Abstract
The long-term survival benefit of treating unresectable hepatocellular carcinoma (HCC) patients with transarterial chemoembolization (TACE) rather than conservative treatment remains controversial. This retrospective case-control study evaluated the survival of patients with unresectable HCC treated with TACE, relative to that of patients who received best supportive care.
From January 2002 to December 2010, 522 of 2386 consecutive patients with unresectable HCC were enrolled. Patients were treated with TACE (n = 347) or best supportive care (non-TACE; n = 175). A survival analysis compared the survival of the 2 groups, as well as only those at Barcelona Clinic Liver Cancer Classification (BCLC)-C and Child-Pugh-B (39 TACE, 61 non-TACE).The median follow-up was 5 months (0.15–106 months).
The overall median survival of the TACE group (8.0 months) was significantly longer than that of the non-TACE (2.0 months; P ≤ .01). Of the patients at BCLC-C and Child-Pugh-B, the overall median survivals of the TACE and non-TACE patients were 6.0 and 2.0 months, respectively (P ≤ .01); and the 1, 2, 3, 5, and 8-year overall survival rates were significantly superior in the TACE group (P ≤ .01). For all the patients, the independent predictors of survival were treatment modalities, portal vein tumor thrombosis, alpha-fetoprotein, and BCLC stage. Regarding the TACE patients, contributors to prognosis were portal vein tumor thrombosis, alpha-fetoprotein level, and the number of TACE procedures.
TACE for unresectable HCC was associated with longer survival compared with best supportive care, especially for patients at BCLC-C and Child-Pugh-B.
Keywords: conservative treatment, hepatocellular carcinoma, prognosis, survival, transarterial chemoembolization
1. Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide and the third leading cause of cancer-related mortality in China.[1,2] The HCC prognosis is very poor, most likely because of late-stage diagnosis and limited access to timely and standard treatments.[3,4] About 80% to 90% of patients with HCC at diagnosis are considered not eligible for any of the available curative treatments, especially liver resection or transplantation. In this setting, the treatment modalities have not been standardized.[3,4] For these patients, the available treatment options are transarterial chemoembolization (TACE), radiofrequency ablation, hepatic arterial infusion chemotherapy, sorafenib, and best supportive care. In actual practice, the choice among these is determined by the clinical situation.[5]
TACE has been proposed for a long time as the criterion standard for palliative treatment of unresectable HCC,[6,7] and has been reported to improve the survival of these patients, compared with supportive treatment.[8,9] According to Barcelona Clinic Liver Cancer (BCLC) tumor staging and management, TACE is recommended as the first-line therapy for unresectable intermediate stage HCC (stage B).[10] Although BCLC guidelines have been extensively validated, a heterogeneous population of patients with intermediate-stage HCC or with advanced-stage HCC has consistently raised issues in clinical practice. Because multiple variables affect the clinical course of HCC,[11] the number of studies comparing TACE to conservative management remains limited.[12,13]
These data prompted us to analyze retrospectively the long-term follow-up of 522 consecutive HCC patients. This study compared the overall survival and survival rates of patients with unresectable HCC who were given either TACE or best supportive care, and assessed the factors that influenced the overall survival of these patients.
2. Materials and methods
The Ethics Committee of the Fourth Hospital of Hebei Medical University approved the study (No. 2014MEC020), which was registered in the Chinese Clinical Trial Registry (ChiCTR-IPC-14005432). Written consent was obtained from each patient.
2.1. Patients
A consecutive cohort of 2386 patients, who were admitted to the Fourth Hospital of Hebei Medical University from January 2002 to December 2010, was investigated. We retrospectively enrolled 522 patients who underwent TACE (n = 347) or conservative management (n = 175) as initial treatment in our hospital. The HCC diagnosis was confirmed by histological or cytological examination or on the basis of the domestic standard criteria of clinical diagnosis and staging[14] (Guangzhou, 2001) and the domestic clinical guidelines (Zhejiang, 2011).[15] Patients were excluded if they had other locoregional or systemic therapy, or previous or current malignancy aside from HCC. The choice of treatment was made at the patients’ request after a full discussion with our multidisciplinary treatment team, which included radiologists, surgeons, hepatologists, and oncologists.
2.2. TACE procedure
TACE was performed using the Seldinger technique. The whole procedure was performed under fluoroscope. A selective catheter was used and visceral angiography was performed to assess the feeding arteries, tumor type and size, tumor number, arterial tumor supply, portal vein tumor thrombus, and arteriovenous fistula. Then the tip of the catheter was advanced into the right or left hepatic artery, or tumor-feeding branches. After safe positioning of the catheter, an emulsion of lipiodol and anticancer agents was infused.
The treatment regimen consisted of iodized oil (10–30 mL), tropisetron (5 mg), and tegafur (1.0 g). Embolization was then performed with injection of absorbable gelatin sponge particles through the angiographic catheter to reach stasis in a tumor-feeding artery. The dose of embolic agents depended on tumor size and blood supply of the hepatic artery. Depending on the patient's situation, 3 kinds of antineoplastic agents were injected into the hepatic artery, among them fluorouracil, doxorubicin, mitomycin, therarubicin, cisplatin, oxaliplatin, or hydroxycamptothecine. The specific dose of antineoplastic agents depended on the patient's body surface area and general condition.
2.3. Conservative management
During the same study period, 175 patients with HCC received best supportive care measures including hepatic protectors, analgesics, Chinese medicine, nutritional support, and management of HCC complications.
2.4. Follow-up
The clinical, laboratory, and radiological data of all the patients were retrospectively reviewed. The survival status and subsequent treatments were followed. The differences in therapeutic efficacy between the 2 groups were evaluated by comparing overall survival and survival rates. Overall survival was a primary endpoint, defined as the time from the date of HCC diagnosis until the date of death from any cause, or the date of the most recent follow-up, and was expressed in months. The follow-up deadline was April 2015.
2.5. Statistical analysis
All statistical analyses were conducted using SPSS 17.0 software (SPSS Inc, Chicago, IL). Continuous variables were compared using Student t test and the χ2 test was used for categorical variables. Survival was calculated with the Kaplan-Meier method and compared statistically using the log-rank test. To identify the independent factors for survival and prognosis, variables that were associated with survival and prognosis in univariate analysis were subsequently included in the multivariate analysis, using the Cox proportional hazard model. A 2-tailed P value <.05 was considered statistically significant.
3. Results
3.1. Patient baseline characteristics
A total of 522 patients were recruited in this study, from January 2002 to December 2010 (Table 1). Of them, 347 patients (66.5%) received TACE treatment (TACE group), and 175 (33.5%) received best supportive care (conservative or non-TACE group). Men were predominate (439, or 84.1%). Patients’ ages ranged from 18 to 94 years.
Table 1.
Baseline characteristics of the entire patient group (n = 522) with unresectable HCC∗.
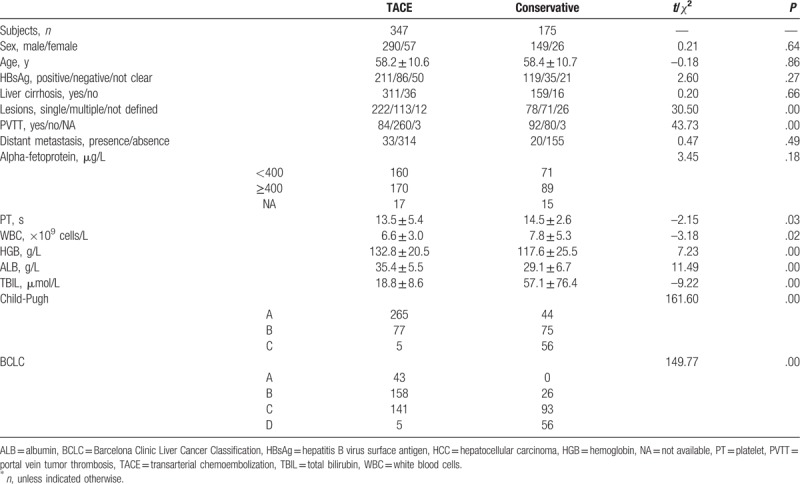
No statistical differences were observed between the 2 groups in terms of general condition, preoperative liver function, tumor features, age, sex, hepatitis B virus infection, presence of cirrhosis, or alpha-fetoprotein (AFP) levels (Table 1). However, some hematological indexes were statistically different between the TACE and non-TACE groups, such as leucocyte count (P < .05) and hemoglobin values (P < .05).
Liver dysfunction was more pronounced in the TACE group, as shown by liver function tests and Child-Pugh scoring. In the TACE group, 265 (76.4%), 77 (22.2%), and 5 (1.4%) were in the Child-Pugh A, B, and C categories, respectively, compared with 44 (25.1%), 75 (42.9%), and 56 (32.0%) in the non-TACE group (P < .05). In the TACE group, 64.0% of patients displayed 1 lesion, compared with 44.6% in the conservative group (P < .05). The ratio of patients with portal vein tumor thrombosis (PVTT) to patients without PVTT was 32.3% in the TACE group, and 115.0% in the non-TACE group (P < .05). According to BCLC,[16] significantly more patients in the TACE group were at BCLC stages B or C (P < .05).
In the TACE group, the mean number of TACE procedures was 2.3 ± 1.8. In all, 155 patients received TACE only once (44.7%), and the other 192 patients received >1 TACE procedure (range, 2–12). TACE was performed every 1 to 3 months.
3.2. Comparison of overall survival in the TACE and conservative groups
The median follow-up duration was 5 months (range, 0.15–106 months), specifically 8 months in the TACE group and 2 months in the conservative group. By the end of the study period, 489 patients (317 TACE, 172 non-TACE) had died. The mortality rates of the TACE and non-TACE groups were 91.4% and 98.3%, respectively. The mean survival periods were 17.3 ± 1.4 months and 5.2 ± 0.7 months (P < .05).
The overall median survival period for the 522 patients was 5.0 months, and was significantly longer in the TACE group (8.0 months) than the conservative (2.0 months; P < .05; Fig. 1). The overall survival rates at 1, 2, 3, 5, and 8 years were each significantly higher in the TACE group than the non-TACE group (P < .05, all). Specifically, in the TACE group, the overall survival rates at 1, 2, 3, 5, and 8 years were 39.4%, 20.1%, 9.5%, 6.9%, and 4.9%, respectively, and in the non-TACE group, the rates were 9.1%, 2.3%, 1.7%, 1.7%, and 1.7%.
Figure 1.
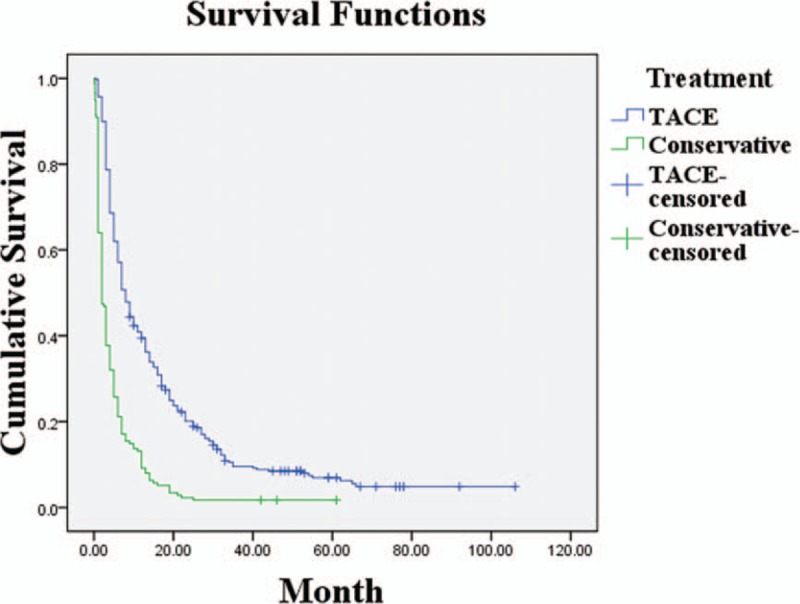
Survival rates of the TACE and conservative treatment groups (P < .05). TACE = transarterial chemoembolization.
3.3. Subgroup analysis of patients with BCLC-C stage and Child-Pugh-B class
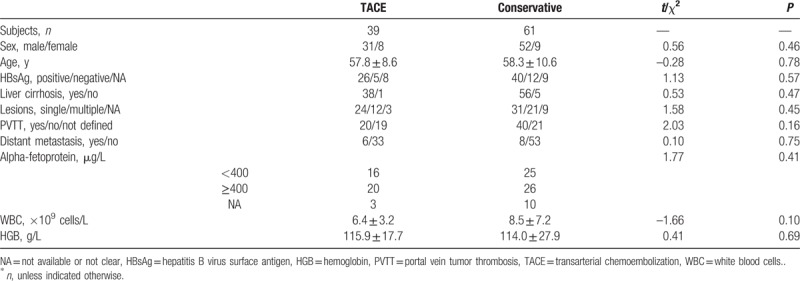
Considering the lack of homogeneity in baseline characteristics of the 522 patients, a subgroup analysis was performed. One hundred consecutive patients with both stage BCLC-C and Child-Pugh-B were enrolled (Table 2). The TACE and non-TACE patients who met this criterion of homogeneity were comparable in all baseline features, including lesion number and presence of PVTT.
Table 2.
Baseline characteristics of the patient subgroup (n = 100)∗.
The mean survival times in this subgroup were 10.7 ± 1.8 months for the TACE patients, and 4.7 ± 0.6 months for the non-TACE (P < .05). The overall median survival of the TACE group was 6.0 months, and that of the non-TACE was 2.0 months (P < .05; Fig. 2). The survival rates at 1, 2, 3, 5, and 8 years were significantly higher in the TACE than the non-TACE group. Specifically, the survival rates of the TACE group at 1, 2, 3, 5, and 8 years were 33.3%, 11.1%, 5.6%, 5.6%, and 5.6%. The survival rates of the non-TACE group at 1 year were 8.2% and 0% after this (Fig. 3).
Figure 2.
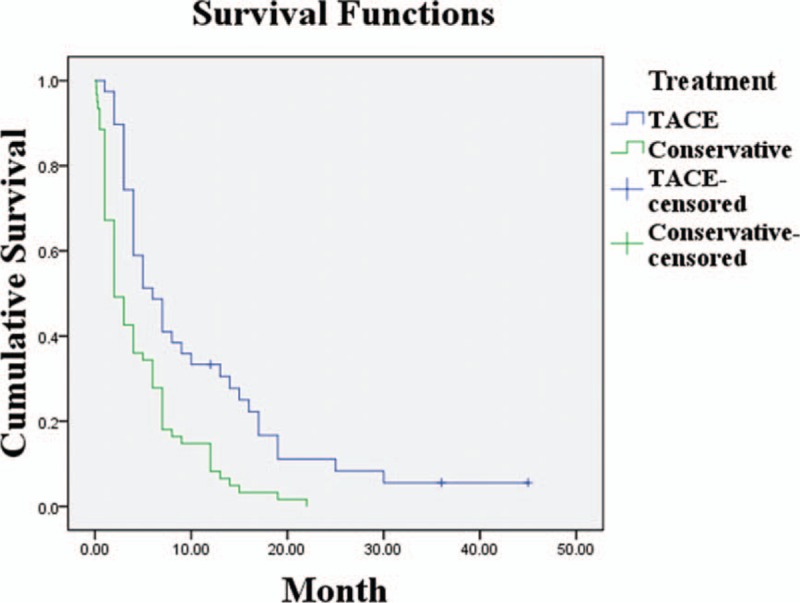
Survival rates of the TACE and conservative treatment groups in 100 patients with unresectable hepatocellular carcinoma of BCLC-C stage and Child-Pugh-B class (P < .05). TACE = transarterial chemoembolization.
Figure 3.
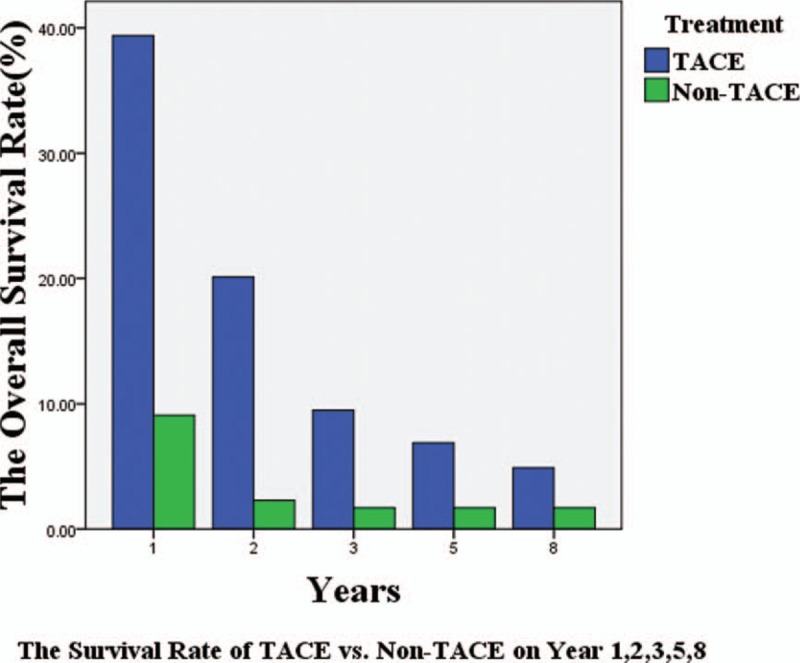
Survival rates of the TACE and non-TACE groups at 1, 2, 3, 5, and 8 years (P < .05). TACE = transarterial chemoembolization.
3.4. Survival and prognostic factors
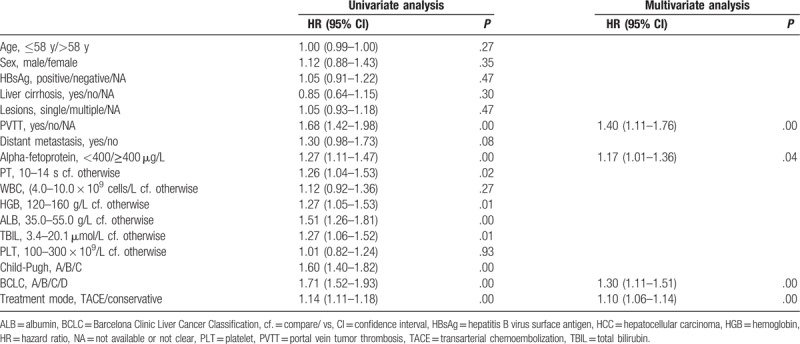
The univariate analysis showed that the following were significantly associated with overall survival: Child-Pugh classification, presence of PVTT, BCLC stage, AFP level, treatment mode, and some hematological indexes (P < .05; Table 2). According to the multivariate analysis, only treatment mode, PVTT, AFP level, and BCLC stage remained as independent predictors of survival (Table 3).
Table 3.
Multivariate analysis of prognostic variables affecting the survival of the HCC patients (n = 522).
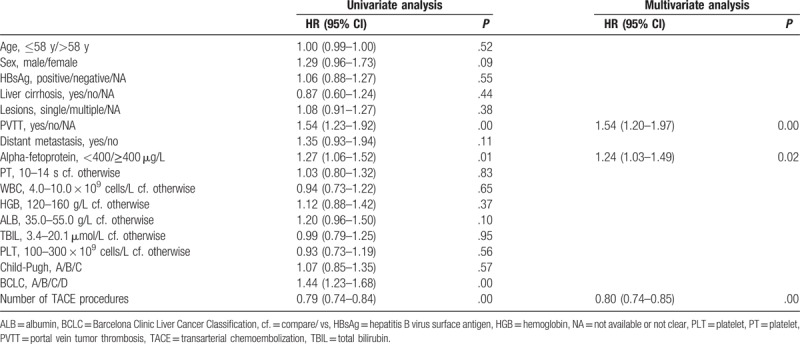
The univariate analysis revealed the following potential prognostic factors in the TACE group: PVTT, AFP levels, BCLC stage, and number of TACE procedures (Table 4). Results of the multivariate analysis indicated that the following were independent factors affecting the efficacy of TACE treatment: portal vein tumor thrombosis, AFP levels, and number of TACE procedures (Table 4).
Table 4.
Multivariate analysis of prognostic variables affecting the survival of patients with TACE treatment (n = 347).
4. Discussion
The annual incidence of HCC in China is estimated to be 50% that of the world.[1] In our experience, a majority of HCC patients do not receive surgical treatment, mostly because of tumor extension, liver dysfunction, or poor general condition. In contrast, TACE has been widely accepted for HCC treatment.[17] We present herein a large-sample retrospective study, evaluating the efficacy of TACE for prolonging survival, compared with best supportive care alone. The results revealed that TACE was an effective treatment option and improved the overall survival for patients with unresectable HCC.
For assessing the efficacy of TACE, the usual evaluated data are imaging, biological response, and quality of life.[5,17,18] However, the ultimate goal of HCC treatment is to prolong survival, and overall survival is therefore the best parameter for evaluating the benefit of TACE. In 2002 in Spain, a randomized controlled trial of patients with unresectable HCC of Child-Pugh class A or B and Okuda stage I or II showed that chemoembolization provided a survival benefit compared with conservative treatment (for death, hazard ratio [HR] = 0.47 [95% confidence interval, CI 0.25–0.91], P = .025). Survival rates at 1 and 2 years were 75% and 50% for embolization, 82% and 63% for chemoembolization, and 63% and 27% for the control (chemoembolization compare/ vs (cf.) control, P = .009).[8] Moreover, a small randomized, controlled trial from Hong Kong, China, reported that the survival benefit of chemoembolization was significant (relative risk of death = 0.49; 95% CI 0.29–0.81; P = .006), with a better actuarial survival (1 year, 57%; 2 years, 31%; 3 years, 26%) than in the control group (1 year, 32%; 2 years, 11%; 3 years, 3%; P = .002).[9] In a recent retrospective comparative study from Guangzhou, China, of a cohort of 287 patients, the 12- and 24-month overall survival rates for the TACE group (18.5% and 2.3%, respectively) were significantly higher than those of the conservative treatment group (12.1% and 0%).[12] However, in the above studies, both the number of patients and the follow-up duration were limited.
Few reports have documented the long-term survival associated with the TACE procedure,[19] and the reported 5-year survival rates of HCC patients after TACE have ranged from 1% to 8%.[19,20] Our present study enrolled 522 patients without previous or current malignancy except for HCC, and included 347 consecutive HCC patients in the TACE group and 175 patients in the conservative group. The longest follow-up was 8.9 years. In agreement with the above studies, TACE treatment offered a survival benefit for patients with unresectable HCC, compared with conservative treatment. The overall median survival was significantly longer in the TACE group, as well as the survival rates at 1, 2, 3, 5, and 8 years (P = .00). It has been observed previously that the overall survival and survival rates were lower in Eastern than in Western HCC patients. These differences may be related to the higher prevalence of hepatitis B virus infection in Eastern countries, which increases the risk of developing HCC.[21]
Because of the significant differences between the treatment groups with regard to prognostic variables such as PVTT, Child-Pugh grade, BCLC stage, and tumor number, we performed a case-control subgroup analysis of the 100 HCC patients graded at BCLC-C and Child-Pugh-B. In this subgroup, there were no significant differences in baseline characteristics between the patients who received TACE and those with conservative care, but the survival outcomes were significantly better for the patients who received TACE.
According to BCLC staging, TACE is recommended for BCLC-B and sorafenib for BCLC-C as standard treatments.[22] However, because of the heterogeneity of patients with advanced-stage HCC and to the short median survival time associated with sorafenib,[5,23] TACE is still used to treat selected HCC patients with vascular invasion or extrahepatic metastases.[24–28] Our present results with TACE indicated a survival benefit which was consistent with previous controlled trials in advanced HCC with PVTT.[25–27] A meta-analysis of 6 studies showed that TACE significantly improved the 6-month overall survival (HR, 0.41; 95% CI: 0.32–0.53; z, 6.28; P = .000) and 1-year overall survival (HR, 0.44; 95% CI: 0.34–0.57; z, 6.22; P = .000) of patients with PVTT, compared with conservative treatment.[25] In another study, Yoo et al[28] reported that patients with advanced HCC treated with TACE alone (without systemic therapy) showed an improved overall median survival of 13.4 months, noting however that patients with advanced HCC (BCLC stage C) did not obtain similar benefits from TACE in the patient subgroup with extrahepatic metastases (median, 8.3 cf. 19.1 months; P = .001). That study suggested that TACE may be an alternative treatment for advanced HCC, particularly for patients with portal vein invasion without extrahepatic metastases.
The main factors affecting survival prognosis are considered the extent of the tumor and severity of hepatic dysfunction.[29–31] Our results show that treatment modality, PVTT, AFP levels, and BCLC were independent survival predictors. Based on the multivariate analysis, the Child-Pugh grade was not an independent prognostic factor. In the TACE group, portal vein tumor thrombosis, AFP levels, and number of TACE procedures were significant prognostic indicators. Another study showed that HCC patients with type I PVTT, Child-Pugh B class, and extrahepatic metastasis may be poor candidates for TACE.[32] Many studies have evaluated the prognostic factors associated with TACE treatment, but the data remain controversial.[7] Therefore, further studies are required for defining the real indications and prognostic factors of TACE.
4.1. Limitations of the study
There were some limitations in our study. First, it is a retrospective, nonrandomized, single-center study, and heterogeneity in the baseline characteristics of the whole cohort exposed the results to a major bias risk. However, this drawback was, at least partly, counteracted by adopting a subgroup analysis strategy focusing on BCLC-C and Child-Pugh-B patients. Second, the study population comprised patients administered TACE alone; patients with other therapies, such as sorafenib and cytotoxic drugs, were excluded by the study design. Sorafenib is recommended as a current standard treatment for BCLC stage C patients, and TACE-based multimodal treatments have been reported as more beneficial than conservative management.[11] Whether combining TACE with systemic therapy would further enhance the survival of patients with advanced HCC deserves further study. Third, patients were only treated with TACE or best supportive care, so that few patients had histopathological data, and in uni- and multivariate analyses, these data were not included as variables. Fourth, in the TACE group, the administered antineoplastic agents and their doses varied. To the best of our knowledge, no standardized protocols exist with regard to the choice of chemotherapeutic agent, dosage, dilution, rate of injection, and optimal retreatment strategy.[33] Moreover, there is no conclusive evidence for TACE superiority over transarterial embolization.[34] Therefore, the influence of this factor in our study is likely to be very small.
5. Conclusion
The present study suggests that TACE significantly improved the survival of patients with unresectable HCC, especially in those graded BCLC-C and Child-Pugh-B. However, because of the study's limitations, prospective, randomized studies are needed before this therapeutic approach can be recommended in clinical practice.
Acknowledgment
The authors thank Xiao-Li Xu and Guo Tian for their most valuable help in the patient follow-up. We are also very grateful to Yue Teng, Chun-Yan Wang, Yue Yao, and Wei-Jing Yan, for their efficient contribution to data collection.
Author contributions
Conceptualization: Sheng-Mian Li.
Data curation: Shu-Mei Li, Jie-Yu Kong.
Formal analysis: Jie-Yu Kong, Hai-Yan Fan, Lan Zhang.
Funding acquisition: Sheng-Mian Li.
Investigation: Jie-Yu Kong, Hui-Jin Zhao.
Methodology: Jie-Yu Kong, Lan Zhang, Hui-Jin Zhao.
Project administration: Sheng-Mian Li, Jie-Yu Kong, Hai-Yan Fan.
Resources: Jie-Yu Kong.
Supervision: Jie-Yu Kong, Sheng-Mian Li.
Validation: Sheng-Mian Li.
Visualization: Sheng-Mian Li.
Writing – original draft: Jie-Yu Kong.
Writing – review & editing: Jie-Yu Kong.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, BCLC = Barcelona Clinic Liver Cancer Classification, HCC = hepatocellular carcinoma, PVTT = portal vein tumor thrombosis, TACE = transarterial chemoembolization.
The authors report no conflicts of interest.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Wang XQ, Sun LJ. Clinical value of percutaneous transcatheter hepatic arterial chemoembolization combined portal vein embolization for advanced hepatocellular carcinoma: a meta-analysis. J Pract Radiol 2012;28:271–9. [Google Scholar]
- [3].Achenbach T, Seifert JK, Pitton MB, et al. Chemoembolization for primary liver cancer. Eur J Surg Oncol 2002;28:37–41. [DOI] [PubMed] [Google Scholar]
- [4].Johnson PJ. Non-surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yim HJ, Suh SJ, Um SH. Current management of hepatocellular carcinoma: an Eastern perspective. World J Gastroenterol 2015;21:3826–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol 2009;15:391–423. [DOI] [PubMed] [Google Scholar]
- [7].Maleux G, van Malenstein H, Vandecaveye V, et al. Transcatheter chemoembolization of unresectable hepatocellular carcinoma: current knowledge and future directions. Dig Dis 2009;27:157–63. [DOI] [PubMed] [Google Scholar]
- [8].Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734–9. [DOI] [PubMed] [Google Scholar]
- [9].Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–71. [DOI] [PubMed] [Google Scholar]
- [10].Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35:519–24. [DOI] [PubMed] [Google Scholar]
- [11].Song do S, Nam SW, Bae SH, et al. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. World J Gastroenterol 2015;21:2395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dai QS, Gu HL, Ye S, et al. Transarterial chemoembolization vs. conservative treatment for unresectable infiltrating hepatocellular carcinoma: a retrospective comparative study. Mol Clin Oncol 2014;2:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Firouznia K, Ghanaati H, Alavian SM, et al. Transcatheter arterial chemoembolization therapy for patients with unresectable hepatocellular carcinoma. Hepat Mon 2014;14:e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang BH, Xia JL. The domestic standard criteria of clinical diagnosis and staging. Chinese J Hepatol 2001;6:324. [Google Scholar]
- [15].China cancer society liver cancer professional committee. Expert consensus on standardization of the management of primary liver cancer. Tumor 2009;4:295–304. [Google Scholar]
- [16].European Association for Study of Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [17].Liu B, Liu GQ, Yue TQ. The evaluation of the efficacy of transarterial chemotherapy and chemoembolization for primary hepatocellular carcinoma. Modern Medicine & Health 2010;16:2469–70. [Google Scholar]
- [18].Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: New clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol 2011;80:40–53. [DOI] [PubMed] [Google Scholar]
- [19].O'Suilleabhain CB, Poon RT, Yong JL, et al. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg 2003;90:325–31. [DOI] [PubMed] [Google Scholar]
- [20].Yamada R, Kishi K, Sato M, et al. Transcatheter arterial chemoembolization (TACE) in the treatment of unresectable liver cancer. World J Surg 1995;19:795–800. [DOI] [PubMed] [Google Scholar]
- [21].Ayub A, Ashfaq UA, Haque A. HBV induced HCC: major risk factors from genetic to molecular level. Biomed Res Int 2013;2013:810461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther 2009;9:739–45. [DOI] [PubMed] [Google Scholar]
- [23].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [24].Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48suppl 1:S20–37. [DOI] [PubMed] [Google Scholar]
- [25].Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413–20. [DOI] [PubMed] [Google Scholar]
- [26].Niu ZJ, Ma YL, Kang P, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol 2012;29:2992–7. [DOI] [PubMed] [Google Scholar]
- [27].Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yoo JJ, Lee JH, Lee SH, et al. Comparison of the effects of transarterial chemoembolization for advanced hepatocellular carcinoma between patients with and without extrahepatic metastases. PLoS One 2014;9:e113926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Calvet X, Bruix J, Gines P, et al. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology 1990;12:753–60. [DOI] [PubMed] [Google Scholar]
- [30].Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer 1989;64:1700–7. [DOI] [PubMed] [Google Scholar]
- [31].Rosellini SR, Arienti V, Nanni O, et al. Hepatocellular carcinoma. Prognostic factors and survival analysis in 135 Italian patients. J Hepatol 1992;16:66–72. [DOI] [PubMed] [Google Scholar]
- [32].Liu L, Zhang C, Zhao Y, et al. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int 2014;2014:194278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paul SB, Manjunatha YC, Acharya SK. Palliative treatment in advanced hepatocellular carcinoma: has it made any difference? Trop Gastroenterol 2009;30:125–34. [PubMed] [Google Scholar]
- [34].Tsochatzis EA, Fatourou E, O’Beirne J, et al. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol 2014;20:3069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


