Abstract
Background:
An assist-as-needed robot-assisted gait training protocol was recently developed. It allows active movement during training, but its exact criteria remain unknown. Asymmetric step length is a common abnormal gait pattern in hemiplegic stroke patients. We compared the effects of assist-as-needed robot-assisted gait training on the unaffected and affected limbs of hemiplegic stroke patients.
Method:
Twenty-four chronic stroke patients with asymmetric step lengths were randomly assigned to 1 of 2 groups. Twelve completed the study protocol. Group 1 underwent 20 sessions of assist-as-needed robot-assisted gait training for the unaffected limb and fully-assisted robot-assisted training for the affected limb. Group 2 underwent 20 sessions of robot-assisted gait training using the opposite protocol. Clinical measurements were obtained and 3-dimensional gait analyses were performed at baseline and after 10 and 20 training sessions.
Results:
Clinical measurements improved in both groups after 20 training sessions. The unaffected limb's step length asymmetry ratio and hip maximal extension moment significantly improved in group 1. The affected limb's maximal dorsiflexion angle for the ankle in the swing phase significantly improved in group 2.
Conclusion:
Application of the assist-as-needed training mode for the unaffected limb helped improve step length asymmetry in chronic stroke patients.
Keywords: assist-as-needed, chronic, gait, rehabilitation, robotics, stroke, symmetry
1. Introduction
Improving gait function in stroke patients is one of the most important goals of rehabilitation therapy. While 60% to 80% of stroke patients are able to ambulate independently, many exhibit a hemiplegic gait, which limits function.[1,2] Several studies have revealed that the unilateral weakness occurring after stroke is likely to result in gait asymmetry.[3,4] Recovery of gait symmetry is important, particularly after a stroke.[4]
An asymmetric gait pattern after stroke mainly depends on muscle strength and the weight-supporting capacity of the affected limb. Hemiplegic patients usually have reduced joint excursion and insufficient forward propulsion, which may lead to an asymmetrical and unstable walking pattern.[5] Since the single support time of the affected limb is significantly shorter than that of the unaffected limb, the unaffected limb's step length is shorter than the affected limb's step length.[4,6] To treat the shorter step length in the unaffected limb, increasing the stability of the affected limb in the stance phase with forward propulsion is needed.
Robot-assisted gait training (RAGT), which repeatedly produces gait motion, is commonly used to improve gait function.[7,8] The guidance of the robot's exoskeleton applied to gait training can be adjusted to the purpose of the treatment. There is a fully assisted mode of RAGT (FA) in which the exoskeleton robotic leg continuously guides the subject to walk with a prescribed gait pattern.[9] This mode has the advantage of repeating ideal movements, but it does not allow the patient to move voluntarily. On the other hand, the assist-as-needed mode of RAGT (AAN) provides assistance while allowing active subject participation. AAN can be used for hemiplegic patients with asymmetric gait patterns in order to facilitate forward propulsion by increasing the affected limb's stability using the exoskeletal robotic leg.
The clinical application of AAN has been rarely reported. Some previous studies have shown that AAN can improve walking function, as measured by walking speed,[5,9,10] functional gait assessment,[9] and kinematic measurements.[5] However, these studies were limited to clinical and/or kinematic measurements, and most recruited a heterogenous subject population to compare FA and AAN. Moreover, there has been no previous study with chronic stroke patients with specific abnormal gait patterns. Therefore, our aim was to investigate the effects of applying AAN to the unaffected or affected limbs of chronic stroke patients with asymmetric step lengths with respect to temporospatial, kinematic, and kinetic gait parameters.
2. Methods
2.1. Subjects and study design
This study was designed as a prospective, single-blind, randomized, and controlled pilot study. Subjects were recruited from an inpatient rehabilitation center at Veterans Health Service Medical Center between October 2015 and April 2017. The inclusion criteria were as follows: patients with first-ever unilateral stroke diagnosed by computed tomography or magnetic resonance imaging, stroke onset at least 6 months previous to study enrollment, sufficient cognition to follow simple instructions and understand both the content and purpose of the study (Korean Mini-Mental State Examination score > 23), the ability to walk at least 10 m independently, and asymmetrical gait with a step length asymmetric ratio > 1.1 (step length asymmetric ratio = affected limb step length/unaffected limb step length).[3] The exclusion criteria were as follows: quadriplegia or double hemiplegia, significant limitation of range of motion of a lower limb joint or severe spasticity of the lower limbs that disallowed RAGT, coexisting neurological and/or orthopedic disease that could influence gait function. Subjects were randomly assigned to group 1 or group 2 through a stratified randomization approach. All subjects in each group underwent 20 sessions of RAGT (2 times per week, 45 minutes per session). Group 1 performed RAGT with AAN applied to the unaffected limb and FA applied to the affected limb. Group 2 performed RAGT with AAN applied to the affected limb and FA applied to the unaffected limb. Clinical measurements were obtained and three-dimensional gait analyses were performed before training (T0), after 10 sessions (T1), and after 20 sessions (T2) of RAGT. All subjects were informed of the purpose and procedure of the study before signing an informed consent form for participation. The institutional review board of our hospital approved the procedures and protocols of this study (approval no. 2015-09-006).
2.2. Robot-assisted gait training
All subjects wore a suspension vest and harness connected to a counterweight system to provide body-weight support and walked on a treadmill with the help of robotic-driven gait orthosis (Walkbot, P&S Mechanics, Seoul, South Korea). The devices were placed on the patient, and then the patient's hip, knee, and ankle joint axes were consistently positioned with the exoskeletal orthosis to adjust joint movements at individualized gait speeds. The torque of the hip, knee, and ankle drives could be set from 100% to 0% for either or both legs. A torque of 100% in the joints signified that the robotic legs guided the subject's legs to move along a predetermined gait trajectory. The predetermined gait trajectory was based on gait patterns programmed into the robot, and these patterns were based on the kinematics exhibited by healthy subjects. The robot was least compliant at 100% of torque, and it only deviated minimally from its reference trajectory even if the patient attempted to deviate from the programmed path. However, the torque algorithms were designed so that at low torque percentages, the robot enforced movement of the subjects and applied AAN torque at all joints. When the subject underwent RAGT, the percentage of torque exerted by the robot was gradually reduced during the training sessions (from 90% assistance at the beginning of training to 60% assistance at the end of training) in order to facilitate active participation. As the subject's function improved, the treadmill speed was increased to a maximum of 2.2 km/h while body-weight support was reduced.
2.3. Clinical measurements
Clinical measurements were obtained by a rehabilitation physician who was blinded to group assignments. Clinical measurements included the National Institutes of Health stroke scale (NIHSS), Fugl–Meyer Motor Assessment—Lower Extremity (FMLE), Functional Ambulation Category (FAC), Motricity Index of the lower extremity (MI), and the Trunk Control Test (TCT).
2.4. Gait analysis
Gait analysis was conducted with a motion analysis system that consisted of eight infrared 60-Hz cameras (Motion Analysis Corporation, Santa Rosa, CA) and 3 force plates (sampling rate 1200 Hz; Kistler Corp., Amherst, NY). Reflective markers were placed on predefined anatomical landmarks of the pelvis, thigh, knee, shank, and foot.[11] Simultaneous recordings of temporospatial lower extremity kinematics and kinetics were obtained as patients walked 6 m while barefoot at a self-selected speed. Joint kinematics and external moments were calculated by the Cortex program (Motion Analysis Corporation, Santa Rosa, CA). Ground reaction forces were normalized to the subject's body weight, while joint moments were reported as N m/kg. This study used the following variables in the gait analysis: temporospatial domain-gait speed (cm/s), cadence (steps/min), step length asymmetric ratio, stride length (cm), percentage of double support and single support time in one gait cycle, kinematic domain-hip/knee maximal extension angle in the stance phase, ankle maximal dorsiflexion angle in the stance phase, hip/knee/ankle maximal flexion angle in the swing phase, and kinetic domain-hip/knee/ankle maximal extension moment (N m/kg) and hip/knee/ankle maximal power generation (W/kg).
2.5. Statistical analysis
The baseline characteristics (age, stroke type, duration after stroke, step length asymmetry ratio, NIHSS, FMLE, FAC, MI, and TCT) of the 2 groups were compared to assess the quality of randomization using the Mann–Whitney U-test or Fisher's exact test. Within-group differences after 10 and 20 sessions of RAGT were evaluated with a Wilcoxon signed-rank test. We analyzed group differences (changes in each score after 10 and 20 RAGT sessions) with a Mann–Whitney U-test. Statistical analysis was performed with SPSS 20.0 (IBM Corp., Armonk, NY). The level of significance was set at P < .05.
3. Results
3.1. Subject recruitment
Twenty-four subjects were enrolled in this study. Twelve subjects completed the AAN protocol, and the remaining 12 dropped out (Fig. 1). Table 1 shows the demographic characteristics of patients who completed the RAGT protocol. There were no statistically significant differences between the groups before treatment (T0). A comparable number of subjects dropped out from each group.
Figure 1.

CONSORT flow diagram.
Table 1.
General characteristics.

3.2. Clinical measures
In group 1, the FMLE, FAC, and MI scores significantly improved at T2 compared to T0. However, only the FMLE score improved significantly at T2 compared to T0 in group 2 (Table 2). There were no significant differences in the between-groups analysis.
Table 2.
Changes in clinical measurements.

3.3. Temporospatial variables
The step length asymmetric ratio in group 1 significantly improved at T1 compared to T0, and the improvements were maintained at T2 (P < .05). There was no significant improvement in temporospatial variables in group 2 throughout the sessions. There were no significant differences in the between-groups analysis (Table 3).
Table 3.
Changes in temporospatial parameters.

3.4. Kinematic variables
There were no significant differences in the within-groups analysis. However, the maximal ankle dorsiflexion angle of the affected limb was significantly improved at T2 compared to T0 (Table 4). In the kinematic curve graph, the angular velocity of hip/knee flexion of the unaffected limb in the swing phase and ankle plantar flexion in the terminal stance were increased and became closer to those of the normal curve in group 1 (Fig. 2, arrowhead). The angular velocity of ankle plantar flexion of the affected limb in the terminal stance increased and became closer to that of the normal curve in group 2 (Fig. 2, arrow).
Table 4.
Changes in kinematic parameters.
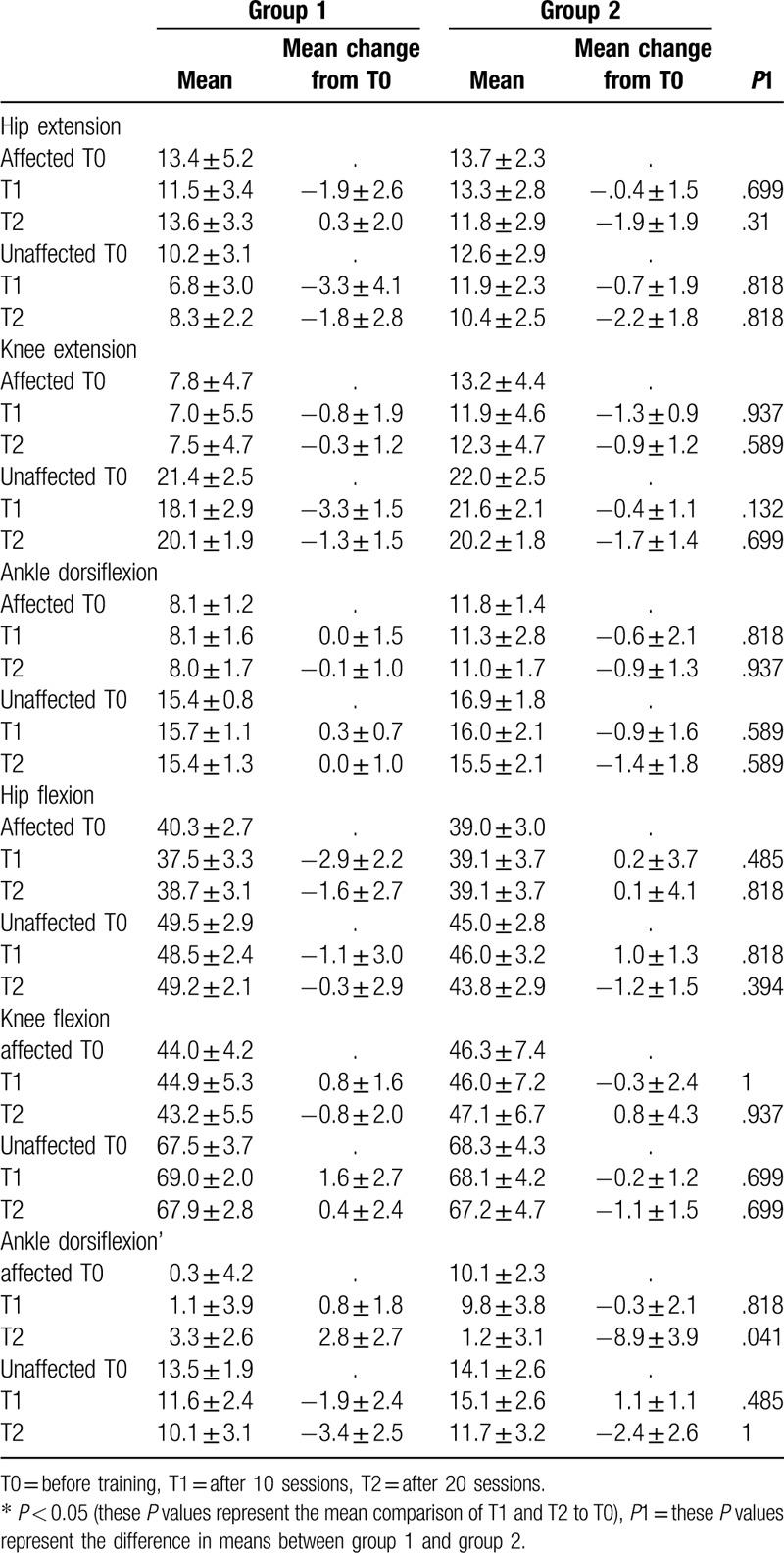
Figure 2.
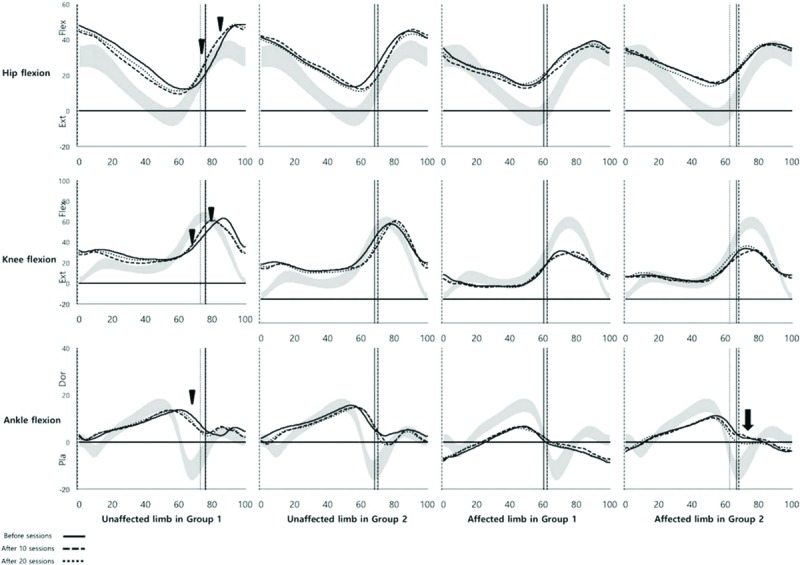
Changes in kinematic variables at baseline, after 10 sessions, and after 20 sessions of training.
3.5. Kinetic variables
In group 1, the hip maximal extension moment of the unaffected limb improved significantly at T1 compared to T0, and the improvements were maintained at T2 (P < .05). There were no significant differences in the between-groups analysis (Table 5).
Table 5.
Changes in kinetic parameters.

4. Discussion
This study was the first to compare the effects of AAN applied to the unaffected limb and to the affected limb in chronic stroke patients. There were significant improvements in the step length asymmetry ratio, hip maximal extension moment of the unaffected limb, and clinical measures after training. The angular velocity of hip and knee flexion of the unaffected limb in the swing phase showed an increasing trend after training.
The application of a specific RAGT protocol to chronic stroke patients should be considered with a clear purpose. Previous studies have shown that robot-assisted gait training was effective in the subacute phase but not in the chronic phase of stroke.[12,13] One reason for this difference may be the heterogeneous study populations recruited by studies with chronic patients. However, a more important reason may be that the same RAGT protocol was applied to subacute stroke patients without considering the specific gait patterns of individuals in the chronic phase. The gait pattern after stroke changes completely during the subacute phase, but most functional recovery, including in gait patterns, takes place within 6 months after stroke onset.[14,15] After that, the gait pattern and function in chronic stroke patients does not change easily, and repeated training is needed to focus on specific gait problems. We focused on the asymmetric step length after stroke, and the treatment protocol involved AAN application on the unaffected or affected limb.
Applying AAN to the unaffected limb led to improvements in step length asymmetry and the maximal hip extension moment of the unaffected limb. Compared to neurologically intact individuals, hemiplegic patients usually have reduced step lengths in the unaffected limb.[3,4,6] Therefore, the improvement of asymmetry observed in this study suggests that AAN may increase the step length of the unaffected limb even if there is reduced hip extension moment in the terminal stance phase. The body weight vector is significantly anterior to the hip joint center with the hip flexed at the initial contact,[16] and the maximal hip extension moment results from the impact of abrupt decrease in body weight. The decrease in the maximal hip extension moment of the unaffected limb may be related to the increase in the weight-supporting capacity of the affected limb, resulting in the weight shifting gradually to the unaffected limb. The FA mode of the exoskeletal robotic leg for the affected limb increases stability during the stance phase, and allowing subjects to voluntarily move their unaffected limb may accelerate hip/knee flexion angular velocity during the swing phase (Fig. 2, arrowhead). These mechanisms may finally result in increased step length and decreased hip moment.
The angle of ankle dorsiflexion in the swing phase of the affected limb improved after AAN applied to the affected limb. The baseline angle of ankle dorsiflexion was approximately 10° in group 2, but the final angle was approximately 1° (Table 4, Fig. 2, arrow). During the swing phase, the normal angle of ankle dorsiflexion is close to 0° for foot clearance of the floor, but the excess angle dorsiflexion does not allow it to reach ideal kinematic movement.[6,17] Voluntary movement with guidance from the exoskeletal robotic leg in the affected limb may induce normalization of ankle kinematic movement during the swing phase.
Further investigation is needed to determine the underlying mechanism of AAN for functional gait recovery and who may benefit from it. Rehabilitation therapy for central nervous system injuries has been emphasized in task-specific training, which allows the patient to repeatedly perform a motion that is as close as possible to the final target motion.[18] RAGT is also based on the repetitive practice of a specific functional task.[17,18] From a motor-learning perspective, AAN may be better than FA at improving the generation of gait. In FA training, task performance was poor when constant guidance was no longer provided.[19,20] However, AAN training may be more likely to encourage independent movement, and task performance was maintained when the robotic-leg was removed.[20] We hypothesized that AAN would promote walking-pattern habituation and strengthening of the legs through repetitive voluntary stepping. After the successful application of AAN, subjects can perform sufficient voluntary leg movements. Subjects with limited voluntary movement in the legs may benefit from FA training.
The drop-out rate was relatively high in this study. The subjects dropped out because AAN was difficult and could not be performed until the end of the 20 sessions. The mean age of the subjects in this study was relatively high (in the mid-60s), and the age of the subjects may have increased the difficulty of completing the protocol. In future studies, it may be necessary to revise the protocol, which started with 90% assistance and gradually decreased the assistance to 60%.
This pilot study had some limitations. First, our study included a small, heterogeneous sample size. Half of the study population dropped out during the application of AAN. Second, we mainly focused on changes in step length asymmetry, so our results are difficult to apply to general treatment protocols for abnormal gait patterns in chronic stroke patients. Despite these limitations, this is the first study to compare the effects of AAN applied to the unaffected/affected limb and to provide insight into the investigation of treatment criteria for RAGT.
Author contributions
Conceptualization: Dae Hyun Kim.
Data curation: Jin Seok Seo, Hee Seung Yang, Suk Jung, Chang Soon Kang, Sunghun Jang.
Formal analysis: Dae Hyun Kim.
Methodology: Jin Seok Seo, Suk Jung.
Writing – original draft: Jin Seok Seo.
Writing – review & editing: Dae Hyun Kim.
Footnotes
Abbreviations: AAN = assist-as-needed mode, ankle dorsiflexion = maximal ankle dorsiflexion in stance phase, ankle dorsiflexion’ = maximal ankle dorsiflexion in swing phase, ankle moment = maximal ankle moment, ankle power = maximal ankle power, FA = fully assisted mode, FAC = functional ambulation category, FMLE = Fugl–Meyer motor assessment of the lower extremity, hip extension = maximal hip extension in stance phase, hip flexion = maximal hip flexion in swing phase, hip moment = maximal hip moment, hip power = maximal hip power, knee extension = maximal knee extension in stance phase, knee flexion = maximal knee flexion in swing phase, knee moment = maximal knee moment, knee power = maximal knee power, MI = motricity index of the lower extremity, NIHSS = NIH stroke scale, RAGT = robot-assisted gait training, T0 = before training, T1 = after 10 sessions, T2 = after 20 sessions, TCT = trunk control tests.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B1003102).
The authors have no conflicts of interest to disclose.
References
- [1].Alexander LD, Black SE, Patterson KK, et al. Association between gait asymmetry and brain lesion location in stroke patients. Stroke 2009;40:537–44. [DOI] [PubMed] [Google Scholar]
- [2].Han EY, Im SH, Kim BR, et al. Robot-assisted gait training improves brachial-ankle pulse wave velocity and peak aerobic capacity in subacute stroke patients with totally dependent ambulation: randomized controlled trial. Medicine (Baltimore) 2016;95:e5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Patterson KK, Gage WH, Brooks D, et al. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture 2010;31:241–6. [DOI] [PubMed] [Google Scholar]
- [4].Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil 2008;89:304–10. [DOI] [PubMed] [Google Scholar]
- [5].Srivastava S, Kao PC, Kim SH, et al. Assist-as-needed robot-aided gait training improves walking function in individuals following stroke. IEEE Trans Neural Syst Rehabil Eng 2015;23:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture 1996;4:136–48. [Google Scholar]
- [7].Hornby TG, Campbell DD, Kahn JH, et al. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 2008;39:1786–92. [DOI] [PubMed] [Google Scholar]
- [8].Mayr A, Kofler M, Quirbach E, et al. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabil Neural Repair 2007;21:307–14. [DOI] [PubMed] [Google Scholar]
- [9].Srivastava S, Kao PC, Reisman DS, et al. Robotic assist-as-needed as an alternative to therapist-assisted gait rehabilitation. Int J Phys Med Rehabil 2016;4:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krishnan C, Kotsapouikis D, Dhaher YY, et al. Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor. Arch Phys Med Rehabil 2013;94:1202–6. [DOI] [PubMed] [Google Scholar]
- [11].Schache AG, Baker R, Vaughan CL. Differences in lower limb transverse plane joint moments during gait when expressed in two alternative reference frames. J Biomech 2007;40:9–19. [DOI] [PubMed] [Google Scholar]
- [12].Mehrholz J, Elsner B, Werner C, et al. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev 2013;7:Cd006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morone G, Bragoni M, Iosa M, et al. Who may benefit from robotic-assisted gait training? A randomized clinical trial in patients with subacute stroke. Neurorehabil Neural Repair 2011;25:636–44. [DOI] [PubMed] [Google Scholar]
- [14].Mulroy S, Gronley J, Weiss W, et al. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture 2003;18:114–25. [DOI] [PubMed] [Google Scholar]
- [15].Jorgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil 1995;76:406–12. [DOI] [PubMed] [Google Scholar]
- [16].Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: SLACK; 2010. [Google Scholar]
- [17].Yang HE, Kyeong S, Lee SH, et al. Structural and functional improvements due to robot-assisted gait training in the stroke-injured brain. Neurosci Lett 2017;637:114–9. [DOI] [PubMed] [Google Scholar]
- [18].French B, Thomas LH, Leathley MJ, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2007. Cd006073. [DOI] [PubMed] [Google Scholar]
- [19].Schmidt RA, Lee TD. Motor learning and performance from principles to application. fifth edition.Champaign: Human Kinetics, cop; 2014. [Google Scholar]
- [20].Lee C, Won D, Cantoria MJ, et al. Robotic assistance that encourages the generation of stepping rather than fully assisting movements is best for learning to step in spinally contused rats. J Neurophysiol 2011;105:2764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


