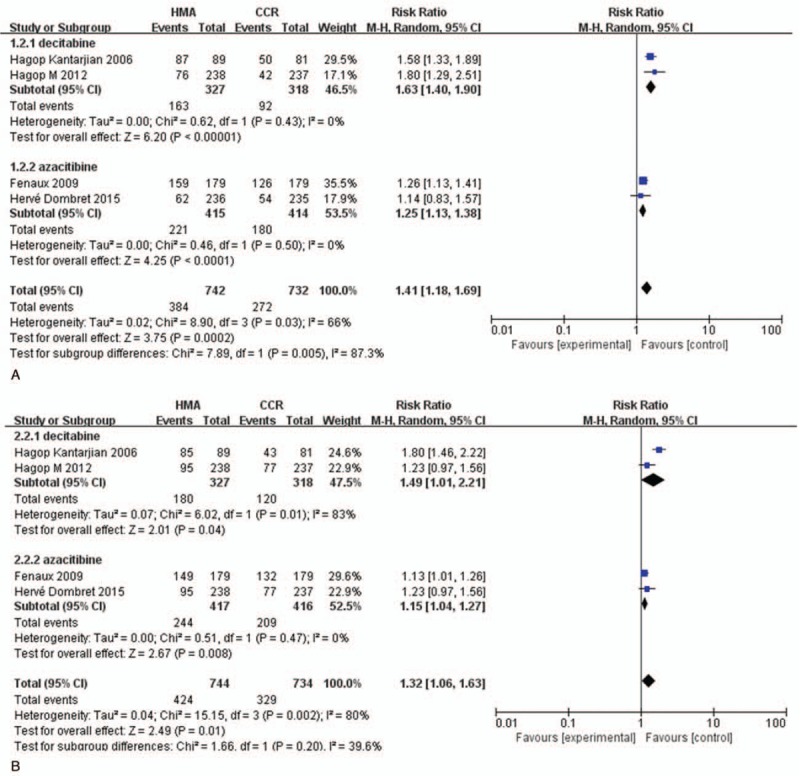
RR of high-grade neutropenia and thrombocytopenia in patients associated with decitabine versus azacitidine. (A) Forest plot of RR of high-grade neutropenia. (B) Forest plot of RR of high-grade thrombocytopenia (sub-grouped by the type of HMAs). HMAs = hypomethylating agents, RR = relative risks.
Figure 7.
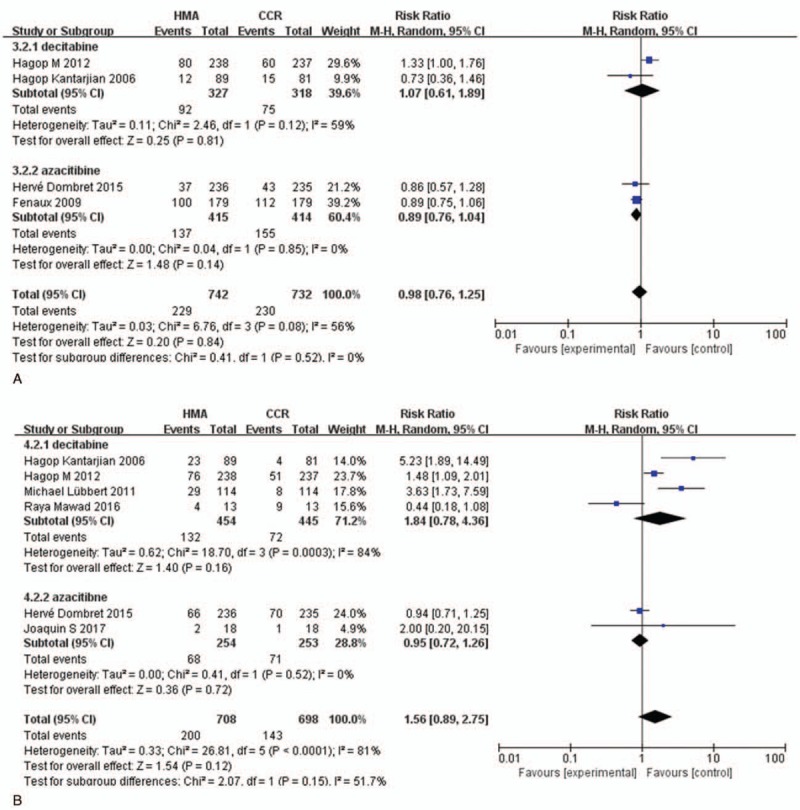
RR of high-grade anemia and febrile neutropenia in patients associated with decitabine versus azacitidine. (A) Forest plot of RR of high-grade anemia. (B) Forest plot of RR of high-grade febrile neutropenia (sub-grouped by the type of HMAs). HMAs = hypomethylating agents, RR = relative risks.
3.9. RR of hematologic toxicities by the type of CCR
To further clarify the relationship between HMAs and different CCR in hematologic toxicities, we performed subgroup analysis based on the type of CCR. The RR of high-grade neutropenia, thrombocytopenia, leukopenia febrile neutropenia, and anemia were 2.83 (95% CI 1.17–2.34; P < .05, 4 studies, 997 pts), 2.64 (95% CI 1.16–2.72; P < .01, 4 studies, 997 pts), 3.19 (95% CI 1.59–6.95; P < .01, 3 studies, 713 pts), 2.16 (95% CI 1.11–8.04; P < .05, 5 studies, 977 pts), and 0.60 (95% CI 0.64–2.3; P > .05, 4 studies, 997 pts), respectively (Fig. 8), suggesting that the HMAs significantly increased the risk compared with BSC except anemia. However, demethylation does not increase the risk of HTEs compared with LDAC or IC (Fig. 8).
Figure 8.
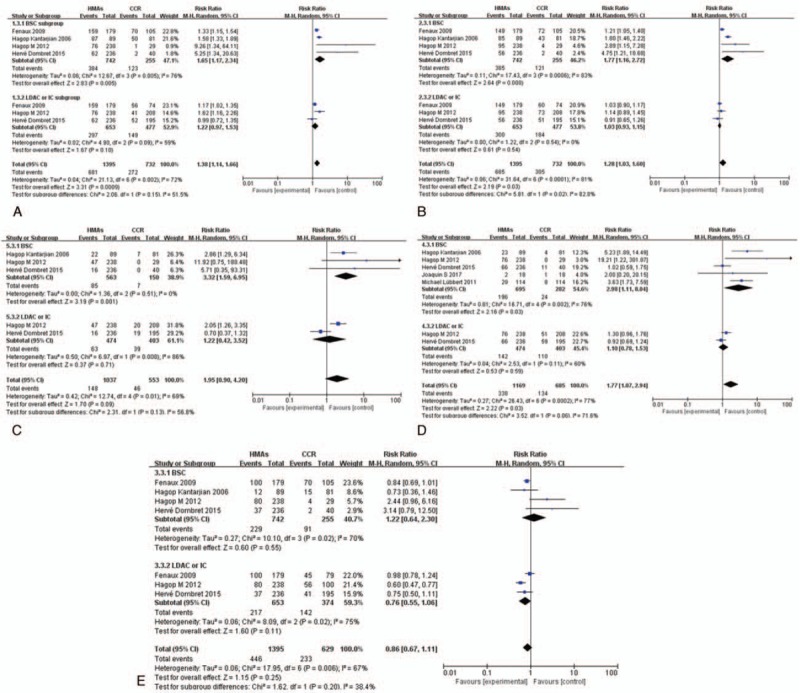
RR of high-grade HTEs in patients associated with HMAs versus CCR (BSC, IC, and LDAC). (A) Forest plot of RR of high-grade neutropenia. (B) Forest plot of RR of high-grade thrombocytopenia. (C) Forest plot of RR of high-grade leukopenia. (D) Forest plot of RR of high-grade febrile neutropenia. (E) Forest plot of RR of high-grade anemia (sub-grouped by the type of CCR). BSC = best supportive care, CCR = conventional care regimens, HMAs = hypomethylating agents, HTEs = hematologic toxicity effects, IC = intensive chemotherapy, LDAC = low-dose cytarabine, RR = relative risks.
4. Discussion
DNA methylation, catalyzed by DNA methyltransferase (DNMT), is one of the most important epigenetic modifications. In normal and cancer cells, DNA methylation regulates gene expression by modifying cytosine, and the silencing of tumor suppressor genes is associated with aberrant promoter DNA methylation.[40–42] Methylation status has been related to the prognosis and pathogenesis of the AML and MDS, with hypermethylation exerting an adverse effect on the results of induction therapy.[43–45] However, DNA methylation can be reversed during DNA synthesis, which makes it a potential therapeutic target. Therefore, demethylation therapy has become a routine treatment in the MDS and AML.
Demethylation therapy has significant overall survival (OS) and complete remission (CR)/partial remission (PR) benefits in comparison to CCR, and it is a preferred therapeutic option especially for the patients not suitable for transplantation and chemotherapy, and for elderly patients with MDS or AML.[18,46] Since HMAs become more commonly used as the routine treatment of MDS or AML, their associated toxicities are being more and more valued in clinical trials. Myelosuppression was the most common adverse effect observed in HMAs treated patients, particularly in early treatment, which frequently led to treatment interruption, discontinuation, disabilities, or deaths.[11,13,26] Intriguingly, the myelosuppression was also the major hematologic adverse effect in clinical trials of HMAs in other diseases.[47,48] Previous studies did not discuss HTEs from using HMAs in detail, and it is difficult for a patient or clinician to judge the risk-benefit balance.
As far as we know, this meta-analysis is among the first to investigate the incidence and risk of HMAs’ hematological toxicities in patients with MDS or AML. Our current meta-analysis included 14 studies, of which 7 studies were randomized controlled trials with decitabine or azacitidine. A total of 2337 patients were included in this meta-analysis and all from prospective phase II and phase III trials, representing a comprehensive meta-analysis of HMAs associated HTEs in MDS and AML patients. Therefore, this study has a higher quality of evidence.
For the incidence of high-grade HTEs analysis, they were 27%, 45%, 38%, and 25% for anemia, neutropenia, thrombocytopenia, and febrile neutropenia, respectively. The incidences of neutropenia and thrombocytopenia were among the highest. Interestingly, this result was similar to the RR of hematologic toxicity. The results of this meta-analysis also demonstrated that the RR of high-grade anemia, leukopenia, and febrile neutropenia in comparison with CCR did not significantly increase among patients receiving HMAs. However, there was a significant difference in the RR of neutropenia and thrombocytopenia between HMAs and CCR (P < .05). In order to further investigate the difference in the RR of neutropenia and thrombocytopenia between HMAs and CCR, we performed subgroup analysis between HMAs and different CCR. Our subgroup analysis results demonstrated that HMAs significantly increased the risk of high-grade neutropenia and thrombocytopenia compared with BSC, but there was no significant difference between HMAs and LDAC or IC. However, a retrospective study showed that the efficacy of decitabine alone or using traditional chemotherapy protocols was equivalent in treating MDS, but decitabine alone regimen was safer,[49] which was inconsistent with our meta-analysis.
Decitabine and azacitidine belong to demethylation drugs, with slightly different structures in which decitabine (5-aza-2′-deoxycytidine) is a deoxyribonucleoside whereas azacitidine is a ribose nucleoside.[50] Although both decitabine and azacitidine act via depletion of DNA methyltransferases, these 2 drugs work through 2 distinct mechanisms: azacitidine is modified into a deoxyribonucleoside triphosphate before incorporating into DNA or directly incorporated into RNA, while decitabine is phosphorylated by different kinases and is directly incorporated into DNA.[51] Azacitidine inhibits protein synthesis as an additional function for incorporation into RNA.[52] Because of these 2 distinct mechanisms of action, the hematologic toxicity from these 2 drugs may show differences. Subsequently, we further conducted specific subgroup analyses to explore such differences in the RR of HTEs between azacitidine and decitabine. Our exploratory subgroup analysis demonstrated an increased risk of high-grade neutropenia in patients receiving decitabine compared with azacitidine, which is consistent with other reported studies.[53] However, the other two meta-analysis showed that there were no differences between decitabine and azacitidine regarding grade 3/4 hematological toxicity.[19,20] But, in the 2 meta-analysis, 1 research included only 3 RCTs, while the other contained only 2 RCTs and the rest of the trials were all non-RCTs about the HTEs research. Therefore, the quality of the conclusion of these 2 meta-analysis was not high, and this might be the reason that was inconsistent with our findings.
Previous research showed that more courses (e.g., ≥3) were needed to achieve optimal response to HMAs.[54,55] However, high-grade HTEs of HMAs did not allow patients to receive more courses of treatment in clinical practice. The high-grade HTEs not only caused discontinuation of treatment, but also led to death, as 24% of the deaths were related to the adverse effects of decitabine.[12] Therefore, in order to prevent the occurrence of adverse reactions, selection of optimized treatment plans was advised to achieve better clinical efficacy for demethylation therapy. A meta-analysis suggested that decitabine at 100 mg/m2/course dosing regimen had a greater clinical benefit in treating MDS than decitabine at 60 to 75 mg/m2/course and 135 mg/m2/course regimens.[56]
Several potential limitations should be considered when interpreting the outcomes of this study. In the first place, significant heterogeneity was detected between studies, which was likely caused by the differences of diseases (MDS or AML), types of HMAs, dose schedules, administrations of HMAs (intravenous or subcutaneous injection), primary or secondary disease, and the phase of trials. Random-effects model was used to minimize the influence, and also we conducted a subgroup analysis to explore the feasible reasons for the heterogeneity. However, such subgroup analysis according to disease types, dose schedules, administrations of HMAs, primary or secondary disease, or the phase of trials was not able to achieve due to the limited number of studies included. Secondly, the safety profile was not in detail or classified according to the age of patients, bone marrow blasts, and frequency of transformation of MDS to AML, etc. In addition, the classification of MDS was according to the recognized French–American–British (FAB) classifications, which contained AML patients (World Health Organization [WHO] criteria). Therefore, we could not differentiate the disease types (MDS vs AML), age of patients, and frequency of transformation of MDS to AML. Thirdly, for RR analysis, this meta-analysis only involved 7 RCT, in which 2 studies had <40 subjects, thus serving as an important contributor for the observed heterogeneity. Moreover, some included studies did not report random sequence generation, concealment allocation, blinding of participants, or personnel and evaluator, contributing to bias in analysis.
5. Conclusion
In summary, our results indicate that there are significant differences in the RR of neutropenia and thrombocytopenia in patients receiving HMAs compared with CCR, and an increased risk of high-grade neutropenia in patients receiving decitabine. Early prevention and effective management of HTEs are feasible and crucial for safe use of HMAs in patients with MDS or AML. The findings of this meta-analysis can provide strong evidence for clinicians when assessing the risk-benefit balance of HMAs in clinical practice.
Acknowledgments
The authors thank Elizabeth Gullen from Yale Medical School for her critical reading and kind revision of the manuscript.
Author contributions
Conceptualization: Chong Gao, Xinyi Chen.
Data curation: Chong Gao, Jia Wang, Jing Wang.
Formal analysis: Chong Gao, Huan Zhao, Jing Wang.
Funding acquisition: Jing Wang.
Investigation: Chong Gao, Ya Li.
Methodology: Chong Gao, Li Hou, Shaodan Tian.
Project administration: Xinyi Chen.
Resources: Ruibai Li, Yayue Zhang, Huan Liang, Chong Wang.
Software: Chong Gao, Jing Wang.
Supervision: Xinyi Chen.
Validation: Xinyi Chen.
Visualization: Chong Gao, Xinyi Chen.
Writing – original draft: Chong Gao.
Writing – review & editing: Chong Gao, Xinyi Chen, Jing Wang.
Author name: ORCID: 0000-0002-3106-2760.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, AEs = adverse events, Allo-HSCT = allogeneic hematopoietic stem cell transplantation, AML = acute myeloid leukemia, BM = bone marrow, BSC = best supportive care, CCR = conventional care regimens, CR = complete remission, CTCAE = common toxicity criteria of adverse events, DNMT = DNA methyltransferase, ED = early death, FAB = French–American–British, FDA = United States Food and Drug Administration, HMAs = hypomethylating agents, HTEs = hematologic toxicity effects, IC = intensive chemotherapy, LDAC = low-dose cytarabine, MDS = myelodysplastic syndromes, OR = odds ratio, ORR = overall response rate, PR = partial remission, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement, RCT = randomized controlled trial, RR = relative risks, WHO = World Health Organization.
CG and JW contribute equally to this article and are listed as the first authors.
No ethical approval.
This work was supported by a Youth Foundation Project of the National Natural Science Foundation of China (No. 81503575), Young Teacher Scientific Program of Beijing University of Chinese Medicine (2015-JYB-JSMS093), 2016 Young Scientists program of Dongzhimen Hospital Affiliated with Beijing University of Chinese Medicine, and National Traditional Chinese Medicine (TCM) Clinical Research Construction Project of State Administration of TCM.
All the authors declare no conflicting interests.
Supplemental Digital Content is available for this article.
References
- [1].Platzbecker U, Santini V, Mufti GJ, et al. Update on developments in the diagnosis and prognostic evaluation of patients with myelodysplastic syndromes (MDS): consensus statements and report from an expert workshop. Leuk Res 2012;36:264–70. [DOI] [PubMed] [Google Scholar]
- [2].McCulloch EA. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood 1983;62:1–3. [PubMed] [Google Scholar]
- [3].Stone RM. How I treat patients with myelodysplastic syndromes. Blood 2009;113:6296–303. [DOI] [PubMed] [Google Scholar]
- [4].Rowe JM, Tallman MS. How I treat acute myeloid leukopenia. Blood 2010;116:3147–56. [DOI] [PubMed] [Google Scholar]
- [5].Issa JP. The myelodysplastic syndrome as a prototypical epigenetic disease. Blood 2013;121:3811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhagat TD, Chen S, Bartenstein M, et al. Epigenetically aberrant stroma in MDS propagates disease via Wnt/beta-Catenin activation. Cancer Res 2017;77:4846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shen N, Yan F, Pang J, et al. Inactivation of receptor tyrosine kinases reverts aberrant dna methylation in acute myeloid leukopenia. Clin Cancer Res 2017;23:6254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schoofs T, Berdel WE, Muller-Tidow C. Origins of aberrant DNA methylation in acute myeloid leukopenia. Leukopenia 2014;28:1–4. [DOI] [PubMed] [Google Scholar]
- [9].Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006;106:1794–803. [DOI] [PubMed] [Google Scholar]
- [10].Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009;10:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukopenia Group and the German MDS Study Group. J Clin Oncol 2011;29:1987–96. [DOI] [PubMed] [Google Scholar]
- [12].Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukopenia. J Clin Oncol 2012;30:2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mawad R, Becker PS, Hendrie P, et al. Phase II study of tosedostat with cytarabine or decitabine in newly diagnosed older patients with acute myeloid leukaemia or high-risk MDS. Br J Haematol 2016;172:238–45. [DOI] [PubMed] [Google Scholar]
- [15].Sanchez-Garcia J, Falantes J, Medina Perez A, et al. Prospective randomized trial of 5 days azacitidine versus supportive care in patients with lower-risk myelodysplastic syndromes without 5q deletion and transfusion-dependent anemia. Leuk Lymphoma 2018;59:1095–104. [DOI] [PubMed] [Google Scholar]
- [16].Oki Y, Aoki E, Issa JP. Decitabine--bedside to bench. Crit Rev Oncol Hematol 2007;61:140–52. [DOI] [PubMed] [Google Scholar]
- [17].Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukopenia. Blood 2007;109:52–7. [DOI] [PubMed] [Google Scholar]
- [18].He PF, Zhou JD, Yao DM, et al. Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukopenia: a systematic review and meta-analysis. Oncotarget 2017;8:41498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gurion R, Vidal L, Gafter-Gvili A, et al. 5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome--a systematic review and meta-analysis. Haematologica 2010;95:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xie M, Jiang Q, Xie Y. Comparison between decitabine and azacitidine for the treatment of myelodysplastic syndrome: a meta-analysis with 1,392 participants. Clin Lymphoma Myeloma Leuk 2015;15:22–8. [DOI] [PubMed] [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukopenia group B. J Clin Oncol 2002;20:2429–40. [DOI] [PubMed] [Google Scholar]
- [26].Boumber Y, Kantarjian H, Jorgensen J, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukopenia in complete remission. Leukemia 2012;26:2428–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Muller-Tidow C, Tschanter P, Rollig C, et al. Azacitidine in combination with intensive induction chemotherapy in older patients with acute myeloid leukopenia: the AML-AZA trial of the Study Alliance Leukopenia. Leukemia 2016;30:555–61. [DOI] [PubMed] [Google Scholar]
- [28].Lubbert M, Ruter BH, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukopenia judged unfit for induction chemotherapy. Haematologica 2012;97:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee JH, Jang JH, Park J, et al. A prospective multicenter observational study of decitabine treatment in Korean patients with myelodysplastic syndrome. Haematologica 2011;96:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thepot S, Ben Abdelali R, Chevret S, et al. A randomized phase II trial of azacitidine +/- epoetin-beta in lower-risk myelodysplastic syndromes resistant to erythropoietic stimulating agents. Haematologica 2016;101:918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA 2010;107:7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol 2009;27:3842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wijermans P, Lubbert M, Verhoef G, et al. Low-dose 5-aza-2’-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol 2000;18:956–62. [DOI] [PubMed] [Google Scholar]
- [34].Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol 2009;27:1850–6. [DOI] [PubMed] [Google Scholar]
- [35].Martin MG, Walgren RA, Procknow E, et al. A phase II study of 5-day intravenous azacitidine in patients with myelodysplastic syndromes. Am J Hematol 2009;84:560–4. [DOI] [PubMed] [Google Scholar]
- [36].Cashen AF, Schiller GJ, O’Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukopenia. J Clin Oncol 2010;28:556–61. [DOI] [PubMed] [Google Scholar]
- [37].Garcia-Manero G, Jabbour E, Borthakur G, et al. Randomized open-label phase II study of decitabine in patients with low- or intermediate-risk myelodysplastic syndromes. J Clin Oncol 2013;31:2548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jacob LA, Aparna S, Lakshmaiah KC, et al. Decitabine compared with low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukopenia: a pilot study of safety, efficacy, and cost-effectiveness. Adv Hematol 2015;2015:167029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu D, Du X, Jin J, et al. Decitabine for treatment of myelodysplastic syndromes in Chinese patients: an Open-Label, Phase-3b study. Adv TherV 32 2015;1140–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Issa JP. DNA methylation as a therapeutic target in cancer. Clin Cancer Res 2007;13:1634–7. [DOI] [PubMed] [Google Scholar]
- [41].Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer 2004;4:143–53. [DOI] [PubMed] [Google Scholar]
- [42].Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012;13:484–92. [DOI] [PubMed] [Google Scholar]
- [43].Grovdal M, Khan R, Aggerholm A, et al. Negative effect of DNA hypermethylation on the outcome of intensive chemotherapy in older patients with high-risk myelodysplastic syndromes and acute myeloid leukopenia following myelodysplastic syndrome. Clin Cancer Res 2007;13:7107–12. [DOI] [PubMed] [Google Scholar]
- [44].Shen L, Kantarjian H, Guo Y, et al. DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol 2010;28:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akalin A, Garrett-Bakelman FE, Kormaksson M, et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukopenia. PLoS Genet 2012;8:e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yun S, Vincelette ND, Abraham I, et al. Targeting epigenetic pathways in acute myeloid leukopenia and myelodysplastic syndrome: a systematic review of hypomethylating agents trials. Clin Epigenet 2016;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schrump DS, Fischette MR, Nguyen DM, et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res 2006;12:5777–85. [DOI] [PubMed] [Google Scholar]
- [48].Gollob JA, Sciambi CJ, Peterson BL, et al. Phase I trial of sequential low-dose 5-aza-2’-deoxycytidine plus high-dose intravenous bolus interleukin-2 in patients with melanoma or renal cell carcinoma. Clin Cancer Res 2006;12:4619–27. [DOI] [PubMed] [Google Scholar]
- [49].Xu ZF, Qin TJ, Zhang HL, et al. The efficacy and safety of the patients of myelodysplastic syndromes-refractory anemia with excess blasts treated with decitabine alone or CAG/HAG regimen. Zhonghua Xue Ye Xue Za Zhi 2017;38:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst 2005;97:1498–506. [DOI] [PubMed] [Google Scholar]
- [51].Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Inter J Cancer 2008;123:8–13. [DOI] [PubMed] [Google Scholar]
- [52].Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukopenia cell lines. PLoS One 2010;5:e9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee YG, Kim I, Yoon SS, et al. Comparative analysis between azacitidine and decitabine for the treatment of myelodysplastic syndromes. Br J Haematol 2013;161:339–47. [DOI] [PubMed] [Google Scholar]
- [54].Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukopenia Group B. J Clin Oncol 2006;24:3895–903. [DOI] [PubMed] [Google Scholar]
- [55].Kantarjian HM, O’Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer 2007;109:1133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang B, Yu R, Cai L, et al. A comparison of therapeutic dosages of decitabine in treating myelodysplastic syndrome: a meta-analysis. Ann Hematol 2017;96:1811–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


