Abstract
Surgical site infections (SSIs) increase the risk of mortality, postsurgery, extend hospital stay, and increase the costs of healthcare. Our aim in this study was to evaluate the effectiveness of a multidisciplinary, evidence-based, surveillance program combined with intrawound application of vancomycin in lowering the incidence rate of SSI after spinal surgery with instrumentation.
We conducted a retrospective analysis of 637 patients who underwent spinal fusion with instrumentation in our institution at 3 different time periods: prior to our surveillance program (control group), surveillance only (surveillance group 1), and surveillance combined with intrawound vancomycin application (surveillance group 2). The following covariates were considered in the evaluation of between-group differences in SSI rate: sex, age, surgical site, National Nosocomial Infection Surveillance (NNIS) risk index, American Society of Anesthesiologists (ASA) physical status classification, and other health comorbidities. The causative organism in cases of SSI was confirmed in all cases.
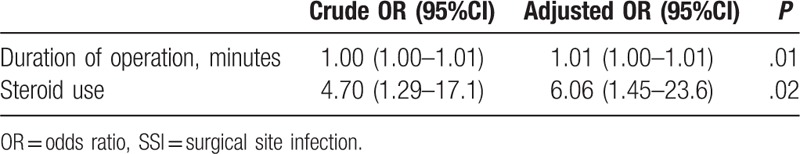
The rate of SSI was significantly lower in the surveillance group 2 (1.4%) than in the control group (4.6%; P = .04). On multivariate logistic regression analysis, steroid use (adjusted odd's ratio (OR), 6.06; 95% confidence interval (CI), 1.45–23.6) and operative time (adjusted OR.1.01; 95% CI, 1.00–1.01) were identified as independent risk factors of SSI. Staphylococcus species and Propionibacterium acnes were the principal causative organisms.
A bundled approach that includes surveillance and intrawound application of vancomycin is an effective strategy to lower the risk of SSI after spinal fusion with instrumentation. The use of steroid and longer operative time are risk factors of SSI.
Our findings support the implementation of a program of surveillance, combined with intrawound vancomycin application, to reduce the incidence rate of SSIs in spinal surgery.
Keywords: duration of operation, intrawound vancomycin application, steroid use, surgical site infection, surveillance
1. Introduction
Surgical site infection (SSI) increases the overall risk of mortality postsurgery,[1,2] extends hospital stay[1–3] and increases the costs of healthcare due to additional treatment needed.[1,3] In their 2014 survey of SSIs across multiple states in the United States, Magill et al[4] reported 66,100 cases of SSI, with an incidence rate of 2.0% to 4.4%.[5–8] The National Nosocomial Infection Surveillance (NNIS) system was established by the Centers for Disease Control and Prevention in 1970 to identify strategies to eliminate SSIs. Cruse and Food[9] reported on the benefit of implementing active surveillance in lowering the incidence rate of SSIs to 1.0% to 2.6%. Similarly, Brandt et al[10] reported a decrease in the risk of SSI to 0.75 after a 3-year program of SSI surveillance, an outcome which was further confirmed by Schneeberger et al[11] for elective orthopedic surgeries. In Japan, the Japan Nosocomial Infections Surveillance (JANIS) program was established by the Ministry of Health, Labor, and Welfare in 2002 to conduct SSI surveillance, using the guidelines and definitions of the NNIS system. In 2007, the JANIS program reported an incidence rate of SSI after spinal fusion of 1.1%. In comparison, the incidence rate of SSI after spinal fusion with instrumentation in our institution, between 2004 and 2007, was as high as 4.6%. To address this issue, we established an SSI surveillance program, in cooperation with the Infection Control Team (ICT), and expanded our perioperative protocol to include the use of antibiotic prophylaxis,[12–15] perioperative glycemic control[16] and intrawound application of vancomycin powder.[17,18] Our aim in this study was to evaluate the effectiveness of our multi-disciplinary, evidence-based surveillance program, combined with intrawound application of vancomycin, in lowering the incidence rate of SSI after spinal surgery with instrumentation.
2. Material and methods
2.1. Patient data and surveillance
We conducted a retrospective analysis of 637 patients who underwent spinal surgery with instrumentation at our institution, between January 2004 and June 2016, during 3 different types of surveillance periods: before formal surveillance and the perioperative protocol were established (January 2004–May 2007, n = 152, control group); surveillance period 1, after implementation of surveillance and the perioperative protocol (June 2007–July 2011, n = 199, surveillance group 1); and surveillance period 2, after implementation of surveillance, the perioperative protocol and intrawound application of vancomycin (August 2011–June 2016, n = 286, surveillance group 2). All procedures of surveillance were performed according to the NNIS guidelines and definitions.[19] The following covariate information was extracted from the medical analysis for inclusion in the analysis: age, sex, body mass index (BMI), current smoking status, use of steroids (defined as any systemic steroid provided at any dosage, but not including locally applied steroids). The following variables were extracted for analysis: use of immunosuppressants, past history of diabetes mellitus (DM), and rheumatoid arthritis (RA), location of surgical site (cervical/thoracic/lumbar), operative time, volume of intraoperative blood loss, number of spinal levels fused; and use of bone graft for fusion. The risk index for SSI was calculated using the methods described in the NNIS system, with the index ranging between “0” and “3,” with higher scores indicative of a higher risk for SSI. Patients’ physical status prior to surgery was assessed using the American Society of Anesthesiologists (ASA) physical status classification system.
Approval by Kyoto university ethics committee was obtained for this study.
2.2. Perioperative protocol
The following perioperative protocol was used for all patients, including: preoperative bathing with soap[20] and intranasal mupirocin treatment, with application of mupirocin ointment for decolonization in patients with nasal Staphylococcus aureus;[12–14] antibiotic prophylaxis, consisting of administration of 1 g of cephazolin 30 minutes before incision, with intraoperative redosing if the procedure exceeded 3 hours, and additional 6 doses administered up to 48 hours after surgery;[12] frequent wound irrigation, using saline, including after instrumentation;[21] double gloving and a change of the outer pair before handling the instrumentation and before wound closing;[22] storage of bone grafts in the plastic box with a cover to prevent bacterial contamination; perioperative glycemic control, measured using an insulin sliding scale;[16] and intrawound application of vancomycin powder. Since August 2011, we added 1 g of vancomycin powder to the bone graft and scattered 1 g of vancomycin around the instrumentation.[17,18]
2.3. Statistical analysis
Differences between the control (before formal surveillance) and the surveillance groups (surveillance period 1 and 2) with regard to age, number of spinal levels fused, volume of blood loss, and BMI were evaluated using Dunnett's test. Between-group differences and sex distribution, rate of SSI, and risk factors were valuated using Pearson's test. Lastly, a chi-squared test was used to compare the NNIS risk index and ASA classification between groups. Univariate analysis and multivariate regression analysis were performed to identify the variables associated with SSI, with variables selected using a stepwise method. All analyses were performed using JMP Pro 11 (SAS, United States), with significance set a P-value of .05. Steroid use and operative time were used for the calculation of the adjusted odds ratio (OR).
3. Results
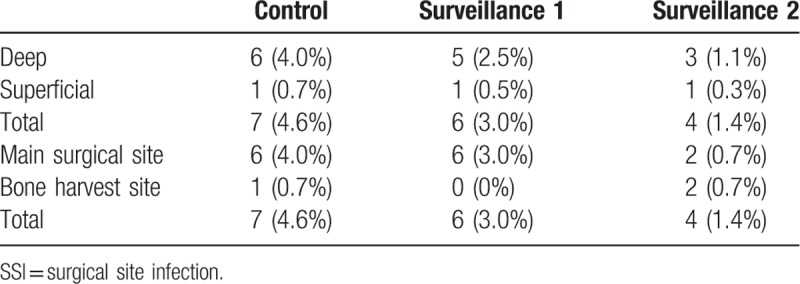
Patient characteristics were comparable between the 3 groups, with the exception of a higher mean age for surveillance 2 group compared to the control group. Surgical characteristics were comparable between groups. The incidence rate of SSI was significantly lower for the surveillance group 2 (1.4%) than for the control group (4.6%; P = .04; Table 1; Fig. 1). Considering the subgroup of patients who developed an SSI only, no between-group differences in operative parameters were identified (Table 2). The distribution of deep SSI was as follows: 6 in the control group, 5 in the surveillance 1 group and 3 in the surveillance group 2 (Table 3). The SSI rate at the main surgical site was significantly lower in the surveillance group 2 than in the control group (P = .02; Table 3; Figure).
Table 1.
Patient demographics in total.
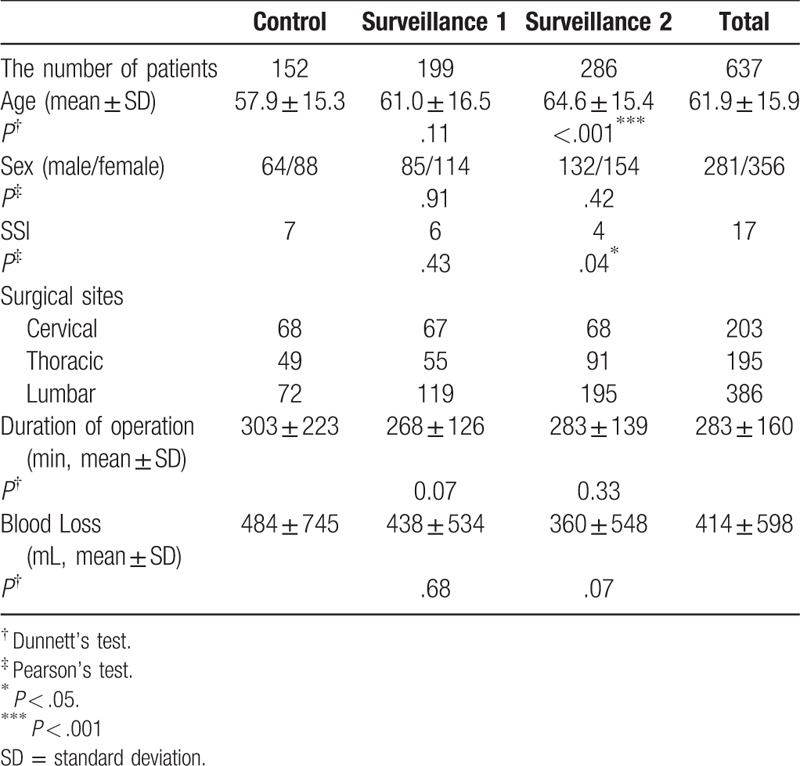
Figure 1.
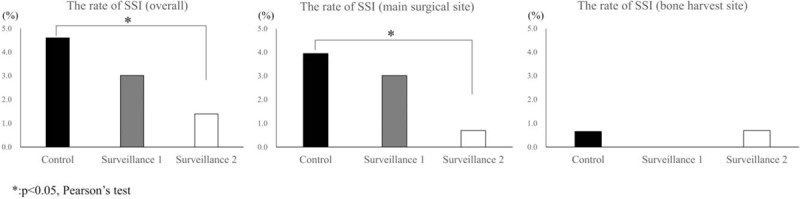
The overall rate of surgical site infection (SSI) was4.6% for the control group, 3.0% for surveillance group 1 and 1.4% for surveillance group 2. The incidence rate of SSI was significantly lower for the surveillance group 2 than the rate for the control group (left panel). The SSI rate at the main surgical site was 4.0%, 3.0%, 0.7%, respectively, for the control, surveillance 1 and surveillance 2 groups. The SSI rate at the main surgical site was significantly lower in the surveillance 2 group than in the control group (middle panel). The SSI rate at the bone harvest site was 0.7%, 0%, 0.7%, respectively. There were no significant differences between groups (right panel).
Table 2.
Patient demographics in SSI subgroup.
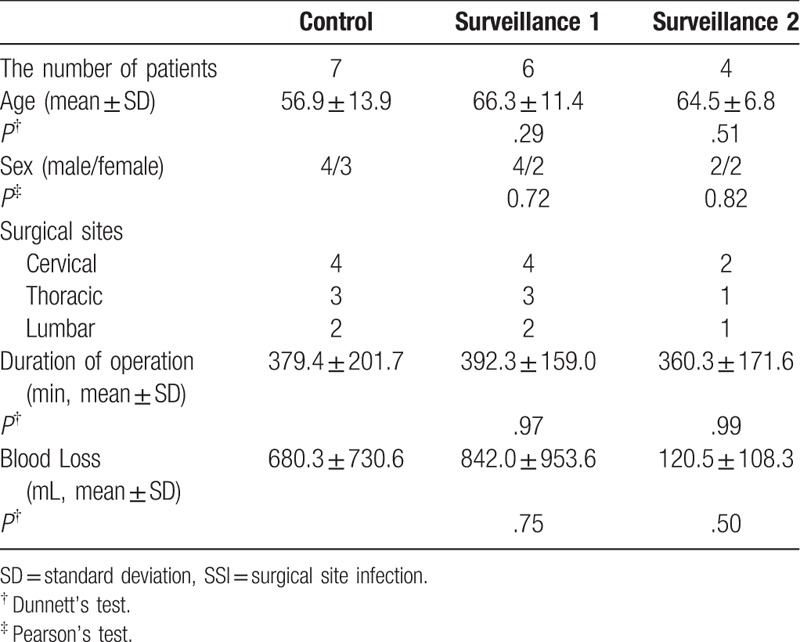
Table 3.
Classification of SSI.
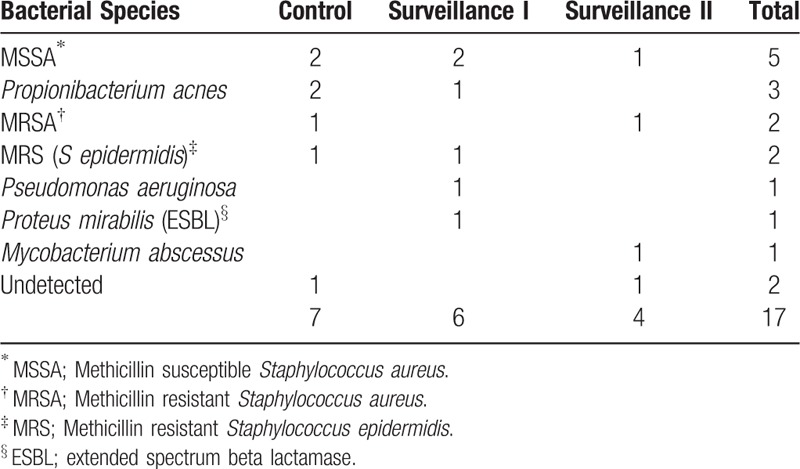
Staphylococcus species and Propionibacterium acnes were major causative organisms of SSI (Table 4). Of note, only 1 case of methicillin resistant S aureus (MRSA) and 1 case of Mycobacterium abscessus were detected from samples of main surgical wounds, with 1 case of methicillin susceptible S aureus s detected at the iliac bone graft site in the surveillance group 2.
Table 4.
Causative organisms of SSI.
In univariate analysis, steroid use and a longer operative time were associated with a higher incidence rate of SSI (P = .01; Table 5). The rate of SSI was also higher among patients with RA (P = .05). Smoking, immunosuppressant therapy, previous surgeries, DM, number of levels of fusion, intraoperative volume of blood loss, BMI, the NNIS risk index, and the ASA classification were not associated with SSI incidence. On multivariate logistic regression analysis, an increase in operative time was associated with an increased incidence of SSI (adjusted OR, 1.01; 95% confidence interval (CI), 1.00–1.01), with steroid use resulting in a 6.06-fold increase in SSI incidence (OR, 6.06; 95%CI, 1.45–23.6; Table 6).
Table 5.
Correlations between risk factors and SSI occurrence during surveillance period.
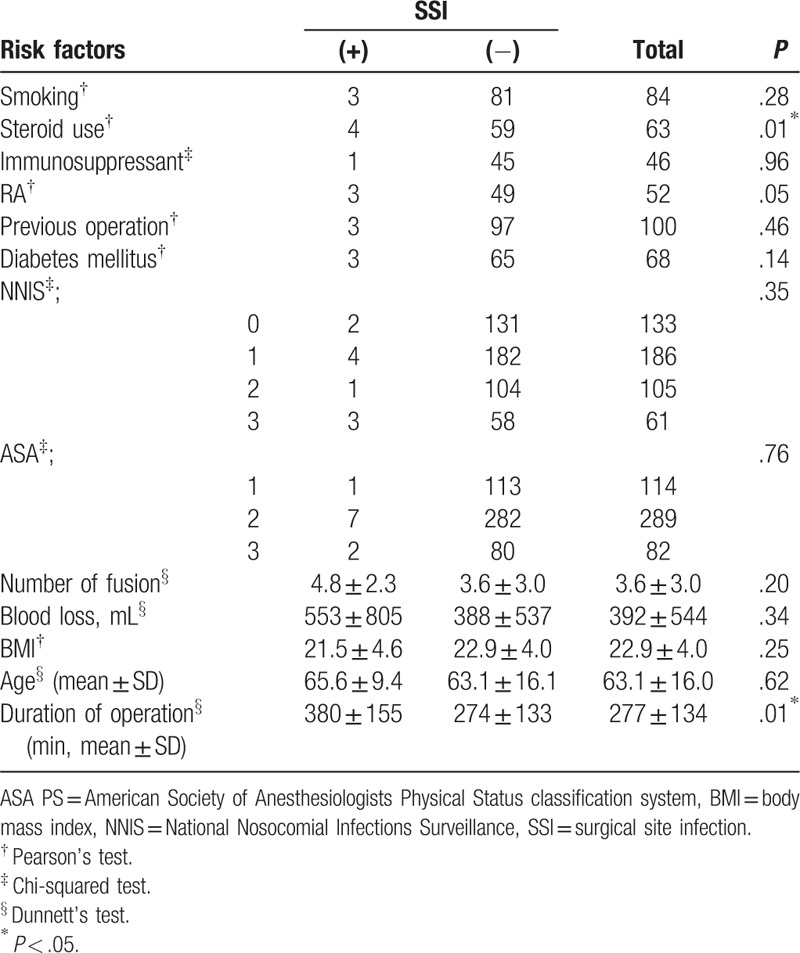
Table 6.
Multivariate logistic analysis of SSI occurrence.
4. Discussion
We were successful in significantly reducing the incidence rate of SSI after spinal instrumentation surgery through a combination of surveillance, implemented by an ICT, and intrawound application of vancomycin powder. Following the American study on the efficacy of nosocomial infection control (SENIC project),[23] active surveillance was implemented in Europe,[10,24–28] Australia,[29] and Asia,[30,31] and has played an important role in the prevention of SSIs. Although most surveillance programs have been effective in reducing the rate of SSI, surveillance alone was insufficient in our study. Although this unexpected result may be due, in part, to the small sample size of our study, the most likely explanation is the increased detection of SSI with surveillance.[32] Moreover, a previously published prospective study reported that surveillance of SSIs by surgeons alone was not as effective as surveillance performed in cooperation with an ICT.[33] Considering this, the rate of SSI might be underestimated when not implemented by an ICT.
Several risk factors have been associated with SSI after spinal surgery, and these can be classified into 3 broad groups: patient characteristics, surgical procedure and postoperative care.[34] At the level of patient characteristics, older age,[8,35] obesity,[5–8,36,37] DM,[2,6–8,21,38] alcohol abuse,[8,39] smoking,[2,8,35,37] low platelet count,[40,41] and previous SSI[7,8] all contribute to increasing the risk for SSI after instrumented spinal surgery. At the level of surgical procedures, use of a posterior approach,[5,35] a volume of intraoperative blood loss >1 L,[7,35] a longer operative time,[2,7,36] and the need for blood transfusion[40,42] have been identified as common risk factor for postsurgical SSI. At the level of postoperative care, not using a postoperative drain,[40] prolonged postoperative wound drainage[39] and postoperative incontinence[5] are recognized as potential risk factors for SSI. In our study cohort, we identified operative time and steroid use as independent risk factors for the development of SSI. Previous studies have reported an OR of operative time for SSI ranging between 1.33 to 2.08,[2,7,36] although different cutoff values have been used in different studies. In our study, we expressed operative time as a continuous variable. Therefore, our OR of 1.01 indicates that extending the operative time by 60 minutes results in a 1.8-fold increase in the rate of SSI. The association between SSI and use of steroids can be explained by the effects of corticosteroids suppressing cell-mediated immunity.[36,43,44]
The combination of systemic antibiotic prophylaxis, consisting of first generation cephalosporins and local vancomycin application, has been associated with excellent outcomes in lowering the incidence rate of SSI after spinal surgery with instrumentation to levels of 0–0.8%.[17,18,35] Both S aureus and S epidermidis bacteria can form a biofilm, increasing their resistance to antibiotics. The combination of vancomycin and β-lactam antibiotics provides a synergistic effect against biofilm forming MRSA.[45] Vancomycin alone, at a concentration >64 μg/mL, can eradicate biofilm forming S epidermidis for up to 4 hours.[46] The average concentration of intrawound vancomycin used 48 h after hip revision surgeries, using impaction bone grafting mixed with vancomycin, is 265 μg/mL.[47] Therefore, an effective local application of vancomycin must be sustained for at least 2 days postsurgery. Considering that a sufficient concentration of vancomycin was used over a sufficient time period in our surveillance 2 protocol, the occurrence of one case of MRSA-associated SSI indicates that not all MRSA infections can be controlled by intrawound application of vancomycin. The issue is to balance an effective local dose of vancomycin with tissue tolerance. An in vitro study reported that human osteoblasts can survive up to vancomycin concentrations of 2000 μg/ml.[48] In humans, thus far, the maximum reported concentration of vancomycin used locally was 1400 μg/mL over a 48-hours period, without any sign of nephrotoxicity.[49] Further research is warranted to clearly delineate the effects of intrawound vancomycin on the bone fusion rate at the graft site.
The limitations of our study need to be acknowledged. First, the bias introduced by the lower mean age of patients in the control versus surveillance group 2 on measured outcomes could not be accounted for in our statistical analysis. Age is an important covariate to consider as higher patient age correlates with higher incidence rates of SSI.[8,35] Moreover, not all risk factors for SSI could be identified from the medical records of patients in the control group, as these risk factors were not systematically recorded prior to the implementation of SSI surveillance. As the majority of our patients underwent spinal fusion with instrumentation of the cervicothoracic and thoracolumbar regions of the spine, a reliable evaluation of the effect of surgical site on the incidence rate of SSI could not be performed. As well, the local or serum concentration of vancomycin was not measured and, therefore, a dose–effect relationship could not be evaluated. Lastly, although steroid use was identified as an independent risk factor for SSI, again, we did not consider the dose-effect. As we were not able to identify research that clearly evaluated the dose effect of steroids on SSI, further research on this issue is needed.
In conclusion, topical administration of vancomycin in combination with surveillance can be effective in lowering the incidence rate of SSI after spinal fusion with instrumentation. Steroid use and longer operative time are risk factors for SSI.
Author contributions
Conceptualization: Takashi Sono, Shunsuke Fujibayashi, Masanori Izeki, Miki Nagao, Satoshi Ichiyama.
Data curation: Takashi Sono, Masanori Izeki, Yu Shimizu, Kazutaka Masamoto, Kazuaki Morizane.
Formal analysis: Takashi Sono.
Investigation: Takashi Sono, Masanori Izeki, Miki Nagao, Satoshi Ichiyama.
Project administration: Satoshi Ichiyama, Shuichi Matsuda.
Supervision: Shunsuke Fujibayashi, Shuichi Matsuda.
Validation: Bungo Otsuki, Shimei Tanida.
Writing - original draft: Takashi Sono.
Writing - review & editing: Shunsuke Fujibayashi, Masanori Izeki, Bungo Otsuki, Shimei Tanida, Miki Nagao, Satoshi Ichiyama.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, DM = diabetes mellitus, ICT = Infection Control Team, JANIS = Japan Nosocomial Infections Surveillance, NNIS = National Nosocomial Infection Surveillance, OR = odds ratio, RA = rheumatoid arthritis, SSI = surgical site infection.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Awad SS. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect 2012;13:234–7. [DOI] [PubMed] [Google Scholar]
- [2].Veeravagu A, Patil CG, Lad SP, et al. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine 2009;34:1869–72. [DOI] [PubMed] [Google Scholar]
- [3].Whitehouse JD, Friedman ND, Kirkland KB, et al. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 2002;23:183–9. [DOI] [PubMed] [Google Scholar]
- [4].Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. New Engl J Med 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003;98:149–55. [PubMed] [Google Scholar]
- [6].Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg 2008;90:62–9. [DOI] [PubMed] [Google Scholar]
- [7].Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine 2009;34:1422–8. [DOI] [PubMed] [Google Scholar]
- [8].Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine 2005;30:1460–5. [DOI] [PubMed] [Google Scholar]
- [9].Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North 1980;60:27–40. [DOI] [PubMed] [Google Scholar]
- [10].Brandt C, Sohr D, Behnke M, et al. Reduction of surgical site infection rates associated with active surveillance. Infect Control Hosp Epidemiol 2006;27:1347–51. [DOI] [PubMed] [Google Scholar]
- [11].Schneeberger PM, Smits MH, Zick RE, et al. Surveillance as a starting point to reduce surgical-site infection rates in elective orthopaedic surgery. J Hosp Infect 2002;51:179–84. [DOI] [PubMed] [Google Scholar]
- [12].Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. New Engl J Med 2010;362:9–17. [DOI] [PubMed] [Google Scholar]
- [13].Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. New Engl J Med 2002;346:1871–7. [DOI] [PubMed] [Google Scholar]
- [14].Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, et al. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis 2002;35:353–8. [DOI] [PubMed] [Google Scholar]
- [15].Allegranzi B, Bischoff P, de Jonge S, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276–87. [DOI] [PubMed] [Google Scholar]
- [16].Rizvi AA, Chillag SA, Chillag KJ. Perioperative management of diabetes and hyperglycemia in patients undergoing orthopaedic surgery. J Am Acad Orthop Surg 2010;18:426–35. [DOI] [PubMed] [Google Scholar]
- [17].Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine 2011;36:2084–8. [DOI] [PubMed] [Google Scholar]
- [18].O’Neill KR, Smith JG, Abtahi AM, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J 2011;11:641–6. [DOI] [PubMed] [Google Scholar]
- [19].Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132. quiz 133-134; discussion 96. [PubMed] [Google Scholar]
- [20].Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev 2015;Cd004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Watanabe M, Sakai D, Matsuyama D, et al. Risk factors for surgical site infection following spine surgery: efficacy of intraoperative saline irrigation. J Neurosurg Spine 2010;12:540–6. [DOI] [PubMed] [Google Scholar]
- [22].Rehman A, Rehman AU, Rehman TU, et al. Removing outer gloves as a method to reduce spinal surgery infection. J Spinal Disord Tech 2015;28:E343–6. [DOI] [PubMed] [Google Scholar]
- [23].Haley RW, Quade D, Freeman HE, et al. The SENIC Project. Study on the efficacy of nosocomial infection control (SENIC Project). Summary of study design. Am J Epidemiol 1980;111:472–85. [DOI] [PubMed] [Google Scholar]
- [24].Marchi M, Pan A, Gagliotti C, et al. The Italian national surgical site infection surveillance programme and its positive impact, 2009 to 2011. Euro Surveill 2014;19:20815. [DOI] [PubMed] [Google Scholar]
- [25].Staszewicz W, Eisenring MC, Bettschart V, et al. Thirteen years of surgical site infection surveillance in Swiss hospitals. J Hosp Infect 2014;88:40–7. [DOI] [PubMed] [Google Scholar]
- [26].Skramm I, Saltyte Benth J, Bukholm G. Decreasing time trend in SSI incidence for orthopaedic procedures: surveillance matters!. J Hosp Infect 2012;82:243–7. [DOI] [PubMed] [Google Scholar]
- [27].Mannien J, van den Hof S, Muilwijk J, et al. Trends in the incidence of surgical site infection in the Netherlands. Infect Control Hosp Epidemiol 2008;29:1132–8. [DOI] [PubMed] [Google Scholar]
- [28].Astagneau P, L’Heriteau F, Daniel F, et al. Reducing surgical site infection incidence through a network: results from the French ISO-RAISIN surveillance system. J Hosp Infect 2009;72:127–34. [DOI] [PubMed] [Google Scholar]
- [29].Worth LJ, Bull AL, Spelman T, et al. Diminishing surgical site infections in Australia: time trends in infection rates, pathogens and antimicrobial resistance using a comprehensive Victorian surveillance program, 2002–2013. Infect Control Hosp Epidemiol 2015;36:409–16. [DOI] [PubMed] [Google Scholar]
- [30].Choi HJ, Adiyani L, Sung J, et al. Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J Hosp Infect 2016;93:339–46. [DOI] [PubMed] [Google Scholar]
- [31].Morikane K, Honda H, Yamagishi T, et al. Factors associated with surgical site infection in colorectal surgery: the Japan nosocomial infections surveillance. Infect Control Hosp Epidemiol 2014;35:660–6. [DOI] [PubMed] [Google Scholar]
- [32].Roberts FJ, Walsh A, Wing P, et al. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine 1998;23:366–70. [DOI] [PubMed] [Google Scholar]
- [33].Rosenthal R, Weber WP, Marti WR, et al. Surveillance of surgical site infections by surgeons: biased underreporting or useful epidemiological data? J Hosp Infect 2010;75:178–82. [DOI] [PubMed] [Google Scholar]
- [34].Boetto J, Chan-Seng E, Lonjon G, et al. Is hospital information system relevant to detect surgical site infection? Findings from a prospective surveillance study in posterior instrumented spinal surgery. Orthop Traumatol Surg Res 2015;101:845–9. [DOI] [PubMed] [Google Scholar]
- [35].Dennis HH, Wei DT, Darren KZ, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine 2016;42:267–74. [DOI] [PubMed] [Google Scholar]
- [36].Sebastian A, Huddleston P, 3rd, Kakar S, et al. Risk factors for surgical site infection after posterior cervical spine surgery: an analysis of 5,441 patients from the ACS NSQIP 2005-2012. Spine J 2016;16:504–9. [DOI] [PubMed] [Google Scholar]
- [37].Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am 2013;95:549–54. [DOI] [PubMed] [Google Scholar]
- [38].Hikata T, Iwanami A, Hosogane N, et al. High preoperative hemoglobin A1c is a risk factor for surgical site infection after posterior thoracic and lumbar spinal instrumentation surgery. J Orthop Sci 2014;19:223–8. [DOI] [PubMed] [Google Scholar]
- [39].Klekamp J, Spengler DM, McNamara MJ, et al. Risk factors associated with methicillin-resistant staphylococcal wound infection after spinal surgery. J Spinal Disord 1999;12:187–91. [PubMed] [Google Scholar]
- [40].Ho C, Sucato DJ, Richards BS. Risk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patients. Spine 2007;32:2272–7. [DOI] [PubMed] [Google Scholar]
- [41].Bronheim RS, Oermann EK, Cho SK, et al. Coagulation profile as a risk factor for 30- day morbidity and mortality following posterior lumbar fusion. Spine 2017;42:950–7. [DOI] [PubMed] [Google Scholar]
- [42].Basques BA, Anandasivam NS, Webb ML, et al. Risk factors for blood transfusion with primary posterior lumbar fusion. Spine 2015;40:1792–7. [DOI] [PubMed] [Google Scholar]
- [43].Cutolo M, Seriolo B, Pizzorni C, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev 2008;8:153–5. [DOI] [PubMed] [Google Scholar]
- [44].Ogihara S, Yamazaki T, Maruyama T, et al. Prospective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adults. J Orthop Sci 2015;20:71–7. [DOI] [PubMed] [Google Scholar]
- [45].Barber KE, Werth BJ, McRoberts JP, et al. A novel approach utilizing biofilm time-kill curves to assess the bactericidal activity of ceftaroline combinations against biofilm-producing methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2014;58:2989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sakimura T, Kajiyama S, Adachi S, et al. Biofilm-forming Staphylococcus epidermidis expressing vancomycin resistance early after adhesion to a metal surface. BioMed Res Int 2015;2015:943056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Buttaro M, Comba F, Piccaluga F. Vancomycin-supplemented cancellous bone allografts in hip revision surgery. Clin Orthop Relat Res 2007;461:74–80. [DOI] [PubMed] [Google Scholar]
- [48].Rathbone CR, Cross JD, Brown KV, et al. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res 2011;29:1070–4. [DOI] [PubMed] [Google Scholar]
- [49].Buttaro MA, Gimenez MI, Greco G, et al. High active local levels of vancomycin without nephrotoxicity released from impacted bone allografts in 20 revision hip arthroplasties. Acta Orthop 2005;76:336–40. [PubMed] [Google Scholar]


