Abstract
Introduction:
Accurate detection and characterization of focal liver lesions, including differentiation between malignant and benign lesions, are particularly important. The objective of this meta-analysis was to evaluate the parameters of intravoxel incoherent motion (IVIM), including apparent diffusion coefficient (ADC), pure molecular diffusion coefficient (D), perfusion-related diffusion coefficient (D∗), and perfusion fraction (f) in differentiating focal liver lesions.
Methods:
IVIM method employed for focal liver lesion and the quality assessment of diagnostic studies were evaluated. Standardized mean differences and 95% confidence intervals were calculated. The heterogeneity was quantified with the I2 statistic.
Results:
The difference between groups was analyzed according to the I2 values from 6 different studies using fixed effects or random effects models. Significant differences in ADC (P < .001) and D (P < .001) were observed between benign and malignant lesions. Moreover, significant differences in ADC (P < .001), D (P < .001), and f (P = .01) were found between hemangioma and hepatocellular carcinoma (HCC). In addition, no significant difference was observed between the metastases and HCC.
Conclusions:
D and ADC values were useful for the differentiation between benignity and malignancy; higher values of ADC, D, and f were observed in hemangioma compared to HCC. Nevertheless, IVIM did not result as the optimal approach for differentiation between the metastases and HCC.
Keywords: diffusion-weighted magnetic resonance imaging, liver neoplasms, magnetic resonance imaging, meta-analysis, perfusion-weighted magnetic resonance imaging
1. Introduction
Noninvasive and real-time imaging methods provide a useful tool for investigating the pathological information on focal liver lesions.[1–5] The accurate detection and characterization of those lesions, including accurate differentiation between malignant and benign lesions, are of particular importance.
Qualitative analysis of diffusion-weighted magnetic resonance (MR) images has become increasingly popular for the evaluation of various liver diseases.[6] Intravoxel incoherent motion (IVIM) imaging, a method based on diffusion-weighted imaging (DWI) with multiple b values representing the degree of diffusion weighting,[7] allows for the separate analysis of 2 components of random water motion in biological tissue, that is, pure molecular diffusion and microcirculation (or perfusion), with the parameters of pure molecular diffusion coefficient (D), perfusion fraction (f), and perfusion-related diffusion coefficient (D∗).[8–10]
IVIM is becoming ever more popular in clinical research as it provides the additional perfusion information without requiring extensive changes in the MR acquisition protocols.[8,11–14] Moreover, IVIM imaging has recently been used for liver imaging,[15] where it has shown to be useful for evaluation of liver fibrosis, nonalcoholic fatty liver disease, and focal liver lesions.[16–19] Furthermore, besides being a good approach for cancerous tumors, IVIM is useful for estimating the diffusion and perfusion of tumor tissue.[20,21] Increased cell density and increased angiogenesis are important pathological processes, accompanied by many types of malignant tumors.[9,22] Nevertheless, different research studies have shown very contradictory data regarding the usage of IVIM for focal liver lesions diagnosis; calling for further investigations into the matter.[22–26]
The aim of this study was to review published data related to IVIM parameters including apparent diffusion coefficient (ADC), D, D∗, and f values, and to evaluate the differences in focal liver lesions among different patients.
2. Methods
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.1. Data sources and keywords
To identify relevant published studies that evaluated the diagnostic value of focal liver lesions, PubMed, Cochrane Library, MEDLINE, Web of Science, EMBASE, and CNKI databases (last updated search: November 1, 2016; data included Chinese and English language articles) were comprehensively explored by 4 experienced radiologists (HW, YL, YG, and WT). The following search terms were used: “liver and intravoxel incoherent motion MR imaging,” “liver and IVIM,” “hepatic or hepatology and intravoxel incoherent motion MR imaging,” “hepatic or hepatology and IVIM,” “liver lesions and intravoxel incoherent motion MR imaging,” “liver lesions and IVIM,” “hepatic lesions and intravoxel incoherent motion MR imaging,” and “hepatic lesions and IVIM.”, “liver or hepatic lesions and DWI or diffusion-weighted imaging.” In addition, bibliographies from prominent studies were searched manually to identify additional relevant studies.
2.2. Quality assessment
The quality assessment of diagnostic studies (QUADAS) was used by 2 independent reviewers (attending radiologists for body imaging with 10 and 17 years of clinical experience, respectively) to assess the quality of each study to be included in this meta-analysis.[27–29] Each item was assigned with “yes,” “no,” or “unclear” (if there was insufficient information to make an accurate judgment) based on QUADAS-2 score. Disagreements were resolved by consensus. All assessment results were imported into RevMan (version 5.2) software.
2.3. Assessment of reporting biases
Since none of the meta-analysis included 10 or more studies, we did not assess publication bias using a funnel plot.[30,31] We performed a comprehensive search strategy to reduce the potential for publication bias.
2.4. Eligibility criteria
Two reviewers who were blinded to the journal, author, institution, and date of publication, independently screened the titles and abstracts and assessed the full text to identify potentially eligible articles; disagreements were resolved by consensus. Studies were included in this analysis if IVIM MR imaging were obtained using either a 1.5 or 3.0-T MR scanner; the diagnostic criteria of the benign and malignant liver lesions were clearly stated; IVIM analysis methods were reported; ADC, D, D∗, and f (%) mean value of hepatic lesions for benign and malignant were summarized. The exclusion criteria included not original research (reviews, editorials, and nonresearch letters); the incomprehensive data; and no summary of benign and malignant or no classification.
2.5. Data extraction
Two reviewers separately collected information from eligible studies. The following data were collected: first author, publication year, study design, ethnicity, number of participants, age, sex, number of lesions, and mean value of the ADC, D, D∗, and f (%). Authors of abstracts and studies with insufficient data were contacted to collect additional information regarding their studies.
2.6. Statistical analysis
For the IVIM parameters [ADC, D, D∗, and f (%)] mean and standard deviation (SD) were extracted or derived using the reported data. To analyze the differences between groups, 2 different approaches were used: fixed-effect and random-effect models. All meta-analyses were performed using a fixed-effect or random-effect model according to the I2 values. Heterogeneity was quantified with the I2, which describes the proportion of the total variation in study estimates caused by heterogeneity.[32,33] If the I2 value was <50%, the heterogeneity was considered acceptable and fixed-effect model was used; and if the I2 value was >50%, it implied the existence of heterogeneity and random-effect models was used.[34] For continuous variables, standardized mean difference (SMD) and 95% confidence intervals (CIs) were calculated. Statistical analyses were conducted using Review Manager (version 5, The Cochrane Collaboration). For all tests, P values <0.05 indicated statistically significant differences.
In this study, 3 main outcome measurements were calculated. Primarily, we assessed the difference of the mean value of IVIM parameters between benign and malignant focal lesions. Then, we focused on the IVIM parameters mean differences between hemangioma and hepatocellular carcinoma (HCC). Finally, we examined performance of IVIM parameters in distinguishing metastases from HCC.
3. Results
3.1. Study selection and data extraction
Titles and abstracts from retrieved references were screened to identify potentially eligible articles for inclusion in the review, whereas potentially relevant full text articles were analyzed based on the inclusion criteria. The initial database search identified 586 relevant articles that were published after November 1, 2016. Consequently, 6 articles were selected for data extraction (Fig. 1 A). Details of QUADAS are shown in Figure 1 B and C.
Figure 1.
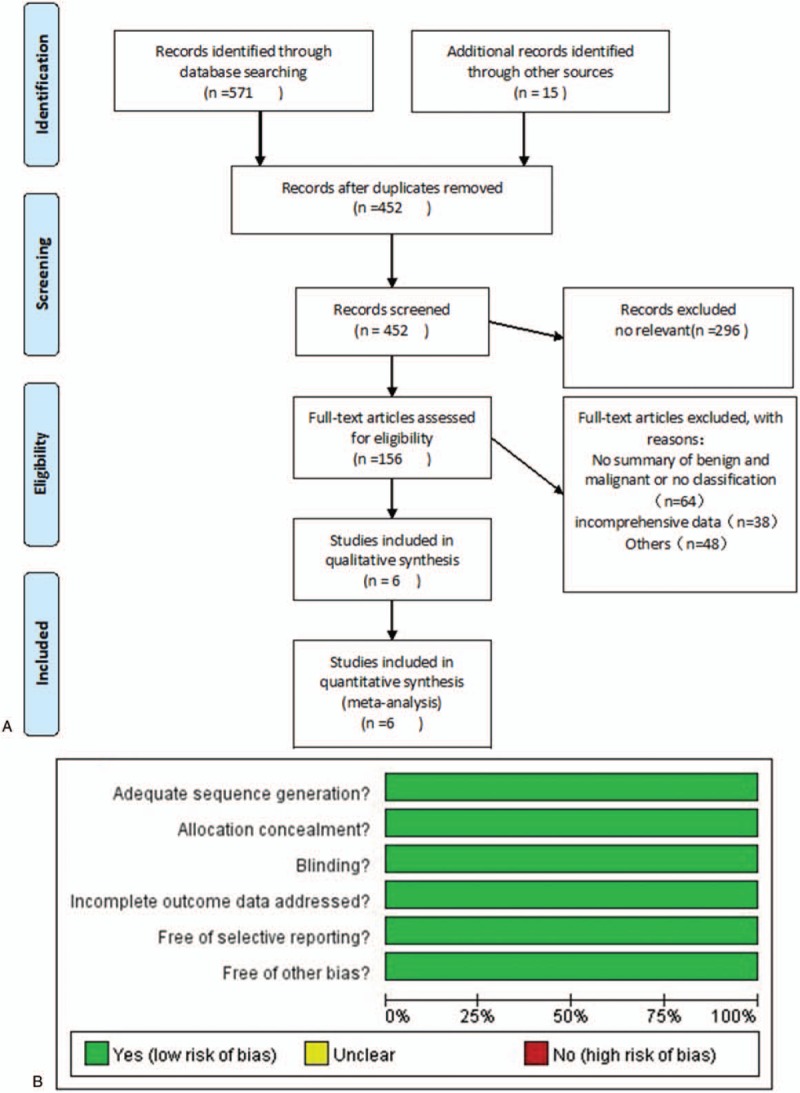
A, Study selection process. The flowchart summarizes the selection of studies including numbers and reasons of exclusion. B, Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies. C, Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.2. Description of the studies
A meta-analysis database was established according to the extracted information from each selected article. Study subjects, study baseline characteristics, and methodological qualities are shown in Table 1.
Table 1.
Characteristics of included studies.

This meta-analysis was performed on the per-lesion basis. Six articles included a total of 484 patients with 582 liver lesions, including 381 malignant and 201 benign lesions. From 381 malignancies, 257 lesions were HCCs, 102 were metastases and 22 were cholangiocellular carcinoma. The benign lesions included 100 hemangiomas, 44 cysts, 37 focal nodular hyperplasia, 14 adenomas, 5 abscesses, and 1 angiomyolipoma. All liver lesions were confirmed by pathology and/or overall analysis combined with medical history, clinical symptoms, and various imaging data.
3.3. Performance of IVIM parameters in distinguishing benign from malignant lesions
Five of 6 studies evaluated the performance of parameters comparing benign with malignant lesions. The random-effect model (I2 > 50%) and SMD were used to perform the meta-analysis. Briefly, the ADC, D, D∗, and f (%) results indicated that the weight of included studies ranged from 14.6% to 27.3%, 12.8% to 23.5%, 9.2% to 27.9%, and 19.6% to 20.5%. The weight derived from SD, which indicates the weight of each study in the combined effect volume can be used to evaluate the quality of references.[35] The values of ADC, D, D∗, and f (%) in benign compared with malignant lesions were 7.3 × 10–4 mm2/s [95% CI = (5.1 – 9.5) × 10–4 mm2/s; test for heterogeneity = 13.64, P = .009, I2 = 71%, test for overall effect: Z = 6.58, P < .001] (Fig. 2A); 5.4 × 10–4 mm2/s [95% CI = (3.3 – 7.4) × 10–4 mm2/s; test for heterogeneity = 26.15, P < .001, I2 = 85%; test for overall effect: Z = 5.17, P < .001] (Fig. 2B); −5.93 × 10–3 mm2/s [95% CI = (−14.19–2.32) × 10–3 mm2/s; test for heterogeneity = 14.33, P = .006, I2 = 72%; test for overall effect: Z = 1.41, P = .16) (Fig. 2C); 4.82(95% CI = −9.50–19.14; test for heterogeneity = 171.46, P < .001, I2 = 98%; test for overall effect: Z = 0.66, P = .51) (Fig. 2D). Furthermore, ADC and D values were significantly higher in benign lesions, while there was no significant difference in the D∗ and f values between the benign and malignant lesions.
Figure 1 (Continued).
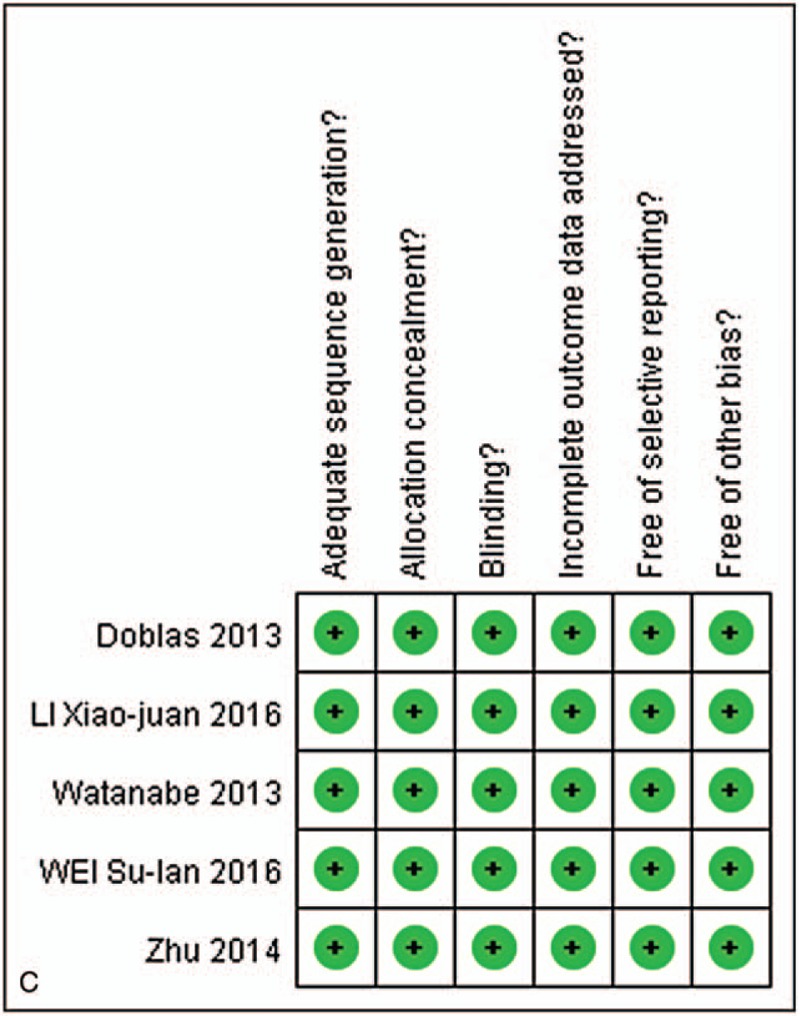
A, Study selection process. The flowchart summarizes the selection of studies including numbers and reasons of exclusion. B, Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies. C, Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.4. Performance of IVIM parameters in distinguishing hemangioma from hepatocellular carcinoma
Five of 6 studies evaluated the performance of parameters in hemangioma compared with HCC. The fixed-effect mode for ADC, D, and D∗ values (I2 < 50%); the random-effect model (I2 > 50%) for f values; and SMD were used to perform the meta-analysis. The results of ADC, D, D∗, and f (%) indicated that the weight of included studies ranged from 5.8% to 49.1%, 4.2% to 48.5%, 3.3% to 41.5%, and 18.6% to 22.4%. The values of ADC, D, D∗, and f (%) in hemangioma compared with HCC were 9.1 × 10–4 mm2/s [95% CI = (7.9 – 10.4) × 10–4 mm2/s; test for heterogeneity = 7.08, P = .13, I2 = 44%, test for overall effect: Z = 14.30, P < .001] (Fig. 3A); 6.2 × 10–4 mm2/s [95% CI = (5.3 – 7.0) × 10–4 mm2/s; test for heterogeneity = 4.89, P = .30, I2 = 18%; test for overall effect: Z = 14.43, P < .001] (Fig. 3B); −5 × 10–4 mm2/s [95% CI = (−7.6 – 6.61) × 10–3 mm2/s; test for heterogeneity = 3.9, P = .42, I2 = 0%; test for overall effect: Z = 0.14, P = .89] (Fig. 3C); 9.73 (95% CI = 2.05–17.41; test for heterogeneity = 21.51, P = .003, I2 = 81%; test for overall effect: Z = 2.48, P = .01) (Fig. 3D). In addition, ADC, D, and f values were significantly higher in hemangioma compared to HCC, whereas there was no significant difference in the D∗ values between the hemangioma and HCCs.
Figure 2.
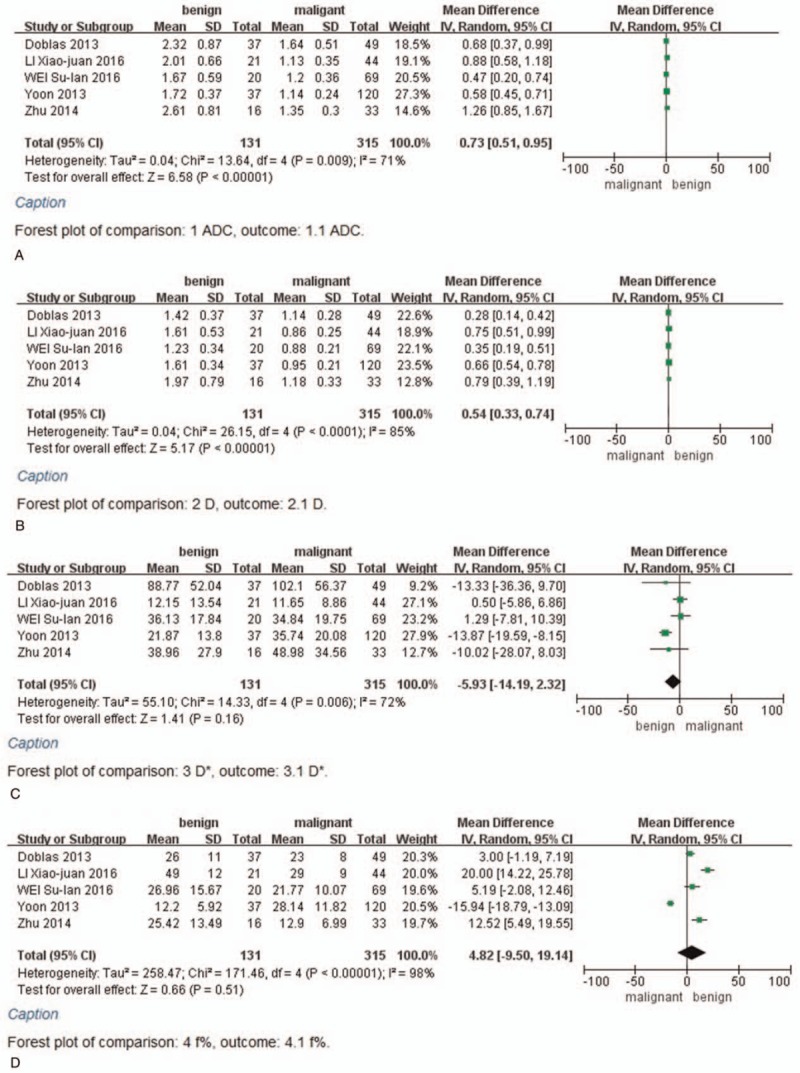
A, Forest plot showing results of the mean apparent diffusion coefficient (ADC) value between benign and malignant focal liver lesions [mean ADC ± standard deviation (SD) × 10–3 mm2/s]. B, Forest plot showing results of the mean D value between benign and malignant focal liver lesions (mean D ± SD × 10–3 mm2/s). C, Forest plot showing results of the mean D∗ value between benign and malignant focal liver lesions (mean D∗ ± SD × 10–3 mm2/s). D, forest plot showing results of the mean f value between benign and malignant focal liver lesions [mean f(%) ± SD].
3.5. Performance of IVIM parameters in distinguishing metastases from hepatocellular carcinoma
Four of 6 studies evaluated the performance of parameters in metastases compared with HCC. The fixed-effect mode for D and D∗ values because of I2 < 50%, the random-effect model (I2 > 50%) for ADC and f values and SMD were used to perform the meta-analysis. The results of ADC, D, D∗, and f (%) indicated that the weight of included studies ranged from 14.3% to 28.8%, 11.7% to 37.4%, 4.7% to 75.0%, and 12.4% to 31.4%. The values of ADC, D, D∗, and f (%) in metastases compared with HCC were 4 × 10–5 mm2/s [95% CI = (−1.6–2.4) × 10–4 mm2/s; test for heterogeneity = 7.13, P = .07, I2 = 58%, test for overall effect: Z = 0.36, P = .72] (Fig. 4A); 8 × 10–5 mm2/s [95% CI = (−1–17) × 10–5 mm2/s; test for heterogeneity = 2.62, P = .45, I2 = 0%; test for overall effect: Z = 1.81, P = .07] (Fig. 4B); −7.61 × 10–3 mm2/s [95% CI = (−16.4–1.18) × 10–3 mm2/s; test for heterogeneity = 3.1, P = .38, I2 = 3%; test for overall effect: Z = 1.70, P = .09] (Fig. 4C); −1.82 (95% CI = −5.85–2.22; test for heterogeneity = 6.74, P = .08, I2 = 55%; test for overall effect: Z = 0.88, P = .38) (Fig. 4D). Furthermore, there were no significant differences in the ADC, D, D∗, and f values between the metastases and HCC.
Figure 3.
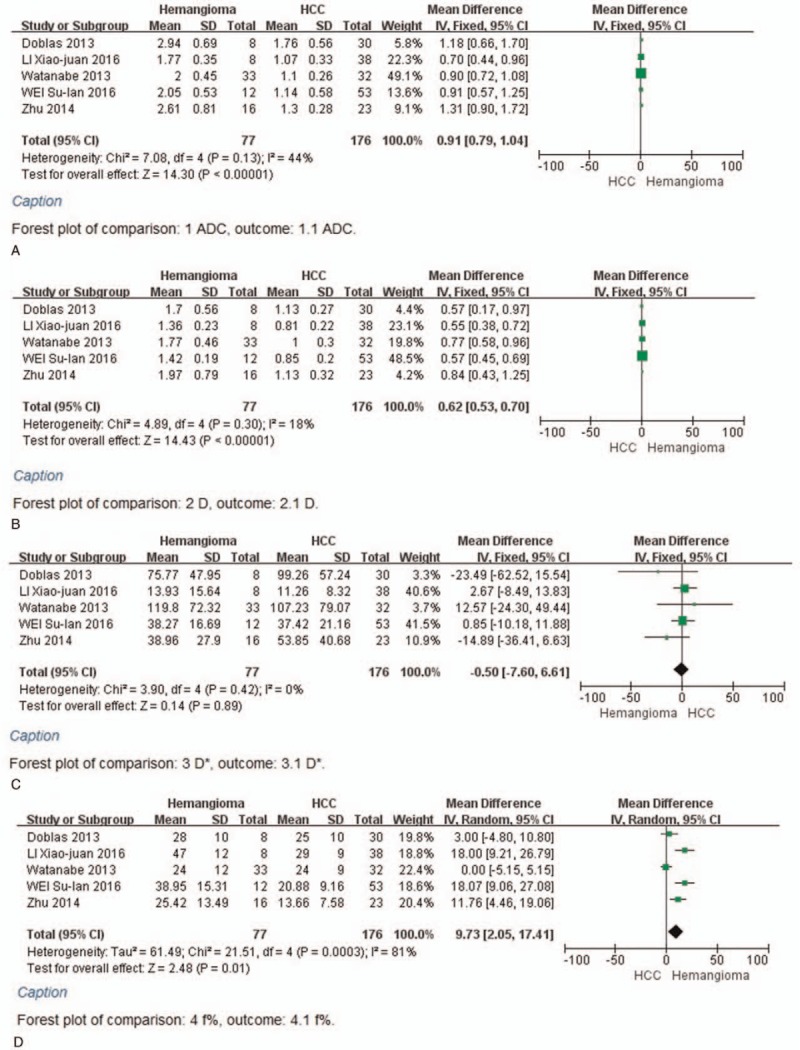
A, Forest plot showing results of the mean apparent diffusion coefficient (ADC) value between hemangioma and hepatocellular carcinoma [mean ADC ± standard deviation (SD)×10–3 mm2/s]. B, Forest plot showing results of the mean D value between hemangioma and hepatocellular carcinoma (mean D ± SD × 10–3 mm2/s). C, Forest plot showing results of the mean D∗ value between hemangioma and hepatocellular carcinoma (mean D∗ ± SD × 10–3 mm2/s). D, Forest plot showing results of the mean f value between hemangioma and hepatocellular carcinoma [mean f (%) ± SD].
Figure 4.
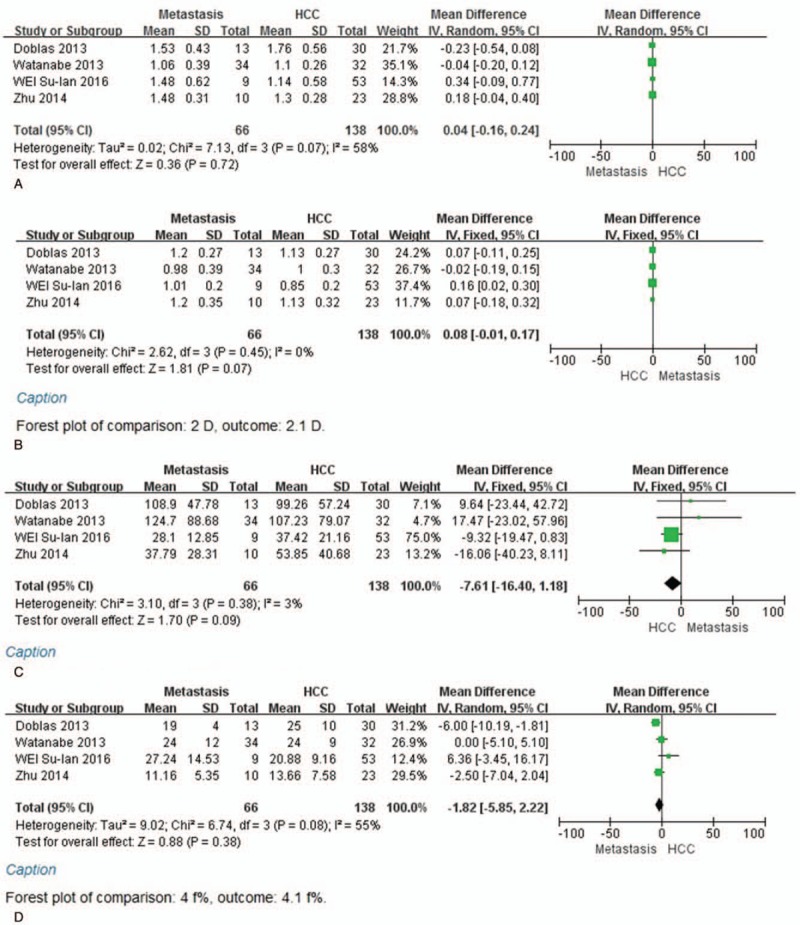
A, Forest plot showing results of the mean apparent diffusion coefficient (ADC) value between metastases and hepatocellular carcinoma [mean ADC ± standard deviation (SD) × 10–3 mm2/s]. B, Forest plot showing results of the mean D value between metastases and hepatocellular carcinoma (mean D ± SD × 10–3 mm2/s). C, Forest plot showing results of the mean D∗ value between metastases and hepatocellular carcinoma (mean D∗ ± SD × 10–3 mm2/s). D, Forest plot showing results of the mean f value between metastases and hepatocellular carcinoma [mean f (%) ± SD].
4. Discussion
IVIM imaging, or DWI with a range of low (i.e., <50 s/mm2) and high (i.e., >200 s/mm2) b-values, was proposed to separately measure diffusion and perfusion-related diffusion.[25,36] IVIM makes it possible to obtain the true diffusion coefficient (D) reflecting cell density and the perfusion fraction (f) reflecting the microcirculation of tumors.[37]
The IVIM parameters D∗ and f describe the microcirculation effect. The D∗ value depends on the mean blood velocity and the length of the microvascular segment, and the diffusion coefficient of the blood, whereas f represents the fraction of the signal originating from perfusion and is expected to reflect the fractional blood volume of capillaries.[38,39]
Our results showed that D and ADC values were helpful for the differentiation between benignity and malignancy according to IVIM MR images, which suggested that true and apparent molecular diffusions may be more informative than pseudodiffusion (D∗) or perfusion fraction (f) in the characterization of liver lesions. The cellular density of malignancy was higher than benignity, whereas the ADC and D values were lower. Mungai et al[40] have reported that ADC is useful in the classification of more than half of noncystic focal liver lesions.
The reason why there was no significant difference in D∗ and f values between liver lesions remains unclear, nevertheless blood volume (f), blood flow (D∗), or secretion could have different effects on perfusion properties in different lesion types.[24,41] For example, metastases and cholangiocarcinomas with low blood supply may be highly cellular and lowly perfused compared with benign tumors.[42]
Secondly, ADC, D, and f values were significantly higher in hemangioma compared to HCC, whereas there was no significant difference in the D∗ values between the hemangioma and HCC. Because D∗ depends on the mean blood velocity and length of microvessel segments (and on the diffusion coefficient of blood),[38,39] and given there are 3 types of hemangiomas: sufficient blood supply, lower blood supply, and lack of blood supply, the value of D∗ might fluctuate over a large range.[43] When the blood supplies of high-flow and middle-flow hemangiomas come from the hepatic artery, they are similar to HCC's blood supply, and they might actually explain why there was no difference in D∗ value between the hemangioma and HCC. Nevertheless, the cellular density and blood volume were different between the hemangioma and HCC, so that the ADC, D, and f values of hemangioma were higher compared to HCC.
Finally, malignant liver tumors can be classified into primary cancers and secondary (metastatic) tumors.[44] In this study, the metaregression analysis indicated there were no significant differences in the ADC, D, D∗, and f values between the metastases and HCCs. The metastases arise from several primary neoplasms such as gastrointestinal, lung, breast, and genitourinary,[45] and may cause a number of variations in the cell density and microcirculation. IVIM parameters are somewhat correlated with histological grade of HCC because of the differences in the cell density and microscopic circulation,[9,41,46,47] nevertheless the studies included in this metaregression analysis did not report on different metastases cell types and HCC histological grades. Consequently, this might explain why there was no difference between the metastases and HCC in IVIM parameters, and it should be addressed by further research.
Since there were <5 studies included in the present data analysis, we did not make the funnel plot for publication bias, because previous studies have reported that funnel plot is not significant with <10 studies.[30] We tried to collect more studies to reduce publication bias. The weight derived from SD indicates the weight of each study in the combined effect volume, and can be used to evaluate the quality of references.[35] In our study, the weight was determined according to the number of cases in each study; the higher the weight, the larger the sample size was.
Our meta-analysis had several limitations. First, one of the relevant studies[48] failed to include the cysts into the benign focal lesions of liver, which might have led to some biased results. Second, the IVIM model is less stable than the monoexponential diffusion model, and it requires the fitting of more variables.[11,49] Free-breathing or respiratory-triggered, multi-b values and cardiac motion artifacts may affect the measurement repeatability of IVIM parameters.[6,50] In the studies we used, the MR scanning parameters were not consistent; 6 studies all together[22,24,42,23,43,48] did not have unified b values and the field strength, which in turn had impact on the results of the meta-analysis. Since IVIM is somewhat a new technology, there are relatively fewer published articles; therefore, this article can serve as a preliminary study. We will continue to follow and collect relevant studies for future analyses.
In conclusion, D and ADC values were helpful for the differentiation between benignity and malignancy on IVIM MR imaging, and thus indicating that true and apparent molecular diffusions may be more informative than pseudodiffusion (D∗) or perfusion fraction (f) in the characterization of liver lesions. Secondly, the ADC, D, and f values of hemangioma were higher compared to HCC, whereas D∗ value showed no difference. This might be due to the types of various blood supplies of hemangioma. However, because of different metastatic cell types and HCC histological grades, IVIM was not very helpful for differentiating the metastases and HCC in the present study, and thus calling for further verifications in the future.
Acknowledgments
The authors thank Lijun Ouyang for proofreading the manuscript.
Author contributions
Data curation: Hongzhen Wu, Xinqing Jiang.
Formal analysis: Hongzhen Wu.
Investigation: Hongzhen Wu, Yingying Liang, Xinqing Jiang, Xinhua Wei, Weifeng Liu, Yuan Guo, Wenjie Tang.
Methodology: Hongzhen Wu, Yingying Liang, Xinqing Jiang, Xinhua Wei, Weifeng Liu, Yuan Guo, Wenjie Tang.
Resources: Hongzhen Wu.
Software: Yu Liu.
Validation: Hongzhen Wu.
Writing – original draft: Hongzhen Wu, Xinqing Jiang, Xinhua Wei, Weifeng Liu, Yuan Guo.
Writing – review and editing: Hongzhen Wu, Yingying Liang, Xinqing Jiang, Xinhua Wei, Yu Liu, Weifeng Liu, Yuan Guo, Wenjie Tang.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, CI = confidence interval, FNH = focal nodular hyperplasia, HCC = hepatocellular carcinoma, IVIM = intravoxel incoherent motion, MR = magnetic resonance imaging, QUADAS = quality assessment of diagnostic studies, SD = standard deviation, SMD = standardized mean difference.
This study was supported by Guangdong modern hospital management institute hospital management research (No.2016009), the National Natural Science Foundation of China (No.81571665) and the Science and Technology Planning Project of Guangzhou (No. 201804010032).
The authors declare no conflicts of interest.
References
- [1].Li R, Wu G, Wang R. Application values of 3.0T magnetic resonance diffusion weighted imaging for distinguishing liver malignant tumors and benign lesions. Oncol Lett 2018;15:2091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Trillaud H, Bruel JM, Valette PJ, et al. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol 2009;15:3748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology 2012;262:520–9. [DOI] [PubMed] [Google Scholar]
- [4].Jahic E, Sofic A, Selimovic AH. DWI/ADC in differentiation of benign from malignant focal liver lesion. Acta Inform Med 2016;24:244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hennedige TP, Hallinan JT, Leung FP, et al. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. Eur Radiol 2016;26:398–406. [DOI] [PubMed] [Google Scholar]
- [6].Lee Y, Lee SS, Kim N, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of the liver: effect of triggering methods on regional variability and measurement repeatability of quantitative parameters. Radiology 2015;274:405–15. [DOI] [PubMed] [Google Scholar]
- [7].Malayeri AA, El Khouli RH, Zaheer A, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011;31:1773–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 2011;196:1351–61. [DOI] [PubMed] [Google Scholar]
- [9].Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505. [DOI] [PubMed] [Google Scholar]
- [10].Turner R, Le Bihan D, Maier J, et al. Echo-planar imaging of intravoxel incoherent motion. Radiology 1990;177:407–14. [DOI] [PubMed] [Google Scholar]
- [11].Ter Voert EE, Delso G, Porto M, et al. Intravoxel incoherent motion protocol evaluation and data quality in normal and malignant liver tissue and comparison to the literature. Invest Radiol 2016;51:90–9. [DOI] [PubMed] [Google Scholar]
- [12].Ebrahimi B, Rihal N, Woollard JR, et al. Assessment of renal artery stenosis using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging analysis. Invest Radiol 2014;49:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hwang EJ, Lee JM, Yoon JH, et al. Intravoxel incoherent motion diffusion-weighted imaging of pancreatic neuroendocrine tumors: prediction of the histologic grade using pure diffusion coefficient and tumor size. Invest Radiol 2014;49:396–402. [DOI] [PubMed] [Google Scholar]
- [14].Klau M, Mayer P, Bergmann F, et al. Correlation of histological vessel characteristics and diffusion-weighted imaging intravoxel incoherent motion-derived parameters in pancreatic ductal adenocarcinomas and pancreatic neuroendocrine tumors. Invest Radiol 2015;50:792–7. [DOI] [PubMed] [Google Scholar]
- [15].Cui Y, Dyvorne H, Besa C, et al. IVIM diffusion-weighted imaging of the liver at 3.0T: comparison with 1.5T. Eur J Radiol Open 2015;2:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murphy P, Hooker J, Ang B, et al. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J Magn Reson Imaging 2015;41:1629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ichikawa S, Motosugi U, Morisaka H, et al. MRI-based staging of hepatic fibrosis: comparison of intravoxel incoherent motion diffusion-weighted imaging with magnetic resonance elastography. J Magn Reson Imaging 2015;42:204–10. [DOI] [PubMed] [Google Scholar]
- [18].Klauss M, Mayer P, Maier-Hein K, et al. IVIM-diffusion-MRI for the differentiation of solid benign and malign hypervascular liver lesions—evaluation with two different MR scanners. Eur J Radiol 2016;85:1289–94. [DOI] [PubMed] [Google Scholar]
- [19].Watanabe H, Kanematsu M, Goshima S, et al. Characterizing focal hepatic lesions by free-breathing intravoxel incoherent motion MRI at 3.0 T. Acta Radiol 2014;55:1166–73. [DOI] [PubMed] [Google Scholar]
- [20].Lai V, Lee VHF, Lam KO, et al. Intravoxel incoherent motion MR imaging in nasopharyngeal carcinoma: comparison and correlation with dynamic contrast enhanced MR imaging. Oncotarget 2017;8:68472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Togao O, Hiwatashi A, Yamashita K, et al. Measurement of the perfusion fraction in brain tumors with intravoxel incoherent motion MR imaging: validation with histopathological vascular density in meningiomas. Br J Radiol 2018;91:20170912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yoon JH, Lee JM, Yu MH, et al. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging 2014;39:276–85. [DOI] [PubMed] [Google Scholar]
- [23].Su-lan W, Feng Y, Xiao-duo Y, et al. Intravoxel incoherent motion diffusion weighted imaging indifferentiation between benign and malignant lesions of liver [in Chinese]. Radiol Practice 2016;04:364–8. [Google Scholar]
- [24].Watanabe H, Kanematsu M, Goshima S, et al. Characterizing focal hepatic lesions by free-breathing intravoxel incoherent motion MRI at 3.0 T. Acta Radiol 2013;55:1166–73. [DOI] [PubMed] [Google Scholar]
- [25].Ichikawa S, Motosugi U, Ichikawa T, et al. Intravoxel incoherent motion imaging of focal hepatic lesions. J Magn Reson Imaging 2013;37:1371–6. [DOI] [PubMed] [Google Scholar]
- [26].Li Y, Chen Z, Wang J. Differential diagnosis between malignant and benign hepatic tumors using apparent diffusion coefficient on 1.5-T MR imaging: a meta-analysis. Eur J Radiol 2012;81:484–90. [DOI] [PubMed] [Google Scholar]
- [27].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [28].Whiting PF, Weswood ME, Rutjes AW, et al. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guo J, Seo Y, Ren S, et al. Diagnostic performance of contrast-enhanced multidetector computed tomography and gadoxetic acid disodium-enhanced magnetic resonance imaging in detecting hepatocellular carcinoma: direct comparison and a meta-analysis. Abdom Radiol (NY) 2016;41:1960–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mbeye NM, Adetokunboh O, Negussie E, et al. Shifting tasks from pharmacy to non-pharmacy personnel for providing antiretroviral therapy to people living with HIV: a systematic review and meta-analysis. BMJ Open 2017;7:e015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abril-Ulloa V, Flores-Mateo G, Sola-Alberich R, et al. Ferritin levels and risk of metabolic syndrome: meta-analysis of observational studies. BMC Public Health 2014;14:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [34].Chen M, Huang J, Zhu Z, et al. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer 2013;13:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Biaoxue R, Shuanying Y, Xiguang C, et al. Differential diagnostic CYFRA 21-1 level for benign and malignant pleural effusions: a meta-analysis in the Chinese population. Arch Med Sci 2012;8:756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401–7. [DOI] [PubMed] [Google Scholar]
- [37].Shirota N, Saito K, Sugimoto K, et al. Intravoxel incoherent motion MRI as a biomarker of sorafenib treatment for advanced hepatocellular carcinoma: a pilot study. Cancer Imaging 2016;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 1992;27:171–8. [DOI] [PubMed] [Google Scholar]
- [39].Penner AH, Sprinkart AM, Kukuk GM, et al. Intravoxel incoherent motion model-based liver lesion characterisation from three b-value diffusion-weighted MRI. Eur Radiol 2013;23:2773–83. [DOI] [PubMed] [Google Scholar]
- [40].Mungai F, Morone M, Villanacci A, et al. Diffusion weighted MR and apparent diffusion coefficient measurement in classification and characterization of noncystic focal liver lesions: does a clinical role exist? Medicine (Baltimore) 2014;93:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wirestam R, Borg M, Brockstedt S, et al. Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiol 2001;42:123–8. [PubMed] [Google Scholar]
- [42].Doblas S, Wagner M, Leitao HS, et al. Determination of malignancy and characterization of hepatic tumor type with diffusion-weighted magnetic resonance imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived measurements. Invest Radiol 2013;48:722–8. [DOI] [PubMed] [Google Scholar]
- [43].Zhu L, Cheng Q, Luo W, et al. A comparative study of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters for the characterization of common solid hepatic tumors. Acta Radiol 2014;56:1411–8. [DOI] [PubMed] [Google Scholar]
- [44].Shi JH, Line PD. Effect of liver regeneration on malignant hepatic tumors. World J Gastroenterol 2014;20:16167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206–13. [DOI] [PubMed] [Google Scholar]
- [46].Woo S, Lee JM, Yoon JH, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology 2014;270:758–67. [DOI] [PubMed] [Google Scholar]
- [47].Matsui O, Kobayashi S, Sanada J, et al. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 2011;36:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xiao-juan L, Xiao-yan M, Xiao C, et al. The diagnostic value of intra-voxel incoherent motion diffusion-weighted imaging in evaluating hepatic lesions. Radiol Practice 2016;6:526–30. [Google Scholar]
- [49].Andreou A, Koh DM, Collins DJ, et al. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol 2013;23:428–34. [DOI] [PubMed] [Google Scholar]
- [50].Kakite S, Dyvorne H, Besa C, et al. Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging 2015;41:149–56. [DOI] [PubMed] [Google Scholar]


