Abstract
Background:
The aim of this study was to find the better treatment for colorectal cancer (CRC) by comparing robot-assisted colorectal surgery (RACS), laparoscopic-assisted colorectal surgery (LACS), and open surgery using network meta-analysis.
Methods:
A literature search updated to August 15, 2017 was performed. All the included literatures were evaluated according to the quality evaluation criteria of bias risk recommended by the Cochrane Collaboration. All data were comprehensively analyzed by ADDIS. Odds ratio (OR), mean difference (MD), and 95% confidence interval (CI) were used to show the effect index of all data. The degree of convergence of the model was evaluated by the Brooks–Gelman–Rubin method with the potential scale reduction factor (PSRF) as the evaluation indicator.
Results:
The PSRF values of operation time, estimated blood loss, length of hospital stay, complication, mortality, and anastomotic leakage ranged from 1.00 to 1.01, and those of wound infection, bleeding, and ileus ranged from 1.00 to 1.02. Open surgery had the shortest operation time compared with LACS and RACS. Furthermore, compared with LACS, the amount of blood loss, complication, mortality, bleeding rate, and ileus rate for RACS were the least, and the length of hospital stay for RACS was the shortest. The anastomotic leakage rate for LACS was the least, but there was no significant difference compared with those of RACS and open surgery. The wound infection rate for LACS was the least, but there was no significant difference compared with that of RACS.
Conclusion:
RACS might be a better treatment for patients with CRC.
Keywords: colorectal cancer, complication, estimated blood loss, length of hospital stay, network meta-analysis, operation time
Highlights
Totally, 40 eligible papers published from 1998 to 2017 were included.
The amount of bleeding and incidence rate of RACS was the least.
The time of hospitalization of patients with RACS treatment was the shortest.
RACS might be a better treatment for CRC patients.
1. Introduction
Colorectal cancer (CRC), one of the most common malignancies in the United States, seriously affects the health of people worldwide.[1] It is the 2nd most commonly diagnosed cancer in women and 3rd in men, with about 571,000 female and 663,000 male patients with CRC in 2012 worldwide.[2] The relative 5-year survival rate for patients with CRC is 65%, while the 10-year survival rate declines to 58%.[1] Risk factors for CRC include human papilloma virus infection, long-term constipation, inadequate physical activity, alcohol intake, smoking, and high protein and fat consumption.[3–6] Most of the patients with stage I and II CRC have to undergo colectomy, and the patients with stage III CRC receive chemotherapy to lower the risk of recurrence.[7] However, for CRC survivors, the risk of chronic diarrhea and cancer recurrence is increased.[8,9] Thus, effective treatment for CRC is needed.
In the early 1990s, laparoscopic surgery was first proposed to be an alternative to open surgery for lesions of the rectum and colon.[10] Kitano indicated that compared with open surgery, laparoscopic surgery was a standard treatment for colon cancer with shorter hospital stay, faster recovery, improved incidence of wound infection, and decreased pain.[11] Furthermore, the long-term outcome with laparoscopic surgery has been identified by randomized controlled trials (RCTs).[12,13] In addition, robotic systems especially Vinci 1 robot (Intuitive Surgical, Sunnyvale, CA), a robot for performing abdominal surgery, have been identified to be an alternative for standard laparoscopic surgery especially in complex pathology.[14,15] Robotic total mesorectal excision may have a better therapeutic effect than laparoscopic total mesorectal excision, especially in special cases, including low and middle rectal cancer.[16] It is necessary to find optimal surgical methods for the treatment of CRC.
A network meta-analysis gains the certainty of all treatment comparisons by combination of direct and indirect evidence based on the randomized nature of the data.[17] Recently, a network meta-analysis focused on the efficacy of laparoscopic and open surgery in CRC; however, the main outcomes were mortality and complications, and operation time, estimated blood loss, and length of hospital stay were not analyzed.[18] Moreover, there was a rare study about the comparisons of laparoscopic-assisted colorectal surgery (LACS), robot-assisted colorectal surgery (RACS), and open surgery. Therefore, in the present study, we evaluated the curative effect of RACS, LACS, and open surgery on CRC using a network meta-analysis to find the best treatment for CRC. Our finding might provide a basis for the future clinical treatment.
2. Methods
This meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[19]
2.1. Data sources
The related clinical researches were obtained from the electronic databases Pubmed (http://www.ncbi.nlm.nih.gov/pubmed/), Embase (http://www.embase.com), and the Cochrane Library (http://www.cochranelibrary.com) updated to August 15, 2017. The keywords were as follows: colorectal cancer (“colorectal cancer” OR “colorectal carcinoma” OR “rectal cancer” OR “rectal carcinoma” OR “colon cancer” OR “colorectal cancer” OR “colorectal carcinoma” OR “carcinoma of colon” OR “colorectal neoplasms”), robot support (“robot” OR “robotic” OR “da vinci” or davinci), laparoscopic support (“Laparoscopies” OR “laparoscopic” OR “laparoscopy”), open surgery (“open” OR “surgery”), and random-control study (Rando∗).
2.2. Informed consent and ethical approval
This study was a meta-analysis of several eligible studies downloaded from the public database including PubMed, Embase, and Cochrane library, thus informed consent and ethical approval were not necessary.
2.3. Inclusion and exclusion criteria
Articles meeting the following criteria were selected (based on the PICOS principle): Published English literatures studied the efficacy of RACS, LACS, and open surgery in patients with CRC (P); participants in each group were patients with CRC treated with RACS, LACS, or open surgery (I, C); the outcomes of the study included operation time, estimated blood loss, and the occurrence of complication (O); and the type was a randomized controlled study (S). The following articles were removed: studies with incomplete data or cannot be used for statistical analysis; and literatures such as reviews, reports, comments, and letters. Besides, if multiple literatures were repeatedly published or from the same population data, only the latest research or the research with complete information was included.
2.4. Data extraction and quality evaluation of literature
Data were independently extracted from the included literatures by 2 reviewers. For each study, the following data were collected: first author, published year, year of study, area of study, staging of CRC, and the total participants in each group, type of treatment, and general demographic data (e.g., sex, age, and body mass index) of participants in each group. The aggregate quality of the included studies was evaluated according to the quality evaluation criteria of bias risk, recommended by Cochrane Collaboration.[20] Disagreement was resolved by discussions with a third reviewer.
2.5. Statistical analysis
All data were comprehensively analyzed by ADDIS (version 1.16.5, http://www.medfloss.org/node/812). ADDIS is a non-programming software used to assess and process data using Markov chain Monte Carlo theory based on the Bayesian framework.[21,22] The network meta-analysis of operation time, estimated blood loss, length of hospital stay and complication, mortality, anastomotic leakage, wound infection, bleeding, and ileus was conducted based on the parameter set up in ADDIS software (number of chains, 4; tuning iterations, 20,000; simulation iterations, 50,000; thinning interval, 10; inference samples, 10,000; variance scaling factor, 2.5). Odds ratio (OR), mean difference (MD), and 95% confidence interval (CI) were used to show the effect index of all data. The test models used in this study were the random effects and consistency models. The degree of convergence of the model was evaluated by the Brooks–Gelman–Rubin method with the potential scale reduction factor (PSRF) as evaluation indicator. PSRF values close to 1 indicates better convergence effect of the model, and generally, PSRF values less than 1.2 are acceptable.
3. Results
3.1. Characteristics of the selected literature
A total of 5237 articles (1687 articles came from PubMed database, 2519 from Embase database, and 1031 from the Cochrane Library) were identified based on the literature search criteria. Among them, 1422 articles were repeated, and 3529 articles were irrelevant after reading the title and abstract. In addition, 246 articles (including 71 case series/reports, 24 letters, 102 literature reviews/meta-analyses, 11 reduplicative studies, 38 articles with irrelevant data) of the remaining 286 articles were removed by reviewing the full text. Finally, 40 eligible papers were included[23–62] (Fig. 1).
Figure 1.
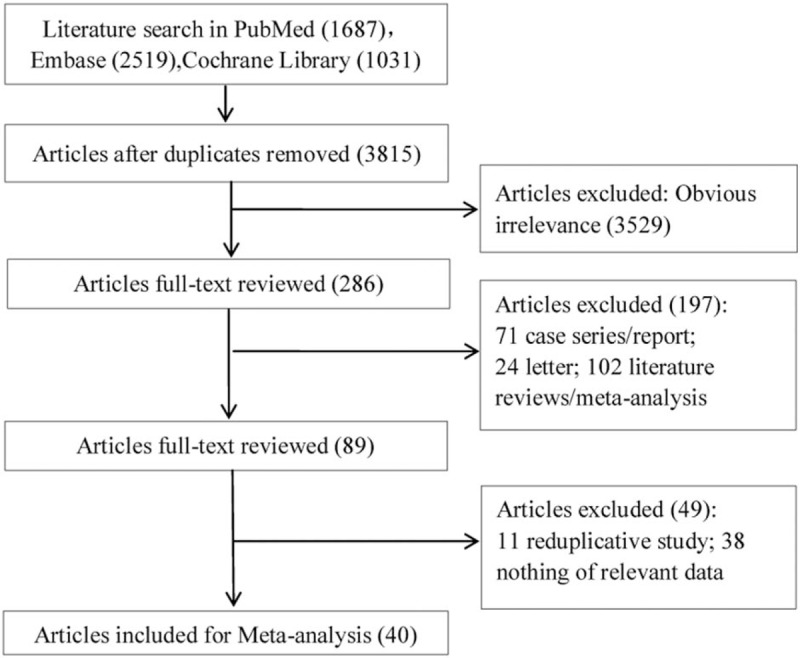
Literature search and study selection.
In total, 12,825 patients with CRC who were mainly concentrated in the I–III periods in staging of CRC were included in the study, including 5947 patients in the open surgery group, 129 patients in the RACS group, and 6749 patients in the LACS group. Among them, there were more male than female patients (including 7249 male and 5576 female patients), but there was no difference for sex in each literature. The baseline characteristics in each group were comparable in terms of age and body mass index, with old age as the main characteristic. Besides, the areas of these studies were Germany, Japan, the United States, China, Spain, South Korea, and so on (Table 1). The result of RCT quality evaluation showed that the quality of the included literatures was not very good; especially, some of the literatures had no detailed description of the blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias, Fig. 2 A, B).
Table 1.
Characteristics of the included literatures.
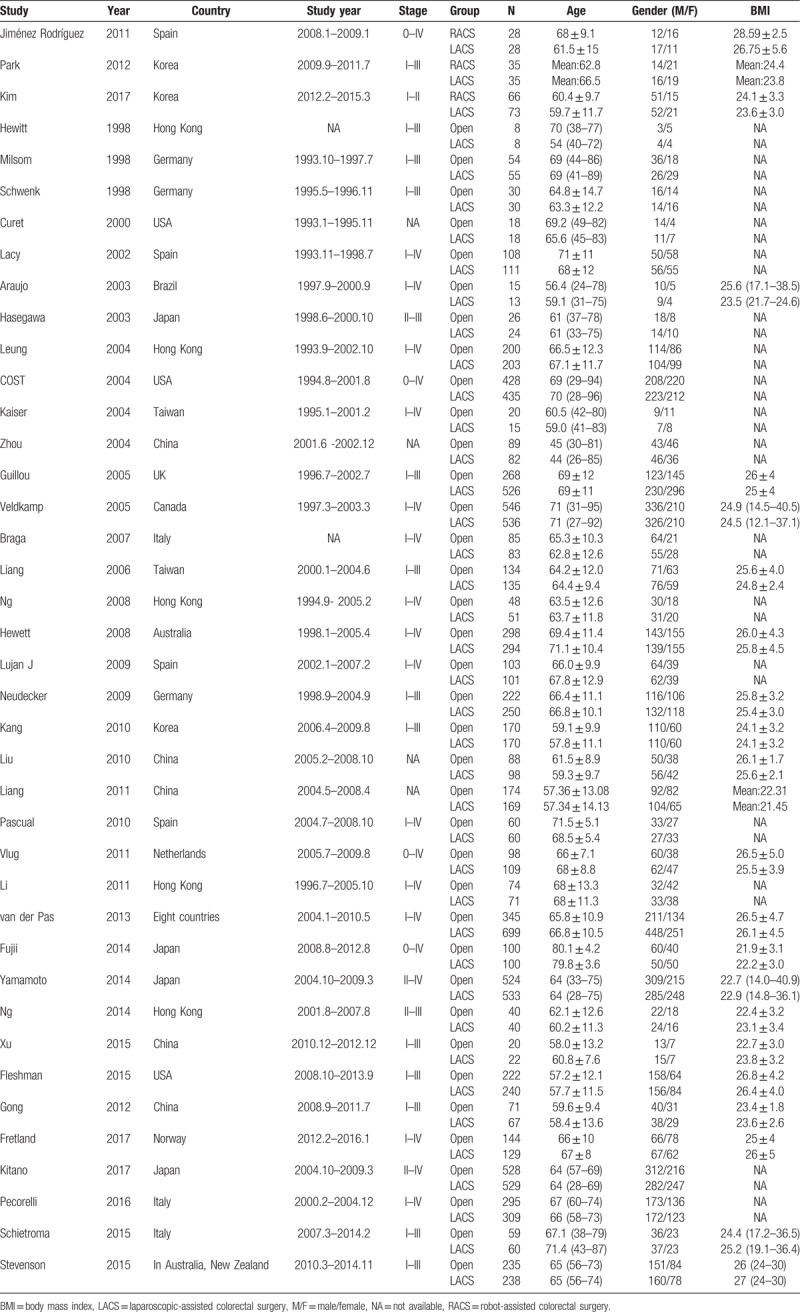
Figure 2.
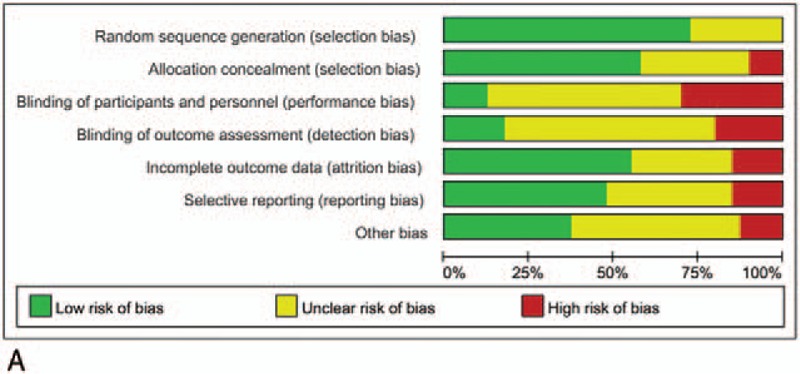
Quality assessments of the included studies. (A) Bias risk of the included studies. (B) Sensitivity and specificity of the included studies. “+,” low risk of bias; “−,” high risk of bias, and “?,” unclear risk of bias.
3.2. Network meta-analysis of operation time
The PSRF value of operation time ranged from 1.00 to 1.01, indicating complete convergence, good iterative effect, and stable results of the model. The results of the meta-analysis revealed that open surgery had the shortest operation time and statistically significant difference compared with LACS (MD = −43.40; 95% CI, −55.24 and −31.78) and RACS (MD = −109.19; 95% CI, −140.16 and −78.96; Table 2, Fig. 3 A).
Table 2.
Results of meta-analysis in operation time.
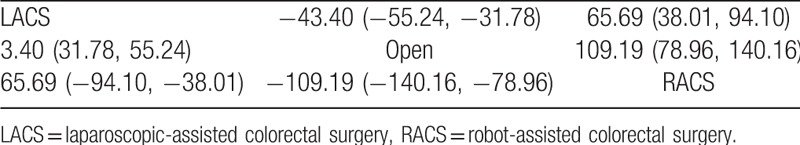
Figure 2 (Continued).
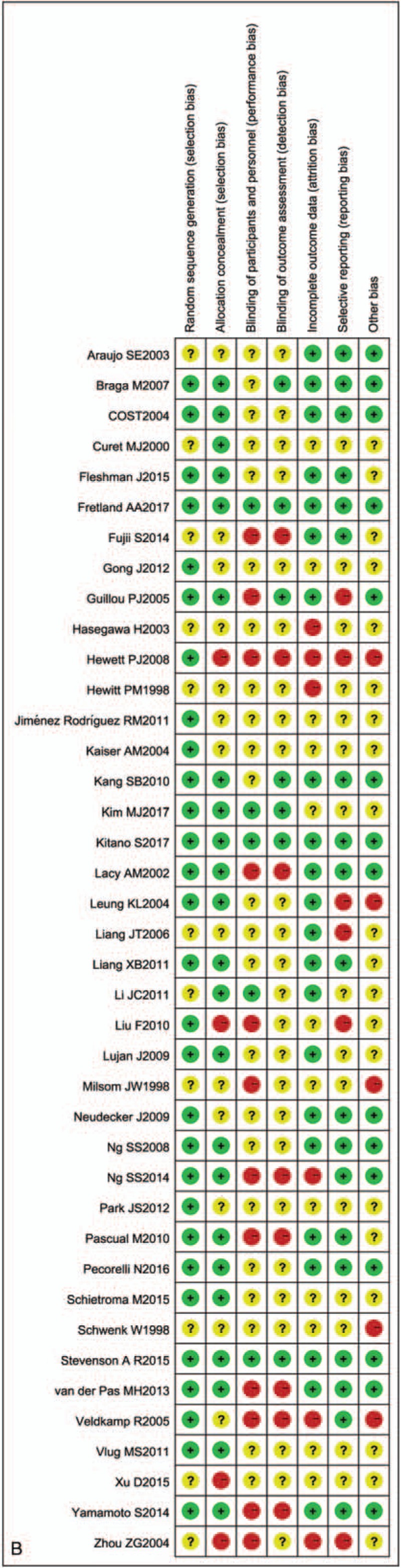
Quality assessments of the included studies. (A) Bias risk of the included studies. (B) Sensitivity and specificity of the included studies. “+,” low risk of bias; “−,” high risk of bias, and “?,” unclear risk of bias.
Figure 3.
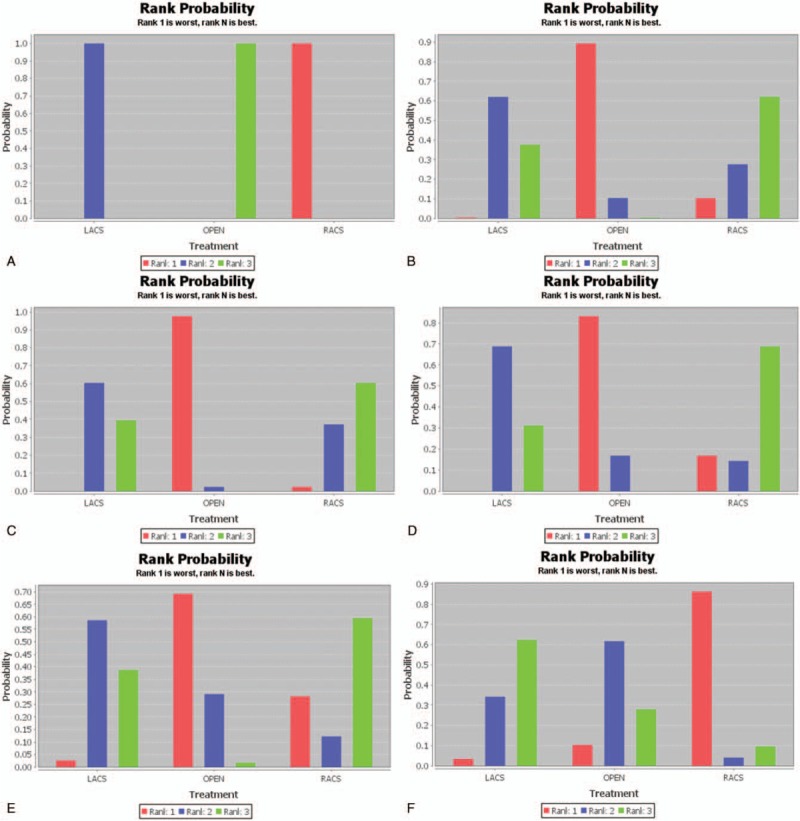
(A) Results of rank probability for operation time. (B) Results of rank probability for estimated blood loss. (C) Results of rank probability for length of hospital stay. (D) Results of rank probability for complication. (E) Results of rank probability for mortality. (F) Results of rank probability for anastomotic leakage. (G) Results of rank probability for wound infection. (H) Results of rank probability for bleeding. I. Results of rank probability for ileus. LACS = laparoscopic-assisted colorectal surgery, RACS = robot-assisted colorectal surgery.
Figure 3 (Continued).
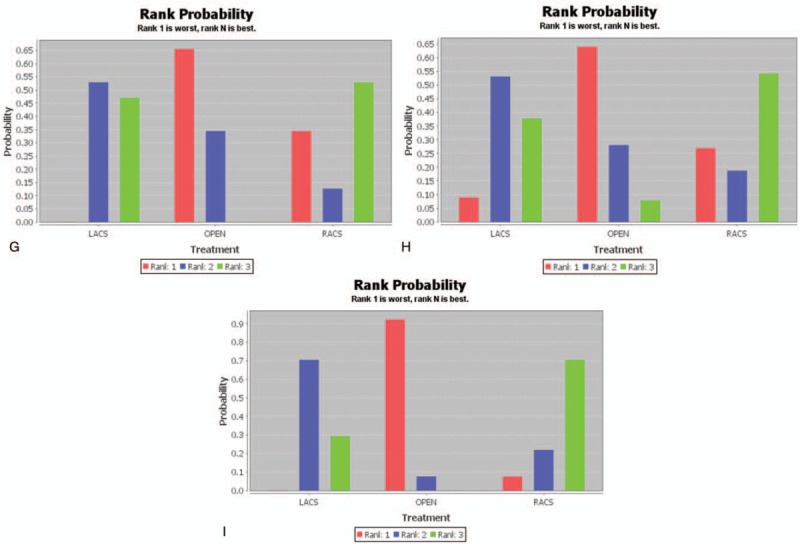
(A) Results of rank probability for operation time. (B) Results of rank probability for estimated blood loss. (C) Results of rank probability for length of hospital stay. (D) Results of rank probability for complication. (E) Results of rank probability for mortality. (F) Results of rank probability for anastomotic leakage. (G) Results of rank probability for wound infection. (H) Results of rank probability for bleeding. I. Results of rank probability for ileus. LACS = laparoscopic-assisted colorectal surgery, RACS = robot-assisted colorectal surgery.
3.3. Network meta-analysis of estimated blood loss
The PSRF value of estimated blood loss ranged from 1.00 to 1.01, indicating complete convergence, good iterative effect, and stable results of the model. The results of the meta-analysis revealed that the amount of bleeding for RACS was the least, but there was no significant difference compared with that for open surgery (MD = −97.55; 95% CI, −260.39 and 68.03) and LACS (MD = −21.12; 95% CI, −175.07 and 133.17; Table 3, Fig. 3 B).
Table 3.
Results of meta-analysis in estimated blood loss.

3.4. Network meta-analysis of length of hospital stay
The model of length of hospital stay had complete convergence, good iterative effect, and stable results with the PSRF value = 1.00. The results of the meta-analysis revealed that patients who underwent RACS had the shortest length of hospital stay. It had a statistically significant difference compared with patients who underwent open surgery (MD = −2.90; 95% CI, −5.85 and −0.06), but had no significant difference compared with patients who underwent LACS (MD = −0.34; 95% CI, −2.93 and 2.21; Table 4, Fig. 3 C).
Table 4.
Results of meta-analysis in length of hospital stay.

3.5. Network meta-analysis of complication
The PSRF value of complication ranged from 1.00 to 1.01, indicating complete convergence, good iterative effect, and stable results of the model. The results of the meta-analysis revealed that the complication rate in patients who underwent RACS was the least, but there was no significant difference compared with those in patients who underwent open surgery (OR = 0.62; 95% CI, 0.21 and 1.68) and LACS (OR = 0.79; 95% CI, 0.28 and 2.13; Table 5, Fig. 3 D).
Table 5.
Results of meta-analysis in complication.

3.6. Network meta-analysis of mortality
The PSRF value of mortality ranged from 1.00 to 1.01, indicating complete convergence, good iterative effect, and stable results of the model. The results showed that the mortality rate in patients who underwent RACS was the least, but there was no significant difference compared with those in patients who underwent LACS (OR = 0.84; 95% CI, 0.20 and 3.37) and open surgery (OR = 0.66; 95% CI, 0.15 and 2.70; Fig. 3 E).
3.7. Network meta-analysis of anastomotic leakage
The PSRF value of anastomotic leakage ranged from 1.00 to 1.01, indicating complete convergence, good iterative effect, and stable result of the model. These results revealed that the rate of anastomotic leakage in patients who underwent LACS was the least, but there was no significant difference compared with those in patients who underwent RACS (OR = 0.46, 95% CI: 0.13, 1.58) and open surgery (OR = 0.92; 95% CI, 0.68 and 1.26; Fig. 3 F).
3.8. Network meta-analysis of wound infection
The PSRF value of wound infection ranged from 1.00 to 1.02, indicating complete convergence, good iterative effect, and stable result of the model. The results revealed that the rate of wound infection in patients who underwent LACS was the least, and there was a statistically significant difference compared with that in patients who underwent open surgery (OR = 0.65; 95% CI, 0.49 and 0.82), but there was no significant difference compared with that in patients who underwent RACS (OR = 1.09; 95% CI, 0.11 and 8.45; Fig. 3 G).
3.9. Network meta-analysis of bleeding
The PSRF value of bleeding ranged from 1.00 to 1.02, indicating complete convergence, good iterative effect, and stable result of the model. Figure 3 H shows that the rate of bleeding in patients who underwent RACS was the least, but there was no significant difference compared with those in patients who underwent LACS (OR = 0.82; 95% CI, 0.05 and 20.06) and open surgery (OR = 0.44; 95% CI, 0.02 and 11.69).
3.10. Network meta-analysis of ileus
The PSRF value of ileus ranged from 1.00 to 1.02, indicating complete convergence, good iterative effect, and stable result of the model. Figure 3 I shows that the rate of ileus in patients who underwent RACS was the least, but there was no significant difference compared with those in patients who underwent LACS (OR = 0.74; 95% CI, 0.20 and 2057) and open surgery (OR = 0.42; 95% CI, 0.10 and 1.49).
Overall, the treatment effect of RACS was the best.
4. Discussion
A network meta-analysis evaluating the curative effect of RACS, LACS, and open surgery was conducted in this study. Our finding revealed that open surgery had the shortest operation time and statistically significant differences compared with LACS and RACS. Furthermore, compared with LACS and open surgery, the amount of bleeding and complication, mortality, bleeding, and ileus rates in RACS were the least, and the length of hospital stay in RACS was the shortest. However, in estimated blood loss and complication, there was no significant difference in RACS compared with open surgery and LACS, and in length of hospital stay, a statistically significant difference was found in RACS and open surgery but not in LACS. The rate of anastomotic leakage in patients who underwent LACS was the least, but there was no significant difference compared with those in patients who underwent RACS and open surgery. The rate of wound infection in patients who underwent LACS was the least, but there was no significant difference compared with that in patients who underwent RACS.
The popularity of RACS, one of the latest developments in laparoscopic surgery, has been increasing since it was first performed in cholecystectomy in 2001.[63] The surgical technique is improved by the properties of the robot system such as ambidextrous capability, 3-dimensional view, and tremor elimination.[43] Similar to our study, previous studies reported that RACS had the longest operation time.[16,64,65] However, it is worth noting that the operation time of RACS is minimal in more complex pelvic processes.[16] Patients who underwent RACS and LACS have a similar quality of life, bowel function recovery, and postoperative morbidity.[16] However, the autonomy of RACS is better than that of LACS.[16] A previous study mentioned that the cost of RACS was much higher than that of LACS.[66] Park et al suggested that the duration of surgery in the RACS group was longer than that in the LACS group, while the number of lymph nodes harvested, resection margin clearance, postoperative pain score, surgical complications, and hospital stay were similar.[43] The operation times were reported to be significantly longer in patients treated with robotic device than that treated with laparoscopy, whereas there were no differences between the 2 groups with regard to complications and hospital stay,[67] which was similar to our results. Two series comparing RACS and LACS in right colectomy have demonstrated that RACS has a longer case time and higher total hospital cost than LACS but similar estimated blood loss and length of hospital stay.[68,69] It is not necessary for RACS and LACS to convert to the open approach.[43] Although there was no significant clinical advantage for RACS in estimated blood loss, length of hospital stay, and complication rate compared with LACS, the lymph nodes around main blood vessels could be cleaned easily based on the stable camera platform. Moreover, RACS provided comfort for the surgeon by providing a better operative performance.
This study first comprehensively compared the efficacy of RACS, LACS, and OPEN in the treatment of CRC in operation time, estimated blood loss, length of hospital stay, complication, mortality, anastomotic leakage, wound infection, bleeding, and ileus using a network meta-analysis, which found that RACS might be the best treatment program for CRC. Our finding may provide a basis for future clinical treatment, that is to say, in the case of better economic conditions RACS is the best method for the treatment of CRC.
However, this research had some limitations as follows: this study did not adjust for covariates, and subgroup analysis was not performed due to the incomplete data of some studies; ADDIS software has the characteristics of easy operation, but because a free program cannot be made using it, the results may have some limitations. For example, only the random effects model can be reported when estimating the effect size, which may lead to slightly conservative results.
In conclusion, the present network meta-analysis suggested that RACS might be a better treatment for CRC. However, more high-quality studies with comprehensive data are needed to further verify our present results.
Author contributions
Conceptualization: Shihou Sheng.
Data curation: Tiancheng Zhao.
Formal analysis: Xu Wang.
Methodology: Xu Wang.
Writing – original draft: Shihou Sheng, Tiancheng Zhao.
Writing – review & editing: Xu Wang.
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, LACS = laparoscopic-assisted colorectal surgery, MD = mean difference, OR = odds ratio, PSRF = potential scale reduction factor, RACS = robot-assisted colorectal surgery.
SS and TZ should be regard as cofirst authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Bray F, Center MM, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87. [DOI] [PubMed] [Google Scholar]
- [3].Battaglia RE, Baumer B, Conrad B, et al. Health risks associated with meat consumption: a review of epidemiological studies. Int J Vit Nutr Res 2015;85:70. [DOI] [PubMed] [Google Scholar]
- [4].Blase JL, Campbell PT, Gapstur SM, et al. Prediagnostic Helicobacter pylori antibodies and colorectal cancer risk in an elderly, Caucasian population. Helicobacter 2016;21:488–92. [DOI] [PubMed] [Google Scholar]
- [5].De RK, De BM, Huybrechts I, et al. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: short review. Mutat Res Rev Mutat Res 2015;766:32. [DOI] [PubMed] [Google Scholar]
- [6].Stefan DC. Red meat, processed meat and cancer in South Africa. S Afr Med J 2015;106:43. [DOI] [PubMed] [Google Scholar]
- [7].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [8].Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263. [DOI] [PubMed] [Google Scholar]
- [9].Tjandra JJ, Chan MKY. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007;50:1783–99. [DOI] [PubMed] [Google Scholar]
- [10].Phillips EH, Franklin M, Carroll BJ, et al. Laparoscopic colectomy. Ann Surg 1992;216:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kitano S, Inomata M, Mizusawa J, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 2017;2:261. [DOI] [PubMed] [Google Scholar]
- [12].Buunen MR, Hop W, Kuhry E, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44. [DOI] [PubMed] [Google Scholar]
- [13].Lacy AM, Delgado S, Castells A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 2008;248:1. [DOI] [PubMed] [Google Scholar]
- [14].Hazey JW, Melvin WS. Robot-assisted general surgery. Semin Laparosc Surg 2004;11:107–12. [DOI] [PubMed] [Google Scholar]
- [15].Ruurda JP, Draaisma WA, Van Hillegersberg R, et al. Robot-assisted endoscopic surgery: a four-year single-center experience. Dig Surg 2005;22:313–20. [DOI] [PubMed] [Google Scholar]
- [16].Kim MJ, Park SC, Park JW, et al. Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg 2018;267:243–51. [DOI] [PubMed] [Google Scholar]
- [17].Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Currie AC, Malietzis G, Jenkins JT, et al. Network meta-analysis of protocol-driven care and laparoscopic surgery for colorectal cancer. Br J Surg 2016;103:1783. [DOI] [PubMed] [Google Scholar]
- [19].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.2. The Cochrane Collaboration 2009; Available from www.cochrane-handbook.org. [Google Scholar]
- [21].Hillege H, Brock Bd, Valkenhoef Gv, Zhao J. ADDIS: an automated way to do network meta-analysis: University of Groningen, Research Institute SOM (Systems, Organisations and Management) 2012. [Google Scholar]
- [22].Van Valkenhoef G, Tervonen T, Zwinkels T, et al. ADDIS: a decision support system for evidence-based medicine. Decis Support Syst 2013;55:459–75. [Google Scholar]
- [23].Braga M, Frasson M, Vignali A, et al. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum 2007;50:464–71. [DOI] [PubMed] [Google Scholar]
- [24].Curet MJ, Putrakul K, Pitcher DE, et al. Laparoscopically assisted colon resection for colon carcinoma: perioperative results and long-term outcome. Surg Endosc 2000;14:1062–6. [DOI] [PubMed] [Google Scholar]
- [25].Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes. JAMA 2015;314:1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic versus open resection for colorectal liver metastases. Ann Surg 2017;1. [DOI] [PubMed] [Google Scholar]
- [27].Gong J. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol 2012;18:7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718–26. [DOI] [PubMed] [Google Scholar]
- [29].Hasegawa H, Kabeshima Y, Watanabe M, et al. Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg Endosc 2003;17:636–40. [DOI] [PubMed] [Google Scholar]
- [30].Hewett PJ, Allardyce RA, Bagshaw PF, et al. Short-term outcomes of the australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer. Ann Surg 2008;248:728–38. [DOI] [PubMed] [Google Scholar]
- [31].Kim MJ, Park SC, Park JW, et al. Robot-assisted versus laparoscopic surgery for rectal cancer. Ann Surg 2017;267:243–51. [DOI] [PubMed] [Google Scholar]
- [32].Kitano S, Inomata M, Mizusawa J, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 2017;2:261–8. [DOI] [PubMed] [Google Scholar]
- [33].Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224–9. [DOI] [PubMed] [Google Scholar]
- [34].Leung KL, Kwok SPY, Lam SCW, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 2004;363:1187–92. [DOI] [PubMed] [Google Scholar]
- [35].Li JC-M, Leung KL, Ng SS-M, et al. Laparoscopic-assisted versus open resection of right-sided colonic cancer – a prospective randomized controlled trial. Int J Colorectal Dis 2011;27:95–102. [DOI] [PubMed] [Google Scholar]
- [36].Liang J-T, Huang K-C, Lai H-S, et al. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol 2006;14:109–17. [DOI] [PubMed] [Google Scholar]
- [37].Liang X, Hou S, Liu H, et al. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A 2011;21:381–5. [DOI] [PubMed] [Google Scholar]
- [38].Lujan J, Valero G, Hernandez Q, et al. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg 2009;96:982–9. [DOI] [PubMed] [Google Scholar]
- [39].Neudecker J, Klein F, Bittner R, et al. Short-term outcomes from a prospective randomized trial comparing laparoscopic and open surgery for colorectal cancer. Br J Surg 2009;96:1458–67. [DOI] [PubMed] [Google Scholar]
- [40].Ng SSM, Lee JFY, Yiu RYC, et al. Laparoscopic-assisted versus open total mesorectal excision with anal sphincter preservation for mid and low rectal cancer: a prospective, randomized trial. Surg Endosc 2013;28:297–306. [DOI] [PubMed] [Google Scholar]
- [41].Ng SSM, Leung KL, Lee JFY, et al. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol 2008;15:2418–25. [DOI] [PubMed] [Google Scholar]
- [42].Park JS, Choi GS, Park SY, et al. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 2012;99:1219–26. [DOI] [PubMed] [Google Scholar]
- [43].Pascual M, Alonso S, Parés D, et al. Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer. Br J Surg 2011;98:50–9. [DOI] [PubMed] [Google Scholar]
- [44].Pecorelli N, Amodeo S, Frasson M, et al. Ten-year outcomes following laparoscopic colorectal resection: results of a randomized controlled trial. Int J Colorectal Dis 2016;31:1283–90. [DOI] [PubMed] [Google Scholar]
- [45].Schietroma M, Pessia B, Carlei F, et al. Laparoscopic versus open colorectal surgery for colon cancer: the effect of surgical trauma on the bacterial translocation. A prospective randomized study. Am J Surg 2015;210:263–9. [DOI] [PubMed] [Google Scholar]
- [46].Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer. JAMA 2015;314:1356. [DOI] [PubMed] [Google Scholar]
- [47].van der Pas MHGM, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–8. [DOI] [PubMed] [Google Scholar]
- [48].Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery. Ann Surg 2011;254:868–75. [DOI] [PubMed] [Google Scholar]
- [49].Xu D, Li J, Song Y, et al. Laparoscopic surgery contributes more to nutritional and immunologic recovery than fast-track care in colorectal cancer. World J Surg Oncol 2015;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yamamoto S, Inomata M, Katayama H, et al. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer. Ann Surg 2014;260:23–30. [DOI] [PubMed] [Google Scholar]
- [51].Zhou ZG, Hu M, Li Y, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 2004;18:1211–5. [DOI] [PubMed] [Google Scholar]
- [52].Araujo SE, Da SEAJ, de Campos FG, et al. Conventional approach x laparoscopic abdominoperineal resection for rectal cancer treatment after neoadjuvant chemoradiation: results of a prospective randomized trial. Clinics 2003;58:133–40. [DOI] [PubMed] [Google Scholar]
- [53].Bland KI. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report - Invited commentary. J Am Coll Surgeons 1998;187:54–154. [DOI] [PubMed] [Google Scholar]
- [54].Fujii S, Ishibe A, Ota M, et al. Short-term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surg Endosc 2014;28:466. [DOI] [PubMed] [Google Scholar]
- [55].Hewitt PM, Ip SM, Kwok SPY, et al. Laparoscopic-assisted vs. open surgery for colorectal cancer. Dis Colon Rectum 1998;41:901. [DOI] [PubMed] [Google Scholar]
- [56].Kaiser AM, Kang JC, Chan LS, et al. Laparoscopic-assisted vs. open colectomy for colon cancer: a prospective randomized trial. J Laparoendosc Adv Surg Tech A 2004;14:329. [DOI] [PubMed] [Google Scholar]
- [57].Kang SB, Ji WP, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637–45. [DOI] [PubMed] [Google Scholar]
- [58].Keats AS. A comparison of laparoscopically assisted and open colectomy for colon cancer. New Engl J Med 2004;350:2050. [DOI] [PubMed] [Google Scholar]
- [59].Liu FL, Lin JJ, Ye F, et al. Hand-assisted laparoscopic surgery versus the open approach in curative resection of rectal cancer. J Int Med Res 2010;38:916–22. [DOI] [PubMed] [Google Scholar]
- [60].Rodríguez RMJ, Pavón JMD, Juan FDLPD, et al. [Prospective randomised study: robotic-assisted versus conventional laparoscopic surgery in colorectal cancer resection]. Cir Esp 2011;89:432–8. [DOI] [PubMed] [Google Scholar]
- [61].Schwenk W, Böhm B, Haase O, et al. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg 1998;383:49. [DOI] [PubMed] [Google Scholar]
- [62].Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477. [DOI] [PubMed] [Google Scholar]
- [63].Weber PA, Merola S, Wasielewski A, et al. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 2002;45:1689–94. [DOI] [PubMed] [Google Scholar]
- [64].Park JS, Choi GS, Lim KH, et al. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 2010;17:3195. [DOI] [PubMed] [Google Scholar]
- [65].Pigazzi A, Ellenhorn JD, Ballantyne GH, et al. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 2006;20:1521–5. [DOI] [PubMed] [Google Scholar]
- [66].Baik SH, Kwon HY, Jin SK, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 2009;16:1480. [DOI] [PubMed] [Google Scholar]
- [67].Rodríguez RMJ, Pavón JMD, Juan FDLPD, et al. [Prospective randomised study: robotic-assisted versus conventional laparoscopic surgery in colorectal cancer resection]. Cir Esp 2011;89:432. [DOI] [PubMed] [Google Scholar]
- [68].AL d, LM P, JJ P, et al. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 2010;53:1000. [DOI] [PubMed] [Google Scholar]
- [69].Rawlings AL, Woodland JH, Vegunta RK, et al. Robotic versus laparoscopic colectomy. Surg Endosc 2007;21:1701–8. [DOI] [PubMed] [Google Scholar]


