Abstract
White matter hyperintensities (WMHs), which are common in elderly people and contribute to age-related disability, can coexist with cardiac injury. It remains unclear whether cardiac biomarkers are associated with WMHs.
To investigate this question, we prospectively recruited patients with cardioembolic stroke due to atrial fibrillation (AF) and/or rheumatic heart disease (RHD). Four cardiac biomarkers were measured: myoglobin, high-sensitivity cardiac troponin T (hs-cTnT), creatine kinase-MB, and terminal pro-brain natriuretic peptide. WMHs in periventricular and deep white matter were assessed separately.
In the entire sample of 171 patients, 120 (70.2%) presented with WMHs, of whom 18 (10.5%) presented with moderate to severe deep white matter hyperintensities (DWMH) and 55 (32.2%) presented with moderate to severe periventricular hyperintensities (PVH). Risk of moderate to severe PVH, after adjusting for confounders, was 2.460-fold higher in patients with high myoglobin levels than in those with low levels, and the risk was 2.608-fold higher in patients with high hs-cTnT levels than in those with low levels. There were no significant associations between any of the 4 cardiac biomarkers and moderate to severe DWMH.
This prospective observational study provides new evidence of the potential relationship of cardiac biomarkers with WMHs in patients with cardioembolic stroke due to AF and/or RHD. We found that elevated myoglobin levels and high hs-TnT levels were independently associated with the presence of moderate to severe PVH. Further studies are required to test our findings and explore whether cardiac biomarkers contribute directly to WMHs pathogenesis.
Keywords: atrial fibrillation, cardiac biomarker, cardioembolic stroke, rheumatic heart disease, white matter hyperintensities
1. Introduction
White matter hyperintensities (WMHs), which are characterized by bilateral and mostly symmetrical hyperintensities on T2-weighted and fluid-attenuated inversion recovery (FLAIR) imaging, are ischemic manifestations of cerebral small vessel diseases.[1] WMHs are common in elderly people and tend to be more extensive and frequent in patients with cerebrovascular diseases, such as stroke patients.[2–5] The Rotterdam Scan Study reported that 95% of participants aged 60 to 90 years presented with WMHs, and that white matter lesion load increased steadily with age.[6] Accumulating evidence suggests that WMHs, especially moderate to severe WMHs, are strongly associated with stroke, cognitive disorders, and age-related disability such as gait and mood disturbances.[7–9] Nevertheless, how WMHs form remains unclear and may be multifactorial.[7]
Cerebrovascular and cardiovascular diseases often share risk factors, such as hypertension and aging,[10–12] so it is conceivable that WMHs coexist with cardiac injury.[13] This raises the question of whether cardiac biomarkers such as amino terminal pro-brain natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT), which are usually used to diagnose chest pain and heart failure related symptoms,[14,15] may also be useful predictors of WMHs or even factors that contribute to WMH pathogenesis. In support of this idea, a community-based study reported that levels of NT-proBNP and hs-cTnT are independently associated with WMHs defined by silent magnetic resonance imaging (MRI),[16] and levels of BNP have been shown to correlate with WMHs in patients with atrial fibrillation (AF) or type 2 diabetes.[17,18] The intriguing results of these studies with 1 or 2 cardiac biomarkers need to be confirmed and extended in more systematic work covering a panel of biomarkers.
Therefore, the present study assessed potential relationships of these cardiac biomarkers and 2 additional ones [myoglobin and creatine kinase-MB (CK-MB)] with risk of WMHs. We examined these relationships in patients with cardioembolic stroke due to AF and/or rheumatic heart disease (RHD), because our previous study found that WMHs can increase risk of hemorrhagic transformation in this population,[19] which can limit the efficacy of oral anticoagulant and thrombolytic therapies.
2. Methods
2.1. Study population
Data on the study population came from the Chengdu Stroke Registry Database, which prospectively collected detailed information on consecutive ischemic stroke patients admitted to the Department of Neurology of West China Hospital (Chengdu, China) since March 2002.[20] This study was approved by the Biomedical Research Ethics Committee of West China Hospital, and the protocol conformed to local ethics criteria for human research. Informed content was obtained from all patients or their guardians.
We enrolled patients with cardioembolic stroke due to AF and/or RHD in the Registry from January 2014 to May 2016. To assess cardioembolic stroke according to the Trial of Org 10172 in acute stroke treatment (TOAST) classification,[21] all patients in our study underwent comprehensive examinations, including brain nonenhanced computed tomography (CT) and MRI scans, imaging of intracranial and extracranial arteries (by MR angiography, CT angiography, or digital subtraction angiography), carotid ultrasound, as well as electrocardiography and echocardiography during hospitalization. To be included, patients experienced ischemic stroke confirmed by brain CT or MRI; had a history of paroxysmal or persistent AF and/or RHD; underwent biochemical blood tests, including myoglobin, hs-cTnT, CK-MB mass, and NT-proBNP within 48 hours of stroke onset; and underwent head MRI scans within 7 days after admission. Whether a patient was assayed for cardiac biomarkers depended on the discretion of the attending physician and consent from the patient or guardian. AF was diagnosed on the basis of electrocardiography and/or 24-hour electrocardiography. RHD was diagnosed according to the criteria of the International Classification of Diseases (10th edition) and confirmed by echocardiography.[22] Patients were excluded if they had stroke due to brain trauma or neoplasm, coma attributable to metabolic disorders or disorders of fluid or electrolyte balance, vasculitis involving the brain or central nervous system infections. Patients who had potential large-artery atherosclerotic sources of thrombosis or embolism were also excluded.
2.2. Data collection and measurements of cardiac biomarkers
Baseline information was collected on age, sex, previous medical history (hypertension, diabetes mellitus, hyperlipidemia, and stroke), therapies before admission, smoking, alcohol consumption, stroke severity, blood pressure on admission, and laboratory tests. Hypertension was defined as current antihypertensive treatment, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Diabetes mellitus was defined as current use of antidiabetic agents or a fasting serum glucose level ≥7.0 mmol/L. Hyperlipidemia was defined as current use of lipid-lowering agents, total cholesterol >6.0 mmol/L, or low-density lipoprotein cholesterol >4.14 mmol/L. Stroke severity was assessed using the National Institutes of Health Stroke Scale and Glasgow Coma Scale. Laboratory data at the first visit included platelet count, blood glucose, international normalized ratio, serum urea, and serum creatinine levels.
All the blood samples in this study were collected within 48 hours of stroke onset and sent to the laboratory to perform the tests in 2 hours. The median time of blood sample collection was 10 hours of onset [interquartile range (IQR) 2–39 hours]. The cardiac biomarkers were tested in the Department of Laboratory Medicine in our hospital. Plasma myoglobin was measured using a microparticle enzyme immunoassay (AxSYM system; Abbot Laboratories, Perth, Australia). Plasma hs-cTnT and CK-MB mass were measured using the Accsee II microparticle chemiluminescence immunoassay (Beckman, Brea, CA). Plasma NT-proBNP was analyzed using an Elecsys 2010 electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany).
2.3. MRI scan and WMH assessment
All MRI examinations were made on a 3-T Siemens scanner, including sequences of axial T1-weighted images (repetition time, 1600 ms; echo time, 8.6 ms), T2-weighted images (repetition time, 4500 ms; echo time, 105 ms), and FLAIR images (repetition time, 6000 ms; echo time, 100 ms). Slice thickness was 5 mm and matrix size was 256 × 256 pixels.
WMHs were defined as the presence of hyperintense lesions on both FLAIR and T2-weighted images, without prominent hypointensities on the T1-weighted image.[7] WMH severity was assessed using the Fazekas scale, which is easy to apply even for inexperienced researchers and has been validated histopathologically.[23] The Fazekas scale classified WMHs as being either in the periventricular or deep white matter.[24] Periventricular hyperintensities (PVH) were graded as absent (grade 0), cap (grade 1), smooth halo (grade 2), or irregular and extending into the subcortical white matter (grade 3), while deep white matter hyperintensities (DWMH) were graded as absent (grade 0), punctate foci (grade 1), early-confluent (grade 2), or confluent (grade 3). As mild WMHs, but not moderate to severe WMHs, can be regarded as nearly normal brain changes in elderly,[9] we focused on the presence of moderate to severe WMHs, defined as having a score ≧2. Two trained neurologists blinded to the clinical data independently examined all images in order to determine WMH severity. Inter-rater reliability was assessed using the interclass correlation coefficient, which was 0.853 for PVH and 0.870 for DWMH.
2.4. Statistical analysis
Results were reported as percentages, mean ± SD, median with IQR, or odds ratio (OR) with 95% confidence interval (95% CI). Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY), and statistical tests were 2-sided. The χ2 test or Fisher exact test was performed to compare categorical data between groups. Student t test or the Mann–Whitney U test was performed to compare continuous data between groups. Binary logistic regression was used to investigate potential associations between cardiac biomarkers and WMH severity. Levels of myoglobin, hs-cTnT, CK-MB mass, and NT-proBNP were dichotomized on the basis of the median values of the whole cohort, rather than on normal reference values taken from healthy populations, similar to the approach in several studies.[16,25,26] This provided a more accurate assessment of our specific patient population, which presented characteristics substantially different from those of typical reference populations. For example, the plasma NT-proBNP level in our entire cohort was 1457 pg/mL (IQR 891–2621 pg/mL), much higher than the reference range usually used at our hospital (0–227 pg/mL).We chose to dichotomize these biomarker levels rather than divide them into tertiles or quartiles because this would have created much smaller subgroups, substantially reducing statistical power and reliability. Variables with P < .10 in the univariate analysis were added to the multivariate models. For multivariate analysis, the significance level was set at P < .05.
3. Results
3.1. Baseline characteristics of subjects
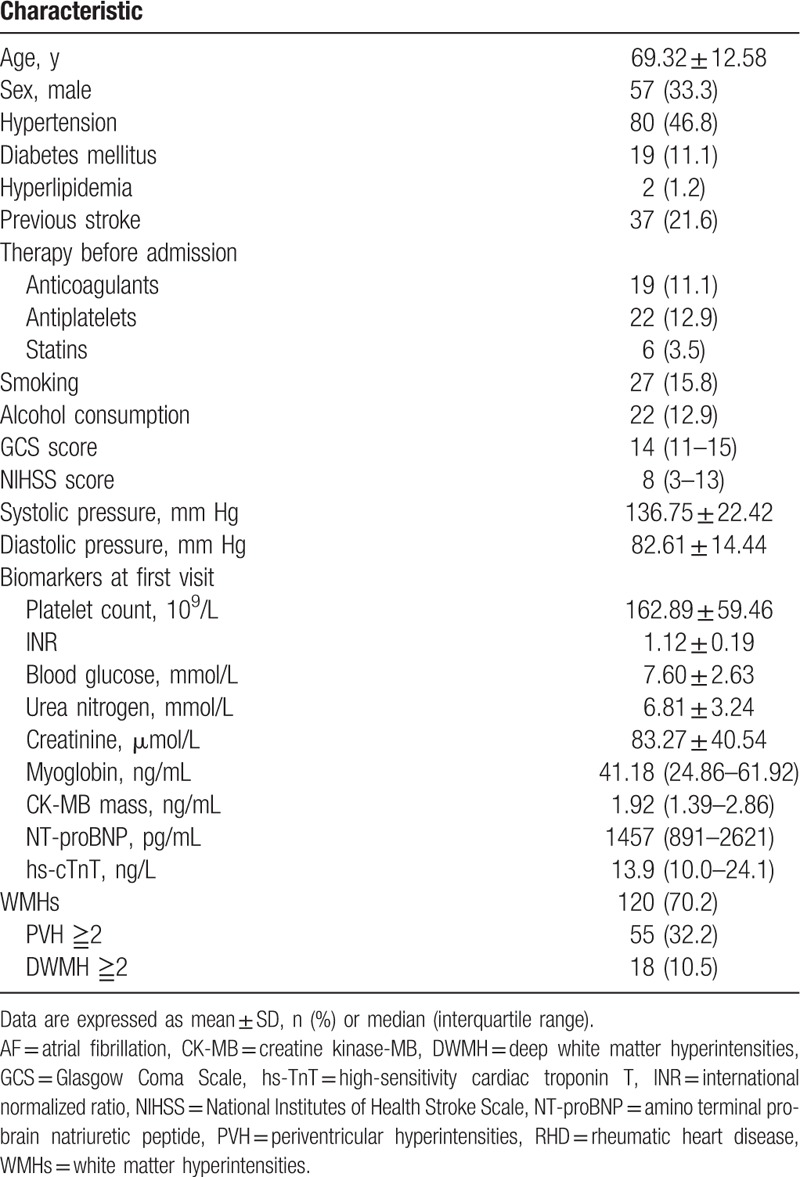
During the study period, 192 cardioembolic stroke patients due to AF and/or RHD underwent MRI scans within 7 days of admission, but 21 were excluded because of the absence of data on levels of plasma myoglobin, hs-cTnT, CK-MB, or NT-proBNP within 48 hours of stroke onset. A total of 171 patients were finally included, of whom 143 had only AF, 8 only RHD, and 20 both conditions. Mean age (±SD) of the whole cohort was 69.32 ± 12.58 years, and 57 (33.3%) patients were male (Table 1). All subjects in the final cohort underwent echocardiography, which gave the following median results (with IQR): ejection fraction, 63% (59–68%); left ventricular wall thickness, 9 mm (8–10 mm); left ventricular end-diastolic diameter, 45 mm (43–48 mm); and end-diastolic volume, 96 mL (81.5–113 mL). The median ratio of mitral peak velocity to annular velocity based on tissue Doppler imaging during early diastolic flow (E/e’) was 13.79 (IQR 11.03–17.82). The median time of the baseline MRI scan was 4 days after admission (IQR 3–6 days). WMHs were detected in 120 (70.2%) patients, of whom 18 (10.5%) presented with moderate to severe DWMH and 55 (32.2%) presented with moderate to severe PVH. Among patients with PVH, 41 (24.0%) had moderate or severe PVH alone, and 14 (8.2%) had moderate or severe PVH as well as DWMH.
Table 1.
Baseline characteristics of patients with cardioembolic stroke due to AF/RHD (n = 171).
3.2. Predictors of moderate to severe PVH or DWMH
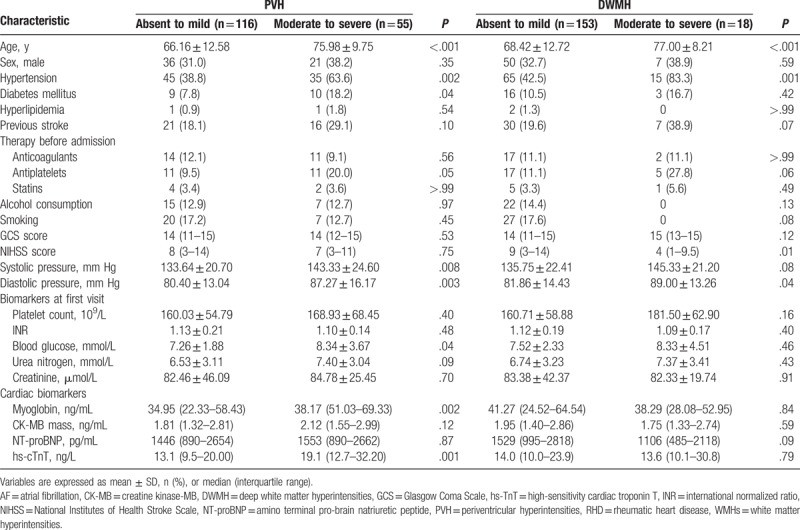
Patients with moderate to severe PVH were more likely to be older, have higher blood pressure and blood glucose on admission, and to have history of hypertension and diabetes mellitus than those without PVH (all P < .05, Table 2). Patients with moderate to severe DWMH were older than those without DWMH (77.00 ± 8.21 vs 68.42 ± 12.72 years, P < .001), had higher diastolic pressure (89.00 ± 13.26 vs 81.86 ± 14.43 mm Hg, P = .04), and were more likely to have a history of hypertension (83.3% vs 42.5%, P = .001).
Table 2.
Univariate analysis to identify predictors of moderate to severe PVH or moderate to severe DWMH in patients with cardioembolic stroke due to AF and/or RHD.
3.3. Relationships between cardiac biomarkers and WMHs
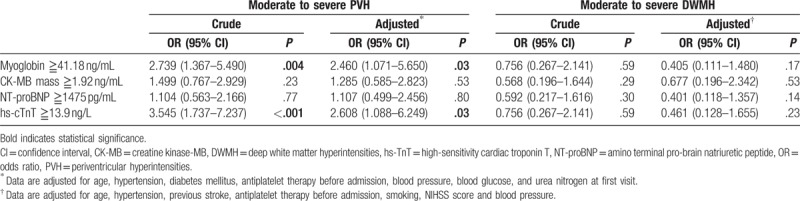
Patients were divided into subgroups having “high” or “low” values of the 4 cardiac biomarkers based on the median values in the entire cohort. Univariate analysis showed that high myoglobin levels (≧41.18 ng/mL) were associated with moderate to severe PVH (OR 2.739, 95% CI 1.367–5.490, P = .004; Table 3). The same was observed for high hs-cTnT levels (≧13.9 ng/L) (OR 3.545, 95% CI 1.737–7.237, P < .001). In contrast, neither CK-MB mass nor NT-proBNP was associated with moderate to severe PVH. The potential risk factors for moderate to severe PVH from univariate analysis were entered into multivariate analysis, in which the following potential confounders were controlled: age, hypertension, diabetes mellitus, antiplatelet therapy before admission, blood pressure, blood glucose, and urea nitrogen at first visit. This multivariate analysis indicated that the risk of moderate to severe PVH was 2.460-fold higher in patients with high myoglobin levels (≧41.18 ng/mL) than in patients with low levels (P = .03, Table 3). Similarly, risk was 2.608-fold higher in patients with high hs-cTnT levels (≧13.9 ng/L) than those with low levels (P = .03). Neither uni- nor multivariate analysis indicated significant associations between any of the 4 cardiac biomarkers and moderate to severe DWMH.
Table 3.
Relationship between cardiac biomarkers and WMHs: binary logistic regression with OR and 95% CI.
4. Discussion
In this prospective observational study, we investigated the possible relationship of cardiac biomarkers with WMHs in cardioembolic stroke patients due to AF and/or RHD. Remarkably, high myoglobin levels (≧41.18 ng/mL) and high hs-cTnT levels (≧13.9 ng/L) were independently associated with moderate to severe PVH. No significant correlation was found between any of the 4 cardiac biomarkers and moderate to severe DWMH.
Previous work showed that hs-cTnT positively correlated with WMHs defined by silent MRI in a community population, in line with our findings.[16] This suggests that hs-TnT contributes to the development of WMHs. We did not observe a significant association between NT-proBNP and WMHs, which contrasts with previous work reporting that BNP is independently associated with WMHs.[16–18] This discrepancy may reflect different study populations. Our study subjects had already suffered stroke, and so their vascular condition was likely to be worse than that of patients with no stroke history, such as those suffering only AF. Another potential explanation for the discrepancy is differences in how WMHs were evaluated. For example, one of the previous studies of BNP assessed WMHs severity using software-automated quantitation of WMHs volumes,[18] whereas we used the semiquantitative Fazekas scale.[24] Further large, preferably multicenter studies are needed to confirm whether BNP is independently associated with WMHs.
We provide new evidence of relationships of myoglobin and hs-cTnT with moderate to severe PVH. It is unclear why elevated levels of these 2 cardiac biomarkers should correlate with WMHs in patients with cardioembolic stroke due to AF and/or RHD. Myoglobin and hs-TnT are preferred biomarkers for diagnosing myocardial infarction.[15] Elevated levels of myoglobin and hs-TnT may reflect cerebral hypoperfusion due to reduced cardiac output, a dysfunctional left ventricle, or other processes due to progressive cardiovascular diseases.[16] Considering that chronic hypoperfusion is important for the pathogenesis of WMHs,[5] it is plausible that moderate to severe WMHs and elevated levels of these 2 cardiac biomarkers occur simultaneously. Indeed, indirect evidence that elevated myoglobin levels and high hs-cTnT levels can coexist with WMHs is the fact that atherosclerosis, which can damage the myocardium, is a pathological feature of WMHs.[1,27] Prospective, long-term studies should aim to clarify when myoglobin and hs-TnT levels become elevated relative to onset of WMHs as a first step to establishing potential causal relationships.
Interestingly, we did not identify significant associations between any of the 4 cardiac biomarkers and DWMH. This may reflect different pathogenesis of PVH and DWMH. PVH are associated with cerebral hypoperfusion due to ischemia in an arterial border zone as well as to diminished cerebral vasomotor reactivity.[28] DWMH are thought to reflect microangiopathy.[29] Cerebral hypoperfusion may be more likely to cause PVH than DWMH. Future work should explore these pathogenetic differences in greater detail and attempt to correlate them with cardiac biomarkers.
This study adds to a growing literature establishing a close relationship between cerebral ischemic lesions and heart disease, especially myocardial infarction.[12,13,30,31] It may be time to consider a more unified clinical approach to treating and managing patients with cerebrovascular disease. It may be appropriate to routinely examine such patients by assaying levels of cardiac biomarkers and by performing electro- and echocardiography, and, when necessary, cardiac MRI, which may be superior to echocardiography for detecting previous myocardial infarction.[32] Cardiac MRI can provide more accurate information on biventricular function and tissue status, helping to establish whether cardiovascular disease contributes to cerebrovascular disease. In the future, the combination of brain and cardiac MRI may be useful for evaluating risk of brain ischemia, guiding the selection of anticoagulant or blood-thinning therapy, and exploring the mechanisms behind cardioembolic stroke, such as unstable hemodynamics. Recently, however, given the expense of cardiac MRI and the technical difficulties of investigating a rapidly moving organ during breathing, assay of cardiac biomarkers may be a reasonable, cost-effective alternative.
Several limitations must be considered in interpreting our findings. First, we enrolled patients with cardioembolic stroke due to AF and/or RHD, which was a relatively small, highly specific population. This may limit the generalizability of our conclusions. Second, we analyzed all patients as a single group, rather than stratifying them on the basis of RHD. We decided not to perform such subgroup analysis because only 28 patients in our cohort had RHD and only 8 had RHD without AF. Therefore, any subgroup analysis would have involved extremely small groups, increasing the risk of unreliable results and inadequate statistical power. Future work should verify and extend our findings in patients with and without RHD. Third, this study relied on a single blood sample, preventing us from assessing potential relationships between fluctuations in cardiac biomarkers and WMHs during hospitalization and after discharge. Finally, our study design allows us to suggest associations between cardiac biomarkers and WMHs, but not to detect causal relationships. Further work is needed to explore whether any of these biomarkers, particularly myoglobin and hs-cTnT, directly participates in WMH pathogenesis.
5. Conclusion
This prospective observational study provides new evidence of the potential relationship of cardiac biomarkers with WMHs in patients with cardioembolic stroke due to AF and/or RHD. We found that elevated myoglobin levels and high hs-TnT levels were independently associated with the presence of moderate to severe PVH. Further studies are required to verify and extent our findings in a larger cohort of patients with other stroke types, and elucidate the exact mechanisms underlying the relationship between cardiac biomarkers and WMHs.
Author contributions
C.W., S.Z., and M.L. conceived and designed the study; C.W. and J.L. collected the data and interpreted the neuroimaging; C.W., S.Z., and R.Y. performed the statistical analysis; C.W. drafted the manuscript; S.Z. contributed to critical revision of the manuscript. All authors reviewed and approved the submitted manuscript.
Conceptualization: Chenchen Wei, Shuting Zhang, Ming Liu.
Data curation: Chenchen Wei.
Formal analysis: Shuting Zhang.
Funding acquisition: Shuting Zhang, Ming Liu.
Investigation: Chenchen Wei, Junfeng Liu.
Methodology: Chenchen Wei, Junfeng Liu, Ruozhen Yuan.
Project administration: Ming Liu.
Software: Ruozhen Yuan.
Supervision: Ming Liu.
Writing – original draft: Chenchen Wei.
Writing – review & editing: Shuting Zhang.
Footnotes
Abbreviations: AF = atrial fibrillation, CI = confidence interval, CK-MB = creatine kinase-MB, CT = computed tomography, DWMH = deep white matter hyperintensities, FLAIR = fluid-attenuated inversion recovery, IQR = interquartile range, MRI = magnetic resonance imaging, NT-proBNP = amino terminal pro-brain natriuretic peptide, OR = odds ratio, PVH = periventricular hyperintensities, RHD = rheumatic heart disease, WMHs = white matter hyperintensities.
Funding/Support: This study was sponsored by National Natural Science Foundation of China (81620108009, 81500923) and National Key Research and Development Program of China, Ministry of Science and Technology of China (2016YFC1300500–505).
The authors of this work have nothing to disclose.
The authors report no conflicts of interest.
CW and SZ contributed equally to this work.
References
- [1].Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- [2].Launer LJ. Epidemiology of white matter lesions. Topics Magn Reson Imaging 2004;15:365–7. [DOI] [PubMed] [Google Scholar]
- [3].Vermeer SE, den Heijer T, Koudstaal PJ, et al. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam scan study. Stroke 2003;34:392–6. [DOI] [PubMed] [Google Scholar]
- [4].Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matsusue E, Sugihara S, Fujii S, et al. White matter changes in elderly people: MR-pathologic correlations. Magn Reson Med Sci 2006;5:99–104. [DOI] [PubMed] [Google Scholar]
- [6].de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ikram MA, van der Lugt A, Niessen WJ, et al. The Rotterdam Scan Study: design update 2016 and main findings. Eur J Epidemiol 2015;30:1299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pantoni L. Leukoaraiosis: from an ancient term to an actual marker of poor prognosis. Stroke 2008;39:1401–3. [DOI] [PubMed] [Google Scholar]
- [10].Verhaaren BF, Vernooij MW, de Boer R, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension 2013;61:1354–9. [DOI] [PubMed] [Google Scholar]
- [11].van Dijk EJ, Breteler MM, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 2004;44:625–30. [DOI] [PubMed] [Google Scholar]
- [12].Ikram MA, van Oijen M, de Jong FJ, et al. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008;39:1421–6. [DOI] [PubMed] [Google Scholar]
- [13].Kim BJ, Lee SH, Kim CK, et al. Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ J 2011;75:451–6. [DOI] [PubMed] [Google Scholar]
- [14].Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330–7. [DOI] [PubMed] [Google Scholar]
- [15].Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–53. [DOI] [PubMed] [Google Scholar]
- [16].Dadu RT, Fornage M, Virani SS, et al. Cardiovascular biomarkers and subclinical brain disease in the atherosclerosis risk in communities study. Stroke 2013;44:1803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gartner W, Zierhut B, Mineva I, et al. Brain natriuretic peptide correlates with the extent of atrial fibrillation-associated silent brain lesions. Clin Biochem 2008;41:1434–9. [DOI] [PubMed] [Google Scholar]
- [18].Reinhard H, Garde E, Skimminge A, et al. Plasma NT-proBNP and white matter hyperintensities in type 2 diabetic patients. Cardiovasc Diabetol 2012;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wei CC, Zhang ST, Wang YH, et al. Association between leukoaraiosis and hemorrhagic transformation after cardioembolic stroke due to atrial fibrillation and/or rheumatic heart disease. J Neurol Sci 2017;378:94–9. [DOI] [PubMed] [Google Scholar]
- [20].Wang D, Hao Z, Tao W, et al. Acute ischemic stroke in the very elderly Chinese: risk factors, hospital management and one-year outcome. Clin Neurol Neurosurg 2011;113:442–6. [DOI] [PubMed] [Google Scholar]
- [21].Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [22].Wang D, Liu M, Hao Z, et al. Features of acute ischemic stroke with rheumatic heart disease in a hospitalized Chinese population. Stroke 2012;43:2853–7. [DOI] [PubMed] [Google Scholar]
- [23].Scheltens P, Erkinjunti T, Leys D, et al. White matter changes on CT and MRI: an overview of visual rating scales. European Task Force on Age-Related White Matter Changes. Eur Neurol 1998;39:80–9. [DOI] [PubMed] [Google Scholar]
- [24].Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6. [DOI] [PubMed] [Google Scholar]
- [25].Tan G, Lei C, Hao Z, et al. Liver function may play an uneven role in haemorrhagic transformation for stroke subtypes after acute ischaemic stroke. Eur J Neurol 2016;23:597–604. [DOI] [PubMed] [Google Scholar]
- [26].Cushman M, Callas PW, McClure LA, et al. N-terminal Pro-B-type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. J Alzheimer Dis 2016;54:497–503. [DOI] [PubMed] [Google Scholar]
- [27].White SJ, Newby AC, Johnson TW. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost 2016;115:509–19. [DOI] [PubMed] [Google Scholar]
- [28].Gerdes VE, Kwa VI, ten Cate H, et al. Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis 2006;186:166–72. [DOI] [PubMed] [Google Scholar]
- [29].Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–9. [DOI] [PubMed] [Google Scholar]
- [30].Scheitz JF, Nolte CH, Laufs U, et al. Application and interpretation of high-sensitivity cardiac troponin assays in patients with acute ischemic stroke. Stroke 2015;46:1132–40. [DOI] [PubMed] [Google Scholar]
- [31].de Leeuw FE, de Groot JC, Oudkerk M, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology 2000;54:1795–801. [DOI] [PubMed] [Google Scholar]
- [32].Haeusler KG, Wollboldt C, Bentheim LZ, et al. Feasibility and diagnostic value of cardiovascular magnetic resonance imaging after acute ischemic stroke of undetermined origin. Stroke 2017;48:1241–7. [DOI] [PubMed] [Google Scholar]


