Abstract
During the perioperative period of cardiac disease, as many risk factors exist, such as primary cardiac diseases, the use of vasopressors, ischemia-reperfusion injury during cardiopulmonary bypass (CPB), and surgical stress, the gut suffered from ischemia, anoxia and oxidative stress, which would lead to the enterocyte injury. The aim of this study was to explore whether serum intestinal fatty acid-binding protein (IFABP), which is excreted specifically from damaged intestinal enterocytes, as a predictor of prognosis in postoperative cardiac surgery patients.
From January 2017 to December 2017, 40 postoperative cardiac surgery patients were enrolled in this observational study. Serum IFABP levels and prognostic biomarkers were recorded at intensive care unit (ICU) admission.
The serum IFABP levels were significantly higher in postoperative cardiac surgery patients who complicated with multiple organ dysfunction syndrome (MODS) (median, 883.20 pg/mL vs 426.10 pg/mL; P < .001), infective complications (median, 917.70 pg/mL vs 409.40 pg/mL; P < .001), or who stayed in ICU beyond 4 days (median, 807.65 pg/mL vs 426.10 pg/mL; P < .001). Moreover, in patients who suffered from right ventricular dysfunction, the serum IFABP levels were significantly higher (median, 737.85 pg/mL vs 445.55 pg/mL; P = .016). The serum IFABP levels also showed great precision for the prediction of MODS (the area under curve, AUC 0.923), infective complications (AUC 0.961) and ICU stay beyond 4 days (AUC 0.853). And it correlated significantly with the acute physiology and chronic health evaluation (APACHE) II score (P < .05), sequential organ failure assessment (SOFA) score (P < .05), and acute gastrointestinal injury (AGI) grade (P < .001).
The serum IFABP level at ICU admission is a valuable, convenient, and objective early predictor of prognosis in postoperative cardiac surgery patients.
Keywords: infective complications., intestinal fatty acid-binding protein, multiple organ dysfunction syndrome, postoperative cardiac surgery
1. Introduction
Critically postoperative cardiac surgery patients usually manifested as suffering from many complications, such as organ dysfunction and infection, which will lead to be longer intensive care unit (ICU) stay and more medical expenditure. Heart, as the motor of oxygen delivery, directly affects other organs function. Both reduced cardiac output (CO) and increased central venous pressure (CVP) are crucial in describing the organ-to-organ crosstalk between the damaged cardiac function and other organs, and vice versa, cardiorenal syndrome is a typically example in cardiology.[1] While accumulating evidence shows that organ crosstalk occurs between the heart and the gut.[2–5] Severe cardiac diseases, such as severe cardiac valve disease or coronary artery disease, at the preoperation, it manifested as intestinal hypoperfusion or congestion, and during the operation, some other factors furtherly cause gut injury, such as cardiopulmonary bypass, vasoactive drugs usage, and surgical stress. Therefore, after operation, gastrointestinal injury, even gastrointestinal dysfunction often appears.[6,7] As is well known, gut is a center organ of surgical stress, and gastrointestinal dysfunction plays a pivotal role in the progression of infective complications and multiple organ dysfunction syndrome (MODS) after operation, which would lead to a poor outcome in postoperative cardiac surgery patients.[8,9] Early identification of postoperative cardiac surgery patients at risk of developing infective complications and MODS is important for the optimization of early aggressive management, which will reduce the length of ICU stay and improve the prognosis.[10]
Intestinal fatty acid binding protein (IFABP), a low-molecular-weight (15 kD) cytosolic protein, is specifically expressed at the tip of intestinal villi.[11] Its circulating levels are very low in healthy individuals, but it rapidly releases into the systemic circulation upon enterocyte injury, and it is thus believed to be an early and useful marker for enterocyte injury.[12] Recently, elevated IFABP levels have been found in patients suffering from acute decompensated heart failure,[3] septic shock,[13] acute pancreatitis,[14] ulcerative colitis,[15] and have been reported as a promising sensitive marker for predicting clinical outcomes.
As enterocyte is very sensitive to ischemia or anoxia, which could be caused by cardiac dysfunction directly. Therefore, we speculate that the circulating IFABP levels may be a predictor of prognosis in postoperative cardiac surgery patients, and the levels of IFABP could be used to help in identifying patients at high risk of developing complications.
2. Methods
2.1. Study population
In this prospective observational study, all consecutive postoperative cardiac surgery patients admitted to our department of cardiovascular ICU, Nanjing First Hospital, with predicted ICU stay beyond 24 hours, were enrolled from January 2017 to December 2017. All enrolled patients, suffered from severe cardiac valve disease and/or coronary artery disease, were performed cardiac valve replacement, and/or coronary artery bypass graft (CABG). Patients with chronic organ dysfunction (e.g., hepatic or renal dysfunction), chronic digestive disease, previous gastrointestinal surgery, confirmed or strongly suspected intestinal ischemia and/or necrosis, confirmed or strongly suspected infection before operation, immunodeficiency, younger than 18 years old or older than 75 years old, were all excluded. All patients received standard medical therapy, including intensive monitoring, controlling arterial blood pressure and pulmonary artery pressure, controlling heart rate and rhythm, glycemic control, bleeding control, oxygen therapy, pain management, organ function maintenance, and antibiotic therapy.[16,17] Moreover, all patients were followed until discharge or hospital death. The study was approved by the institutional review board of our hospital. Informed consent was taken from all participants.
2.2. IFABP measurements
Blood samples were collected at ICU admission, and were clotted at room temperature for 30 minutes. Samples were then centrifuged for 10 minutes at 3000g, and serum was collected in 2.0 mL eppendorf tubes and stored at −80°C until assayed. Serum IFABP levels were analyzed with a human FABP2/IFABP enzyme-linked immunosorbent assays (ELISA) with commercially available kits (Biocalvin Company, Suzhou, China) according to the manufacturer's protocol, and 450 nm with a correction wavelength set at 570 nm on a spectrophotometer (Beckman Du 530 Life Science UV/Visible spectrophotometer; Beckman Coulter) was used. Attending intensivists were blind to patient IFABP results, and the results therefore had no influence on the treatment of patients.
2.3. Data collection
At ICU admission, some baseline values were collected, including demographic data: age, sex, body mass index (BMI), edema of lower extremities or not, preoperative left ventricular ejection fraction (LVEF); operation data: operation time, cardiopulmonary bypass (CPB) time, cross-clamp time; disease severity evaluation within 24 hours after ICU admission: acute physiology and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score, acute gastrointestinal injury (AGI) grade; hemodynamic data: mean arterial pressure (MAP), heart rate, CVP, inotropic score[Inotropic score calculated as (dopamine dose × 1) + (dobutamine dose × 1) + (milrinon dose × 10) + (epinephrine dose × 100) + (norepinephrine dose × 100), where all doses are expressed as micrograms per kilogram per minute.)];[18] laboratory data: white blood cell count (WBC), platelet count, hemoglobin, arterial lactate, creatinine, blood urea nitrogen (BUN), albumin, procalcitonin (PCT), serum N-terminal pro-B-type natriuretic peptide (NT-proBNP), IFABP, oxygenation index. Besides, some prognostic markers were also collected, like the duration of ventilatory support, the number of patients suffered from MODS, infective complications, hospital mortality, duration of ICU stay and hospital stay. The definitions of organ dysfunction were based on a score of 2 or more in the SOFA scoring system.[19] MODS was defined as the combined dysfunction of 2 major organ systems. Infection was diagnosed by synthesizing clinical signs and symptoms, imaging examinations and specimen bacterial culture. The AGI grade was from the Recommendations of the ESICM Working Group on Abdominal Problems in 2012.[20]
2.4. Statistical analysis
Data were presented as medians (interquartile ranges), unless stated otherwise. Categorical variables were expressed as absolute numbers or in percentages, and were analyzed using χ2 test or Fisher's exact test. Continuous variables were compared by the Mann–Whitney U test or Kruskal–Walls test. Receiver operating characteristic (ROC) curves were used to evaluate the ability of some parameters or scoring systems to predict the severity and prognosis. Pearson test was used to analyze the correlations between the 2 variables. We used the Statistical Package for the Social Sciences (SPSS, version 19.0, Chicago, IL) software for statistical analysis. P < .05 was considered significant.
3. Results
3.1. Study population
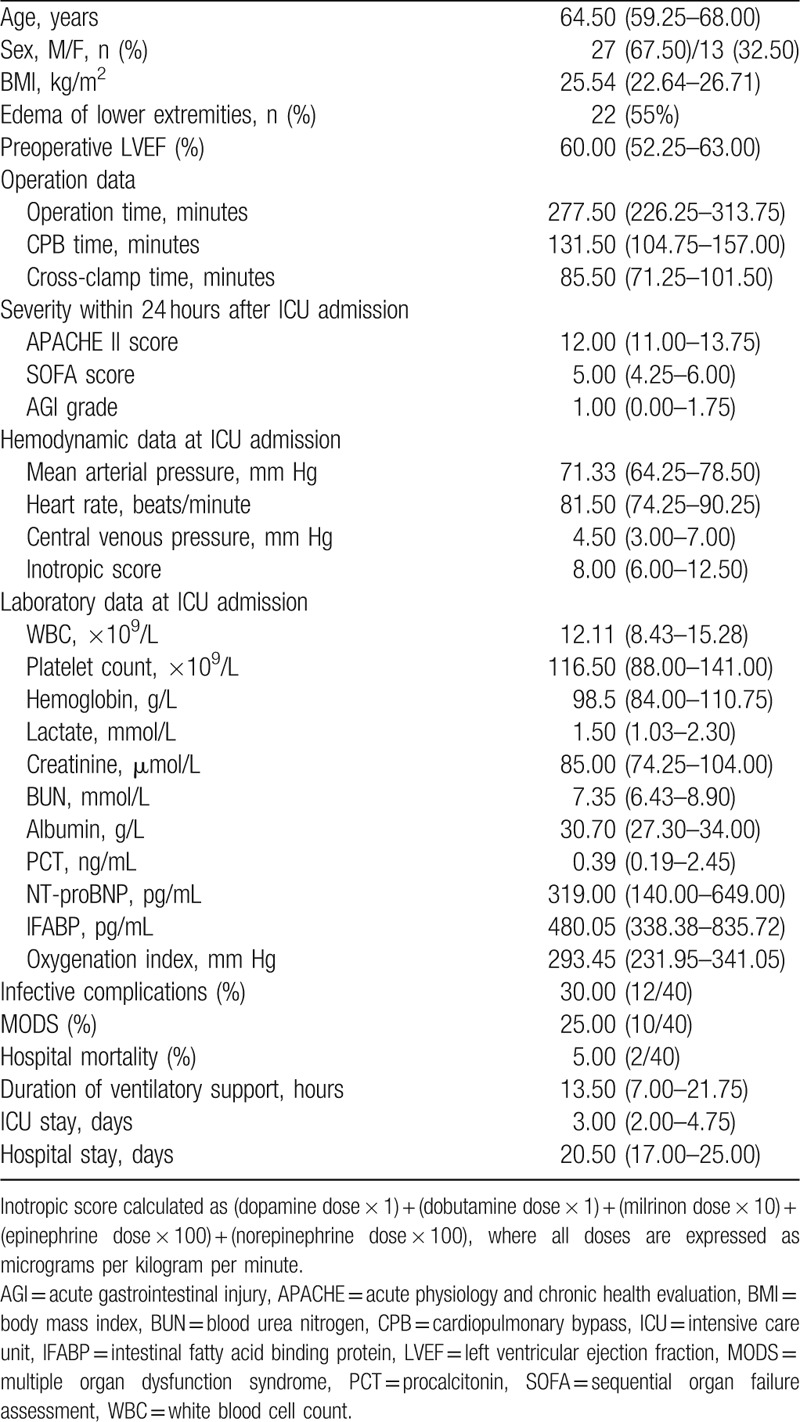
As shown in Figure 1, a total of 40 eligible postoperative cardiac surgery patients were enrolled in this prospective, observational study during the specified period. Table 1 provides some information of patients in this study group in details, including the demographic parameters, clinical data, severity scores and outcomes. Ultimately, the median age was 64.5 years old, and the median APACHE II score was 12 (interquartile range, IQR, 11–13.75) and SOFA score was 5 (IQR, 4.25–6), and the median IFABP concentration was 480.05 pg/mL (IQR, 338.38–835.72). Around 2 patients (5.0%) suffered from hospital death, and 10 patients (25.0%) were complicated with MODS, while 12 patients (30.0%) developed infective complications. The median ICU stay was 3.0 days (IQR, 2.0–4.75).
Figure 1.
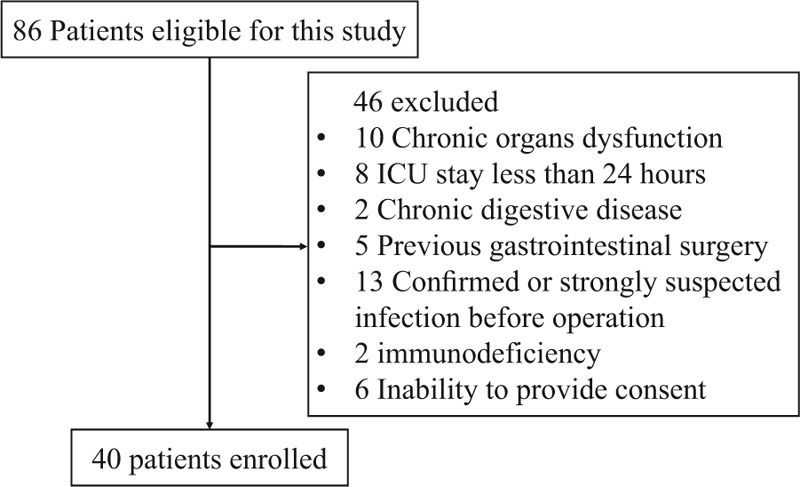
The flow diagram of participants.
Table 1.
Demographic data at ICU admission and clinical parameters.
3.2. IFABP and presence or absence of clinical variables
As shown in Table 2, the serum IFABP levels were significantly higher in postoperative cardiac surgery patients who complicated with MODS (median, 883.20 pg/mL vs 426.10 pg/mL; P < .001), infective complications (median, 917.70 pg/mL vs 409.40 pg/mL; P < .001), positive blood cultures (median, 1096.10 pg/mL vs 473.45 pg/mL; P < .001), or who stayed in ICU beyond 4 days (median, 807.65 pg/mL vs 426.10 pg/mL; P < .001). Moreover, in patients who suffered from right ventricular dysfunction, such tricuspid valve disease or right coronary artery disease, the serum IFABP levels were significantly higher (median, 737.85 pg/mL vs 445.55 pg/mL; P = .016). And in patients who needed higher doses of vasoactive drugs (inotropic scores more than 10) to maintain hemodynamic stability at ICU admission, the serum IFABP levels were also significantly higher (median, 716.45 pg/mL vs 422.05 pg/mL; P = .004). Patients who died in hospital also had higher serum IFABP levels (P < .001) (Fig. 2).
Table 2.
Values of serum IFABP in relation to the presence or absence of clinical variables.
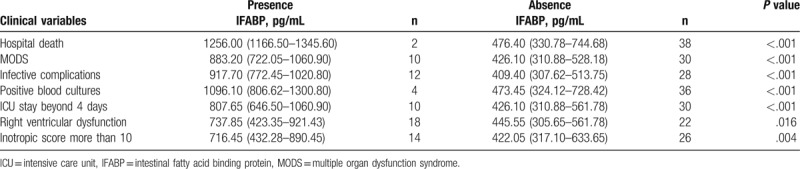
Figure 2.
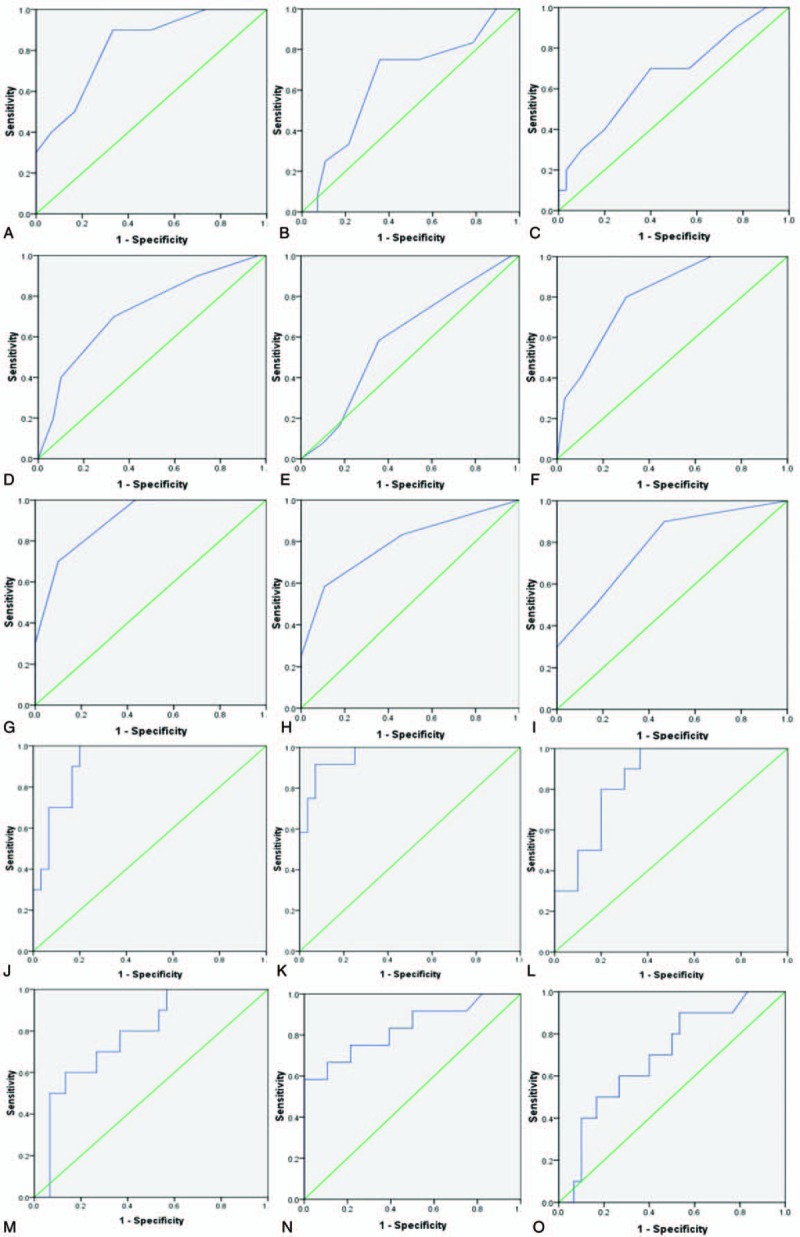
Receiver operating characteristic (ROC) curves for acute physiology and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score, acute gastrointestinal injury (AGI) grade, intestinal fatty acid-binding protein (IFABP), and procalcitonin (PCT) level in the prediction of multiple organ dysfunction syndrome (MODS), infective complications and intensive care unit (ICU) stay beyond 4 days. (A) ROC curve for APACHE II score in the prediction of MODS; (B) ROC curve for APACHE II score in the prediction of infective complications; (C) ROC curve for APACHE II score in the prediction of ICU stay beyond 4 days; (D) ROC curve for SOFA score in the prediction of MODS; (E) ROC curve for SOFA score in the prediction of infective complications; (F) ROC curve for SOFA score in the prediction of ICU stay beyond 4 days; (G) ROC curve for AGI grade in the prediction of MODS; (H) ROC curve for AGI grade in the prediction of infective complications; (I) ROC curve for AGI grade in the prediction of ICU stay beyond 4 days; (J) ROC curve for IFABP in the prediction of MODS; (K) ROC curve for IFABP in the prediction of infective complications; (I) ROC curve for IFABP in the prediction of ICU stay beyond 4 days; (M) ROC curve for PCT in the prediction of MODS; (N) ROC curve for PCT in the prediction of infective complications; (O) ROC curve for PCT in the prediction of ICU stay beyond 4 days. AGI = acute gastrointestinal injury, APACHE = acute physiology and chronic health evaluation, ICU = intensive care unit, IFABP = intestinal fatty acid binding protein, MODS = multiple organ dysfunction syndrome, PCT = procalcitonin, ROC = receiver operating characteristic, SOFA = sequential organ failure assessment.
3.3. Prediction of MODS, infective complications and ICU stay beyond 4 days
As shown in Table 2, postoperative cardiac surgery patients who presented with MODS during hospitalization, compared with those without MODS, had a higher level of IFABP at ICU admission, so were those who suffered from infective complications, and whose ICU stay beyond 4 days. In the prediction of the development of MODS, all the variables studied were valuable (P < .05), including the APACHE II score, SOFA score, AGI grade, IFABP and PCT. And the serum IFABP levels presented the best predictive value with the biggest areas under the curve (AUCs) (0.923), and showed a good sensitivity (100%) and specificity (80%). In the prediction of the presence of infective complications after operation, IFABP, AGI grade, and PCT was found to be valuable (P < .05), and the serum IFABP levels manifested a better predictive value (AUC = 0.961) than PCT (AUC = 0.833) with a better sensitivity, but its specificity (75%) is inferior to that of PCT (89%). As the same, in the prediction of ICU stay beyond 4 days, together with the SOFA score and the AGI grade, the serum IFABP levels had a great predictive value (P < .05), and the AUC of the serum IFABP levels was 0.853 with a good sensitivity (100%), although its specificity (63%) is less than that of the SOFA score (70%). The accuracies of the serum IFABP levels, with the optimal cut-off values, derived from the ROC curves, in the prediction of the occurrence of MODS, infective complications and ICU stay beyond 4 days are shown in Table 3 in details. On account of the limited number of dead cases (n = 2), the prediction for hospital death was not done.
Table 3.
Values of APACHE II score, SOFA score, AGI grade, IFABP, and PCT in prediction of the development of MODS, infective complications and ICU stay beyond 4 days.
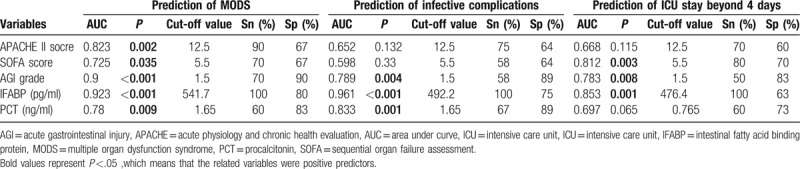
3.4. Comparison with some common markers of severity
As presented in Figure 3, the serum IFABP levels were positively correlated with the common severity scoring systems (P < .05), such as the APACHE II score (R2 = 0.116), SOFA score (R2 = 0.125), and AGI grade (R2 = 0.584). Moreover, the serum IFABP levels were also well correlated with PCT (P = .002, R2 = 0.231), a widely-used infection prognostic marker. As shown in Table 3, according to the AUCs of each parameter, the serum IFABP levels seemed to have a better predictive power for prognosis than that of others.
Figure 3.
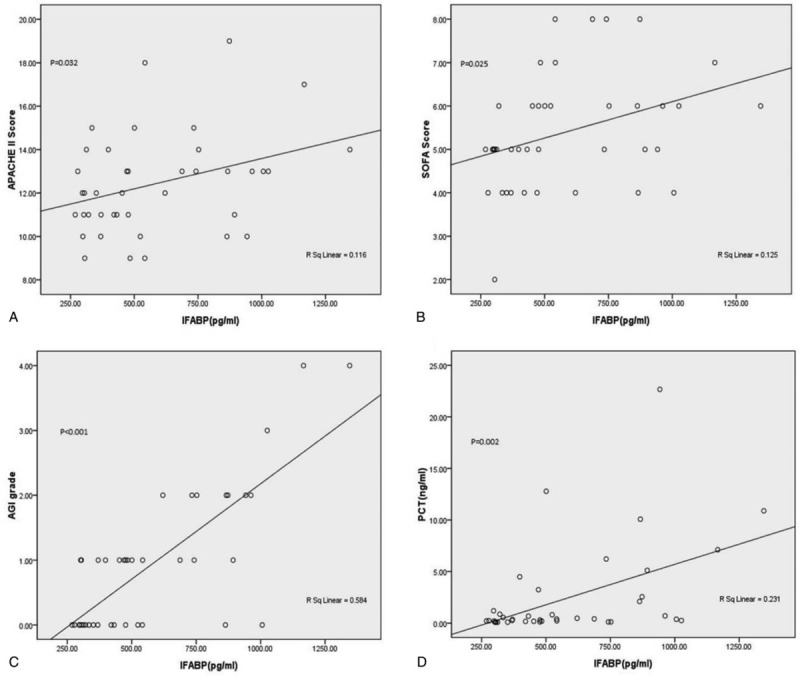
Correlation of serum IFABP level with APACHE II score, SOFA score, AGI grade and serum PCT level. (A) Positive correlation of serum IFABP level with APACHE II score; (B) positive correlation of serum IFABP level with SOFA score; (C) positive correlation of serum IFABP level with AGI grade; (D) positive correlation of serum IFABP level with serum PCT level. APACHE = acute physiology and chronic health evaluation, IFABP = intestinal fatty acid binding protein, PCT = procalcitonin, SOFA = sequential organ failure assessment.
4. Discussion
As many risk factors exist, such as primary cardiac diseases, the use of vasopressors, ischemia-reperfusion injury during CPB, and surgical stress, postoperative cardiac surgery patients usually patients manifested as abnormal appetite, nausea and vomiting, abdominal distension, bowel sound attenuation, which means gastrointestinal dysfunction.[6,7] Also gastrointestinal dysfunction nowadays is considered to be a major risk factor for MODS and gut derived infection in patients with critical illness, and is related to poor prognosis.[9] As is well known, the gastrointestinal function is very complicated, and many researchers tried to establish different evaluating systems to assess it in ICU. The AGI grade, from the Recommendations of the ESICM Working Group on Abdominal Problems,[20] was the classical and well-accepted one. The AGI grade, which includes the abdominal signs and symptoms, intra-abdominal pressure (IAP) and organs function, was considered as an important indicator on assessing gastrointestinal function of ICU patients. Our results revealed that AGI grade beyond 1.5 showed an optimal predictive value for development of outcomes, as evidenced by AUC of 0.9 for the prediction of MODS (P < .001), AUC of 0.789 for the prediction of infective complications (P = .004), AUC of 0.783 for the prediction of ICU stay beyond 4 days (P = .008). However, the main limitation of AGI grade was its complexity and subjectivity. Therefore, a valuable, convenient, and objective gastrointestinal function predictor is looked forward to.
With the further understanding of the pathophysiological basis of gastrointestinal dysfunction during perioperative period of cardiac disease, it is found to result from the gastrointestinal ischemia, anoxia and oxidative stress, which would lead to the enterocyte injury.[6,7] IFABP, a novel and objective biomarker of enterocyte injury, has been reported to be able to use for predicting outcomes of critical illness, such as acute decompensated heart failure,[3] cardiac arrest,[11] septic shock,[13] acute pancreatitis.[14] However, an association between IFABP and postoperative cardiac surgery patients has not been established. In this study, we found that serum IFABP level was well correlated with the AGI grade (P < .001), and significantly higher in patients with inotropic score more than 10 (P = .004), or patients who suffered from right ventricular dysfunction (P = .016). It may attribute to gastrointestinal ischemia or congestion. And it was also showed that serum IFABP level was well correlated with the common severity scores, like the APACHE II score and SOFA score (P < .05).
For postoperative cardiac surgery patients, MODS and postoperative infective complications were the 2 main causes of poor prognosis, which would lead to longer ICU stay. ICU stay beyond 4 days is an important quality indicator of ICU treatment in China, because longer ICU stay means more expense. Therefore, the predictive value of the IFABP level for these 3 phenomena is of specific interest, as the use of this parameter may enable to screen individual patients for prompt aggressive intensive care measures, which may help to shorten ICU stay. In this study, the results of ROC curve analysis show that serum IFABP level could be a better predictor of prognosis than the APACHE II score, SOFA score or PCT, as evidenced by greater AUCs for the prediction of MODS, infective complications and ICU stay beyond 4 days. Moreover, the measurement of serum IFABP is easy, objective, and inexpensive, and even able to become a bed-side testing.
The APACHE II score, including 14 parameters, was considered as a typical and widely used indicator on assessing disease severity and outcomes of critically ill patients.[21] Our results revealed that APACHE II score beyond 12.5 showed an optimal predictive value for development of MODS, as evidenced by AUC of 0.823 (P = .002), but no predictive values for infective complications (P = .132) or ICU stay beyond 4 days (P = .115). Similarly, the SOFA score, a common scoring system of evaluating organs function and outcome,[22] is also found to be able to predict the development of MODS (P = .035) in our study. As organ dysfunction is a main factor of longer ICU stay, therefore, we found that the SOFA score shows a great predicting ability for ICU stay beyond 4 days, as evidenced by AUC of 0.812 (P = .003). PCT has been demonstrated its usefulness in the diagnosis of infections and directing antibiotics therapy, and is currently one of the most investigated biomarkers for infection and has already been integrated in treatment algorithms for ICU patients.[23] Our results showed that PCT manifested being valuable prediction for infective patients after cardiac surgery (P = .001), with the optimal cut-off values of 1.65 ng/ml, so was for MODS (P = .009). Maybe it is just because infection is an important factor for organ dysfunction.
Several limitations of this study need to be discussed. Despite the clear evidence that serum IFABP level was a valuable and objective early predictor of prognosis in postoperative cardiac surgery patients, the one parameter alone maybe just provided a reference, and more often it was better to combine with other markers or evaluation systems to make assessment. Moreover, on account of the relatively small sample size and single-center design of this study, a nonparametric test had to be chosen, which resulted in lower statistical power and some uncertainty to our conclusion. The accuracy would be verified by further large sample and multicenter research. Finally, as some patients may present MODS or infective complications in many days after ICU admission, the serum IFABP level values should be used flexibly, using a combination of IFABP value at ICU admission and consecutive values in clinical practice.
5. Conclusion
In conclusion, the results of this study show that the serum IFABP level at ICU admission is a valuable, convenient, and objective early predictor of prognosis in postoperative cardiac surgery patients.
Author contributions
Conceptualization: Cui Zhang, Xinwei Mu.
Data curation: Liang Hong.
Formal analysis: Jiakui Sun, Xinwei Mu.
Funding acquisition: Lei Zou.
Investigation: Lei Zou, Liang Hong.
Methodology: Lei Zou.
Project administration: Xiaochun Song.
Resources: Xiaochun Song.
Software: Liang Hong.
Supervision: Cui Zhang, Xinwei Mu.
Validation: Xiaochun Song, Xiao Shen, Jiakui Sun.
Visualization: Xiao Shen.
Writing – original draft: Lei Zou.
Writing – review & editing: Xiao Shen.
Footnotes
Abbreviations: AGI = acute gastrointestinal injury, APACHE II = acute physiology and chronic health evaluation II, AUC = area under curve, BMI = body mass index, BUN = blood urea nitrogen, CABG = coronary artery bypass graft, CO = cardiac output, CPB = cardiopulmonary bypass, CVP = central venous pressure, ELISA = enzyme-linked immunosorbent assays, IAP = intra-abdominal pressure, ICU = intensive care unit, IFABP = intestinal fatty acid-binding protein, IQR = interquartile range, LVEF= left ventricular ejection fraction, MAP = mean arterial pressure, MODS = multiple organ dysfunction syndrome, NT-proBNP = N-terminal pro-B-type natriuretic peptide, PCT = procalcitonin, ROC = receiver operating characteristic, SOFA = sequential organ failure assessment, WBC = white blood cell count.
Funding: The project of this thesis was supported by the Foundation for Development of Science and Technology from Nanjing Medical University, Nanjing, Jiangsu Province, China (Grant No. 2015NJMU057).
The authors have no conflicts of interest to disclose.
References
- [1].Thind GS, Loehrke M, Wilt JL. Acute cardiorenal syndrome: mechanisms and clinical implications. Cleve Clin J Med 2018;85:231–9. [DOI] [PubMed] [Google Scholar]
- [2].Tang WH. We are not alone: understanding the contributions of intestinal microbial communities and the congested gut in heart failure. JACC Heart Fail 2016;4:228–9. [DOI] [PubMed] [Google Scholar]
- [3].Kitai T, Kim YH, Kiefer K, et al. Circulating intestinal fatty acid-binding protein (I-FABP) levels in acute decompensated heart failure. Clin Biochem 2017;50:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luedde M, Winkler T, Heinsen FA, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail 2017;4:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valentova M, von Haehling S, Bauditz J, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J 2016;37:1684–91. [DOI] [PubMed] [Google Scholar]
- [6].Habes QLM, Linssen V, Nooijen S, et al. Markers of intestinal damage and their relation to cytokine levels in cardiac surgery patients. Shock 2017;47:709–14. [DOI] [PubMed] [Google Scholar]
- [7].Warwick R, Mediratta N, Chalmers J, et al. Virchow's triad and intestinal ischemia post cardiac surgery. Asian Cardiovasc Thorac Ann 2014;22:927–34. [DOI] [PubMed] [Google Scholar]
- [8].Wilmore DW, Smith RJ, O’Dwyer ST, et al. The gut: a central organ after surgical stress. Surgery 1988;104:917–23. [PubMed] [Google Scholar]
- [9].Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med 2014;20:214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vincent JL, Pelosi P, Pearse R, et al. Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit Care 2015;19:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grimaldi D, Guivarch E, Neveux N, et al. Markers of intestinal injury are associated with endotoxemia in successfully resuscitated patients. Resuscitation 2013;84:60–5. [DOI] [PubMed] [Google Scholar]
- [12].Matsumoto S, Sekine K, Funaoka H, et al. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg 2014;101:232–8. [DOI] [PubMed] [Google Scholar]
- [13].Sekino M, Funaoka H, Sato S, et al. Intestinal fatty acid-binding protein level as a predictor of 28-day mortality and bowel ischemia in patients with septic shock: a preliminary study. J Crit Care 2017;42:92–100. [DOI] [PubMed] [Google Scholar]
- [14].Goswami P, Sonika U, Moka P, et al. Intestinal fatty acid binding protein and citrulline as markers of gut injury and prognosis in patients with acute pancreatitis. Pancreas 2017;46:1275–80. [DOI] [PubMed] [Google Scholar]
- [15].Wiercinska-Drapalo A, Jaroszewicz J, Siwak E, et al. Intestinal fatty acid binding protein (I-FABP) as a possible biomarker of ileitis in patients with ulcerative colitis. Regul Pept 2008;147:25–8. [DOI] [PubMed] [Google Scholar]
- [16].Sousa-Uva M, Head SJ, Milojevic M, et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:5–33. [DOI] [PubMed] [Google Scholar]
- [17].Carl M, Alms A, Braun J, et al. S3 guidelines for intensive care in cardiac surgery patients: hemodynamic monitoring and cardiocirculary system. Ger Med Sci 2010;8:Doc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA 2009;301:2445–52. [DOI] [PubMed] [Google Scholar]
- [19].Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 2009;37:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med 2012;38:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meyer AA, Messick WJ, Young P, et al. Prospective comparison of clinical judgment and APACHE II score in predicting the outcome in critically ill surgical patients. J Trauma 1992;32:747–53. discussion 753–744. [DOI] [PubMed] [Google Scholar]
- [22].Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754–8. [DOI] [PubMed] [Google Scholar]
- [23].Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011;39:2048–58. [DOI] [PubMed] [Google Scholar]


