Abstract
Rationale:
Only 4.5% of brown tumors involve facial bones; of these, solitary bone involvement is usual. Brown tumors of multiple facial bones are extremely rare. Here, we report the case of a brown tumor of multiple facial bones initially misdiagnosed as an odontogenic cyst.
Patient concerns:
A pregnant 26-year-old woman was referred to our hospital with painful swelling of multiple facial bones, anemia, urinary calculi, marasmus, and a history of multiple bone fractures. Laboratory examination revealed an elevated serum calcium level of 3.09 mmol/L (normal range: 2.0–2.8 mmol/L) and a low phosphorus level of 0.62 mmol/L (normal range: 0.81–1.65 mmol/L). The serum alkaline phosphatase concentration was 397 IU/L (normal range: 24–82 IU/L) and parathyroid hormone level was 267 pg/mL (normal range: 14–72 pg/mL). Cone beam computed tomography revealed multiple ossifying fibromas of the maxilla and mandible. Incisional biopsy revealed abundant spindle cells with areas of hemorrhage and haphazardly arranged diffuse multinucleated giant cells.
Diagnoses:
The patient was diagnosed with primary hyperparathyroidism (HPT).
Interventions:
She was treated by parathyroidectomy.
Outcomes:
The multiple osteitis fibrosa cystica gradually resolved as bone re-mineralized. The patient has been followed up for 2 years without evidence of tumor recurrence.
Lessons:
As multiple osteolytic lesions of facial bones can be caused by primary HPT, serum calcium and parathyroid hormone assays should be performed routinely when investigating these lesions.
Keywords: brown tumor, facial bone, primary hyperparathyroidism
1. Introduction
Brown tumors are rare giant-cell lesions which result from abnormally high parathyroid hormone levels (hyperparathyroidism [HPT]). All forms of HPT (primary, secondary, and tertiary) are associated with hyperparathyroid bone disease, which manifests as slowly enlarging painful masses or fractures. Primary HPT is characterized by abnormal parathyroid hormone secretion and has an annual worldwide incidence of approximately 5 in 10, 000 people, most of whom are women aged 60 years or older.[1] Co-occurrence of HPT and brown tumors is now rare due to routine screening for serum calcium in recent years.[2] Nearly 85% of cases are caused by benign parathyroid tumors, and a fewer than 1% by parathyroid malignancies.[3]
The common brown tumor sites are the long bones, such as the ribs, clavicles, tibia, and pelvic girdle. Although tumors of the head and neck are rare, when they occur, the mandible is usually involved.[4] Concurrent involvement of multiple facial bones is exceptional. Here, we present the case of 26-year-old woman with painful swelling of multiple facial bones caused by a brown tumor. Despite investigations, including biopsy, the patient was initially not diagnosed with HPT. Therefore, in order to make an accurate diagnosis, HPT should be considered when multiple cystic lesions are located in the facial bones.
2. Case report
A 26-year-old woman was referred to our hospital with painful swelling of multiple facial bones and limited mouth opening. The patient was 5-months pregnant. She reported that, 3 months before seeking medical attention, she noticed swelling of the left mandible that continued to increase in size and caused constant pain, occasional hemorrhages, and malocclusion. She had a history of rib fracture 5 years previously and pelvic bone fracture 7 years previously. A painless mass in the arterial neck region around the thyroid-cartilage level was noted 2 years previously. Her family history was not significant.
Intraoral examination revealed 1.5 cm × 1.5 cm and 4 cm × 2 cm painless sessile swellings on the posterior region of the left maxilla and the retromolar area of the left mandible, respectively. These masses had expanded buccolingually and the oral mucosa was ulcerated and purplish-red; 37, 38 were mobile. There was no lymphadenopathy in the submandibular region on palpation, but a 1.5 cm × 1 cm node was found at the level of the thyroid cartilage.
Laboratory tests revealed an elevated serum calcium level of 3.09 mmol/L (normal range: 2.0–2.8 mmol/L) and a low phosphorus level of 0.62 mmol/L (normal range: 0.81–1.65 mmol/L). The serum alkaline phosphatase concentration was 397 IU/L (normal range: 24–82 IU/L). Subsequent examination revealed a parathyroid hormone (PTH) level of 267 pg/mL (normal range: 14–72 pg/mL). Plain radiographs of the chest showed multiple osteolytic lesions in the right sixth to tenth ribs and a 5.9 cm × 4 cm oval calcified nodule in the right upper abdomen (Fig. 1).
Figure 1.

Chest x-ray demonstrated multiple osteolytic lesions in the right ribs (star) and calcified nodule in the right upper abdominal region (arrow).
Cone beam computed tomography (CBCT) of the skull revealed a 1.8 cm × 1.7 cm × 1.4 cm expansile lucent lesion on the left maxilla. This destructive osteolytic lesion had expanded into the buccolingual bone. The cortex of the mandible was thin bilaterally and the lesion extended from the molar alveolar process into the ramus (Fig. 2).
Figure 2.

Cone beam computer tomography scan of maxillofacial bone. A, Axial section showing an osteolytic lesion of the left maxillary (arrow). B, Axial section showing an osteolytic lesion with bucco-lingually bone expanding of the right mandible (arrow). C, Axial section showing an osteolytic lesion with bucco-lingually bone expanding of the left mandible (arrow). D, Coronal section showing bucco-lingually bone expanding of the mandible and tooth displacement (arrow).
An excisional biopsy was performed on the left mandible. Pathological analysis revealed abundant spindle cells with areas of hemorrhage and haphazardly arranged diffuse multinucleated giant cells (Fig. 3).
Figure 3.
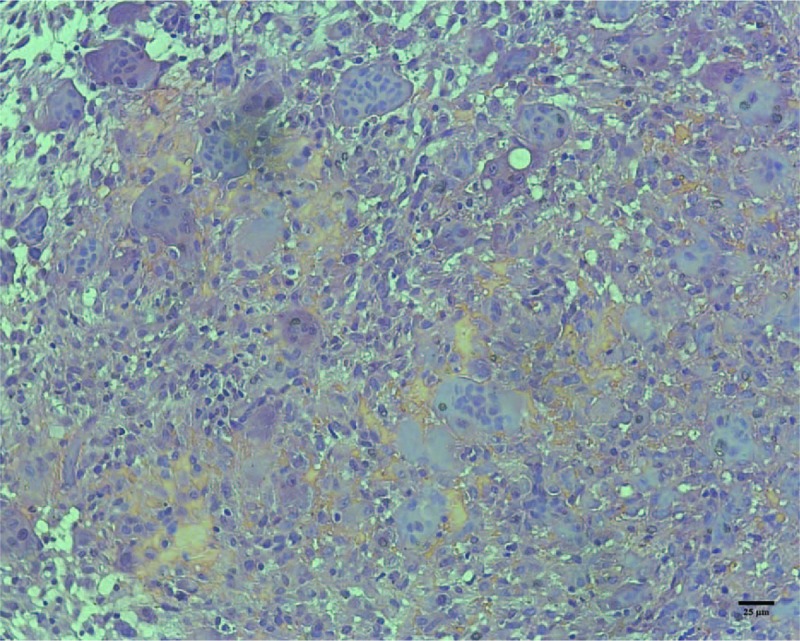
Brown tumor with multinucleated giant cells, and deposits of hemosiderin (hematoxylin and eosin stain).
The patient was referred to the endocrine department of a general hospital for further treatment and a parathyroidectomy was performed. Histopathological examination confirmed a parathyroid adenoma. The patient reported marked absence of pain and serum calcium levels underwent an uneventful recovery. Noticeable recalcification of the osteolytic lesion was noted during follow-up.
3. Discussion
Although brown tumors have been reported extensively, only 4.5% of reported cases have involved the facial bones.[5] Tumors of the maxillofacial region are more likely to affect women than men, with a reported female: male ratio of approximately 1.7:1 and mean age at diagnosis of 34 years.[6] Maxillofacial tumors usually involve solitary bones, with the mandible being the most frequently affected.[7] Tumors may cause loosening and displacement of the teeth, resultant malocclusion, and facial deformity. Patients are often referred to hospital complaining of painful bone swelling or of loose teeth. Associated symptoms, which are caused by elevated serum calcium levels, include anorexia, nausea, vomiting, constipation, fatigue, and urinary calculi.
Our patient presented with a particularly interesting case of brown tumor caused by primary HPT. First, multiple facial bones were involved, which is extremely rare and could be confused with odontogenic cysts or tumors, bone metastasis, or multiple myeloma.[8] The radiological diversity of brown tumors makes accurate diagnosis via imaging challenging. Brown tumors are benign reactive osteolytic lesions rather than true neoplasms. Some brown tumors have sclerotic margins, while others are expansive soft tissue masses which cause cortical destruction. Moreover, the radiological appearance of the lesions may vary according to the stage of HPT. The presence of a sclerotic margin makes it easier to exclude bone metastasis but rather difficult to exclude odontogenic cysts or tumors. However, some tumors which present as soft tissue masses with cortical destruction may mimic malignant tumors.
A fewer than 5% of cases of primary HPT with multiple maxillofacial bone involvement are diagnosed as primary hyperparathyroidism-jaw tumor syndrome (HPT-JT).[9] This syndrome is characterized by the presence of primary HPT, ossifying fibroma of the mandible and maxilla, and, occasionally, renal tumors and uterine fibroids. It is an autosomal dominant disorder associated with mutations of the CDC73 gene. Approximately 80% of HPT-JT patients have primary HPT and the average age at diagnosis is 32 years.[10] Genetic sequencing for CDC73, screening for renal and uterine tumors, and a detailed review of family history would be beneficial for young HPT patients with multiple-facial-bone involvement. Our patient had no family history of primary HPT, so we did not perform genetic tests.
Second, tumor growth was accelerated due to the patient's pregnancy, and many of her clinical symptoms, which included anorexia, nausea, vomiting, constipation, and fatigue, had been incorrectly attributed to her pregnancy at previous hospital visits.
A diagnosis of brown tumor should be based on elevated serum calcium, alkaline phosphatase and parathyroid hormone levels, low serum phosphate levels, and histological features. Computed tomography or bone scans are helpful in making a differential diagnosis and identifying the extent of the lesion. Although fine needle aspiration biopsy has been used to diagnose brown tumors,[11] it poses the risk of uncontrolled local hemorrhage, which unfortunately occurred in our case.
Primary HPT is usually cured by parathyroidectomy. After surgery, excessive parathyroid hormone levels return to normal and osteolytic lesions are gradually re-mineralized without surgery,[12] which was the case with our patient.
4. Conclusion
Multiple osteolytic lesions of facial bones are common in patients attending maxillofacial clinics. Although the incidence of brown tumors resulting from primary HPT is very low, they should be included in a differential diagnosis of odontogenic cysts or tumors, bone metastasis, or multiple myeloma. This report highlights the importance of reviewing the patient's history to guide appropriate investigations and aid diagnosis. Serum calcium and parathyroid hormone assays should be performed routinely for multiple osteolytic lesions. Such an approach will reduce the rate of misdiagnosis of this disease, avoid unnecessary harm to patients, and enable appropriate treatment to be given.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. This case was communicated as a poster on the 23th international conference on oral and maxillofacial surgery.
Author contributions
Conceptualization: Haixiao Zou, Li Song.
Data curation: Haixiao Zou, Li Song.
Formal analysis: Mengqi Jia, Li Wang, Yanfang Sun.
Investigation: Mengqi Jia.
Methodology: Yanfang Sun.
Resources: Li Wang, Yanfang Sun.
Writing – original draft: Haixiao Zou.
Writing – review & editing: Yanfang Sun.
Footnotes
Abbreviations: CBCT = cone beam computer tomography, CT = computer tomography, HPT = hyperparathyroidism, HPT-JT = hyperparathyroidism-jaw tumor syndrome, PTH = parathyroid hormone.
The study had obtained the approval from ethics committee of School and Hospital of Stomatology, Wuhan University.
The authors declare no conflicts of interest.
References
- [1].Bilezikian JP, Silverberg SJ. Clinical practice. Asymptomatic primary hyperparathyroidism. N Engl J Med 2004;350:1746–51. [DOI] [PubMed] [Google Scholar]
- [2].Silverberg SJ, Bilezikian JP. Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab 2003;88:5348–52. [DOI] [PubMed] [Google Scholar]
- [3].Syed H, Khan A. Primary hyperparathyroidism: diagnosis and management in 2017. Pol Arch Intern Med 2017;127:438–41. [DOI] [PubMed] [Google Scholar]
- [4].Artul S, Bowirrat A, Yassin M, et al. Maxillary and frontal bone simultaneously involved in brown tumor due to secondary hyperparathyroidism in a hemodialysis patient. Case Rep Oncol Med 2013;2013:909150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rai S, Rattan V, Bhadada SK. Giant cell lesions associated with primary hyperparathyroidism. J Maxillofac Oral Surg 2015;14:930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Palla B, Burian E, Fliefel R, et al. Systematic review of oral manifestations related to hyperparathyroidism. Clin Oral Investig 2018;22:1–27. [DOI] [PubMed] [Google Scholar]
- [7].Brabyn P, Capote A, Belloti M, et al. Hyperparathyroidism diagnosed due to brown tumors of the jaw: a case report and literature review. J Oral Maxillofac Surg 2017;75:2162–9. [DOI] [PubMed] [Google Scholar]
- [8].Naiman J, Green WR, d’Heurle D, et al. Brown tumor of the orbit associated with primary hyperparathyroidism. Am J Ophthalmol 1980;90:565–71. [DOI] [PubMed] [Google Scholar]
- [9].Carpten JD, Robbins CM, Villablanca A, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 2002;32:676–80. [DOI] [PubMed] [Google Scholar]
- [10].Simonds WF, James-Newton LA, Agarwal SK, et al. Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002;81:1–26. [DOI] [PubMed] [Google Scholar]
- [11].Selvi F, Cakarer S, Tanakol R, et al. Brown tumour of the maxilla and mandible: a rare complication of tertiary hyperparathyroidism. Dentomaxillofac Radiol 2009;38:53–8. [DOI] [PubMed] [Google Scholar]
- [12].Leppla DC, Snyder W, Pak CY. Sequential changes in bone density before and after parathyroidectomy in primary hyperparathyroidism. Invest Radiol 1982;17:604–6. [DOI] [PubMed] [Google Scholar]


