Supplemental Digital Content is available in the text
Keywords: clinicopathological factors, meta-analysis, perihilar cholangiocarcinoma, preoperative management, prognosis
Abstract
The refinement in surgical techniques combined with the preoperative management has improved the resectability rate of perihilar cholangiocarcinoma (pCCA). However, the prognosis of pCCA with curative resection is still dismal. This meta-analysis was performed to investigate the prognostic clinicopathological factors in resectable pCCA.
PubMed, the Cochran Library, ScienceDirect, and Web of Science were searched systematically to identify reports focusing on studying the prognostic clinicopathological factors in resectable pCCA. The hazard ratios (HRs) and its 95% confidence interval (95%CI) from the identified studies using Cox proportional hazard regression model were extracted for overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS) analysis.
Three prospective and 35 retrospective cohort studies including 5681 resectable pCCA were included in the pooled analysis. Among more than 20 clinicopathological factors associated with negative survival of pCCA, only 6 were included in quantitative analysis which showed that lymph node involvement was associated with a reduced OS (HR = 2.04; 95%CI: 2.10–2.62), DSS (HR = 1.80; 95%CI: 1.39–2.34), DFS (HR = 4.38; 95%CI: 1.89–10.14), negative resection margin (HR = 2.04; 95%CI:1.73–2.41), operative transfusion (HR = 1.82; 95%CI: 1.06–3.11), and T3 or T4-stage (HR = 2.04; 95%CI: 2.04–2.53) were poor prognostic factors of OS, and poor or moderate differentiation was also an adverse prognostic factor of OS (HR = 2.71; 95%CI: 1.80–4.07) and DSS (HR = 1.74; 95%CI: 1.25–2.44). The overall median resectability rate (95CI%), R0 resection (95CI%), and 5-year OS (95CI%) in Eastern and Western countries were 74.9 (66.4–78.4) % and 41.3 (32.6–80.8) %, 70.7 (65.6–80.8) % and 75.9 (64.0–80.4) %, and 33.0 (29.7–39.7) % and 25.5 (20.0–31.6) %, respectively.
Negative resection margin, lymph node involvement, poor or moderate differentiation grade was identified as the negative predictor factors of resectable pCCA. Operative transfusion and T3/T4 stage were also associated with a reduced survival of resectable pCCA.
1. Introduction
Perihilar cholangiocarcinoma (pCCA), also named as hilar cholangiocarcinoma, is the most common type of biliary tract cancer, which is a neoplasm originating from the degree of 2nd-order bile duct proximally to the insertion of cystic duct into the extrahepatic bile duct.[1] The pCCA is labeled as “silent” cancer on account of the symptoms may unnoticed until the carcinoma has been found in the advanced stage when it got metastasis. Due to the major hepatectomy combined with preoperative management strategies development, the resectability rate of pCCA has increased gradually in recent years worldwide.[2] However, the 3- and 5-year overall survival (OS) of most previously published studies varied considerably, ranging from 27% to 56% and 13% to 42% respectively in resectable pCCA,[3–17] which indicates that the prognosis of pCCA who underwent an operation with curative intention remains gloomy.
Surgical resection with negative resection margins (R0) is the only way to cure this intractable neoplasm since the chemotherapy with or without radiation, neoadjuvant chemotherapy or photodynamic therapy is less effective.[18] Nevertheless, the postoperative recurrence rate of this silent cancer is not rare even with using of R0 resection that comprises major hepatectomy combined with en bloc resection of the extrahepatic bile duct, and caudate lobe and lymphadenectomy.[19] In addition, majority of previously published research studies was carried out in past 2 decades[2–6,10,12–17,20–25] with varied hazard ratio (HR) found that positive surgical resection margins and lymph nodes involvement were associated with the prognosis of resectable pCCA, but the prognosis of pCCA nor merely associated with positive surgical resection margins and lymph nodes involvement, there are many other clinicopathological factors surrounding (such as histological grade, operative transfusion, male gender, T-stage, no-hepatectomy, caudate lobe invasion, etc.), as many previously published studies described,[2,5,8–10,12–14,16,22,24–31] were associated with a reduced OS of resectable pCCA. For those reason depicted above, we, therefore, performed a prognostic systematic review and meta-analysis to investigate the clinicopathological factors associated with the prognosis of resectable pCCA postoperatively.
2. Materials and methods
2.1. Search strategy
A comprehensive literature search on studies in human subjects was performed to identify all original reports that focus on assessing the prognostic clinicopathological factors associated with the prognosis of resectable pCCA. In November 2017, we searched 4 main electronic databases (PubMed, ScienceDirect, The Cochrane Library, and Web of Science), restricted to articles published in English. The following keywords were used for search: (“Bile duct adenoma” OR “Bile duct neoplasms” OR “Cholangiocarcinomas” OR “Cholangiocellular carcinoma” OR “Biliary tract cancer” OR “ Bile duct cancer”) AND (“obstructive jaundice” OR “cholestasis” OR “jaundice”) AND (“survival” OR “prognostic” OR “prognosis”). Moreover, we scrutinized the reference lists of the identified reports, meta-analysis, reviews, and other relevant publications to identify additional pertinent studies missed on the initial search. This study did not require ethical approval as all the data used have been published previously, and hence are already in the public domain.
2.2. Inclusion criteria
Published studies were included if they met the following criteria: prospective or retrospective cohort design with a well-defined study population with justification for excluded cases; all subjects with pCCA or hilar cholangiocarcinoma or klatskin tumor were diagnosed by pathologist postoperatively; clear description of surgical resection approach, perioperative mortality rate, and 5-year survival rate of pCCA; statistical analysis using multivariate Cox proportional hazards model that adjusted for clinical prognostic factors; and reporting HRs including 95% confidence interval (95%CI) and the corresponding P value. We excluded studies based on following criteria: animal studies; review articles, case reports, comments, or case series with less than 10 patients; duplicated publications; and subjects with intrahepatic cholangiocarcinoma, distal cholangiocarcinoma, primary gallbladder carcinoma, or carcinoma of pancreas.
2.3. Data extraction
Data were extracted from all included manuscripts by 2 investigators independently (Zengwei Tang and Yuan Yang), with the discrepancies resolved by the consensus of these 2 investigators (any disagreement on a conflicting study was resolved by a full discussion). Besides the HR and 95%CI and corresponding P value, the following characteristics were recorded: the first author of study, year of publication, study design and duration, country, sample size in each study with mean age and gender, median follow-up time, resectability rate and R0 resection rate, 30-day and/or 90-day mortality or death in hospital, 3- and 5-year OS rate, indication of preoperative biliary drainage (PBD) and type of PBD, indication of preoperative portal vein embolization (PVE), operative technique and type of hepatectomy, and any prognostic clinicopathological factors with or without statistical significance in multivariate analysis using Cox proportional HR model.
2.4. Quality assessment across studies and publication bias
The methodological quality of included studies was assessed by using the modified risk of bias tool recommended by the Cochrane Collaboration as described previously.[32,33] Furthermore, Begg funnel plot and the Egger linear regression test were applied to evaluate potential publication bias for eligible studies using OS as an endpoint.
2.5. Statistical analysis
The statistical analysis was performed according to the guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology group (MOOSE).[34] The pooled HR with 95%CI was calculated with a random-effect model according to the DerSimonian-Laird method to assess the prognostic significance of clinicopathological factors associated with survival of resectable pCCA.[35] By definition, the value of HR > 1 indicates a worse outcome for patients with this risk factor, and this would be considered statistically significant if the 95%CI of the HR did not overlap 1. The heterogeneity among included studies was measured using the Q tests and I2 statistic to assess the extent of the inconsistency.[36] A probability value of P < .1 and I2 >50% indicated the existence of significant heterogeneity.[35] Publication bias was evaluated for OS analysis by Egger and Begg test. Moreover, a P < .01 for Egger test was considered representative of significant publication bias.[18] Pearson correlation coefficient analysis was used to determine the correlation between the age of resectable pCCA patients and OS. Column scatters graphs were performed to investigate the difference in resectablity rate, R0 resection, and 5-year OS rate between Eastern and Western countries. Furthermore, Mann–Whitney U test was used to test the difference between these 2 groups. All statistical analyses were performed with Stata/MP 14.0 (StataCorp, Parallel Edition) and GraphPad Prism Software (CA) Version 7.0.
3. Results
3.1. Study selection
The initial literature research yielded 2458 articles by using 4 primary electronic databases, of which 315 papers were selected for full-text review, the remaining 1873 publications were excluded by reviewing the titles and abstracts. Among these publications retrieved from the full-text review, according to the inclusion and exclusion criteria, 38 articles were eligible for this meta-analysis. The detailed screening process is visualized in Fig. 1.
Figure 1.
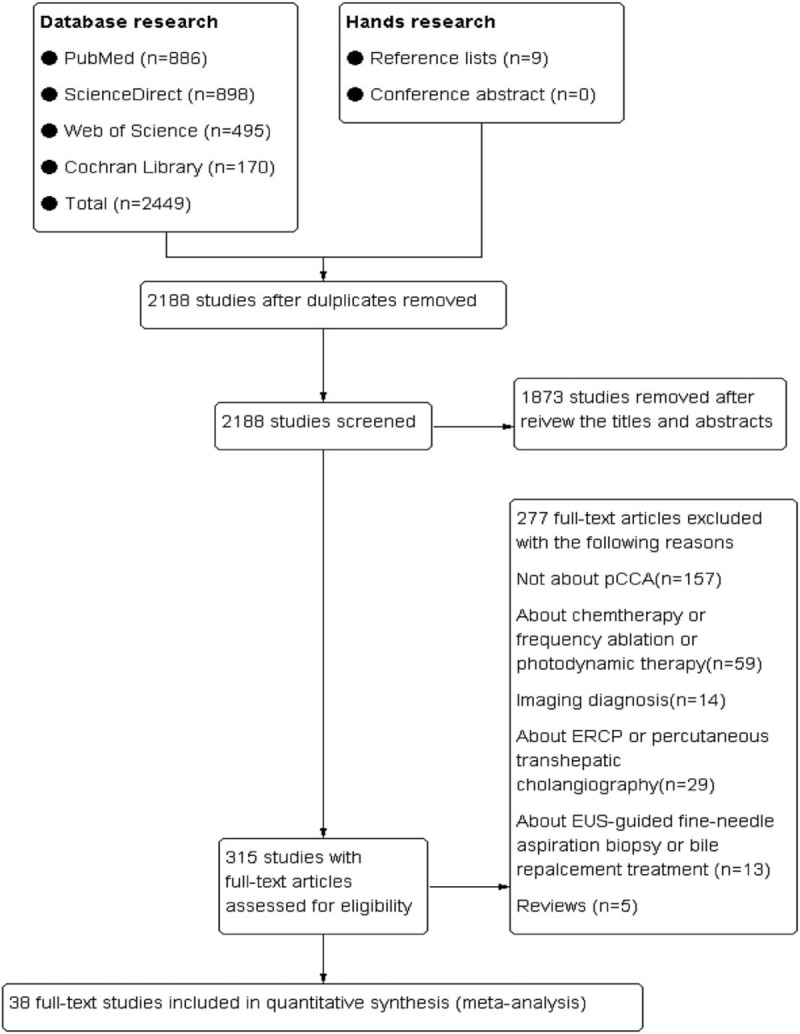
Search flow diagram.
3.2. Study and participants characteristics
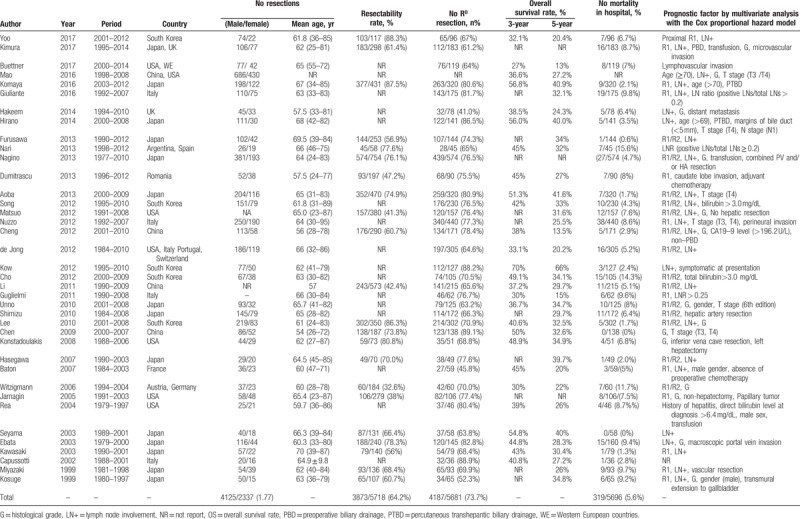
A total of 38 observational studies, studying the prognostic survival factors, including 2 prospective cohorts[31,37] and 36 retrospective cohort studies[2–17,20–30,38–46] met the inclusion criteria. Of those publications, 29 studies[3–7,9,12,13,15–17,20,21,23–26,28,30,31,37–41] used OS as endpoint alone, 5 studies[8,10,11,22,29] used both OS and disease-free survival (DFS), 3 studies[2,27,42] used disease-specific survival (DSS), and only 1 study[14] reported both OS and DSS. In addition, 21 studies (55%) were carried out in Eastern countries,[3–5,9,12,16,17,20,21,23–25,27–31,39,40,44,45] 16 studies (42%) were performed in Western countries,[6–8,10,11,13–15,22,26,37,38,41–43,46] and the remaining 1 study (3%) including 2 cohorts was from Japan and UK, respectively.[2] The sample size, mean age, the ratio of men to women, and R0 resection rate included studies varied greatly, ranging from 36 to 1116, 54 to 70, 1.2 to 3.7, and from 46% to 89%, respectively. Furthermore, the resectability rate, caudate lobe resection rate, and major hepatectomy rate also differed greatly among included studies, ranging from 32% to 88%, 33% to 100%, and 67% to 100%, respectively. The detailed characteristics of eligible studies included in this meta-analysis were presented in Table 1 and Supplementary Tables 1–3.
Table 1.
Summary of reports on surgical management of perihilar cholangiocarcinoma.
3.3. Summary of indication of preoperative management strategies: preoperative biliary drainage (PBD) and portal vein embolization (PVE)
Among these 38 included studies, 27 studies[2–4,6,8,9,12,16,17,20–23,25–31,38,39,42–46] had a detail data on indication of PBD. The main indication of PBD was the level of serum total bilirubin concentration which ranged from >2.0 to >15 mg/dL differed greatly. In addition, 17 out of the 27 studies including 2478 resectable pCCA described the type of PBD,[2–4,6,8,12,16,17,20,21,25,27–30,44,45] in which 1427 subjects underwent percutaneous transhepatic biliary drainage (PTBD), 850 subjects underwent endoscopic biliary drainage (EBD), and remaining 201 patients required both type of biliary technique. The ratio of PTBD to EBD was 1.7 (1427/850). Furthermore, the volume of future liver remnant ranging from 20% to 50% was only criteria of preoperative PVE, which also varied greatly among 19 included studies.[2,3,5,9,16,17,20,22,23,25–30,39,40,44,46]
3.4. Summary of the prognostic clinicopathological factors of resectable pCCA
The prognostic value of quantitative analysis of clinicopathological parameters were performed, which including the positive surgical resection margins, lymph node involvement, histological grade, operative transfusion, and T-stage (T3/T4) based on American Joint Committee on Cancer 6th/7th edition, and the male gender. Moreover, as mentioned only by the following groups, the age of resectable pCCA (≥70 or ≥69),[12,14] caudate lobe invasion,[10] papillary tumor,[42] adjuvant chemotherapy,[10] symptomatic at presentation,[29] history of hepatitis,[43] transmural extension of gall bladder,[25] surgical margins of bile duct <5 mm,[12] the preoperative serum total bilirubin (>3.0 mg/dL) and CA19–9 level (>196 U/L),[5,28,30] no hepatic resection,[7] portal vein and/or hepatic artery resection,[24,27,40] PTBD,[12,16] and the ratio of lymph nodes involved to lymph nodes retrieved[13,26,38] (>0.2 or >0.25) were reported as the risk factor of prognosis in resectable pCCA as well. However, it can thus be conceivably hypothesized that pooled analysis would be conducted to determine the correlation between these clinical parameters and the prognosis of resectable pCCA patients once the sample size increases.
4. Meta-analyses
4.1. The prognostic clinicopathological factors associated with OS
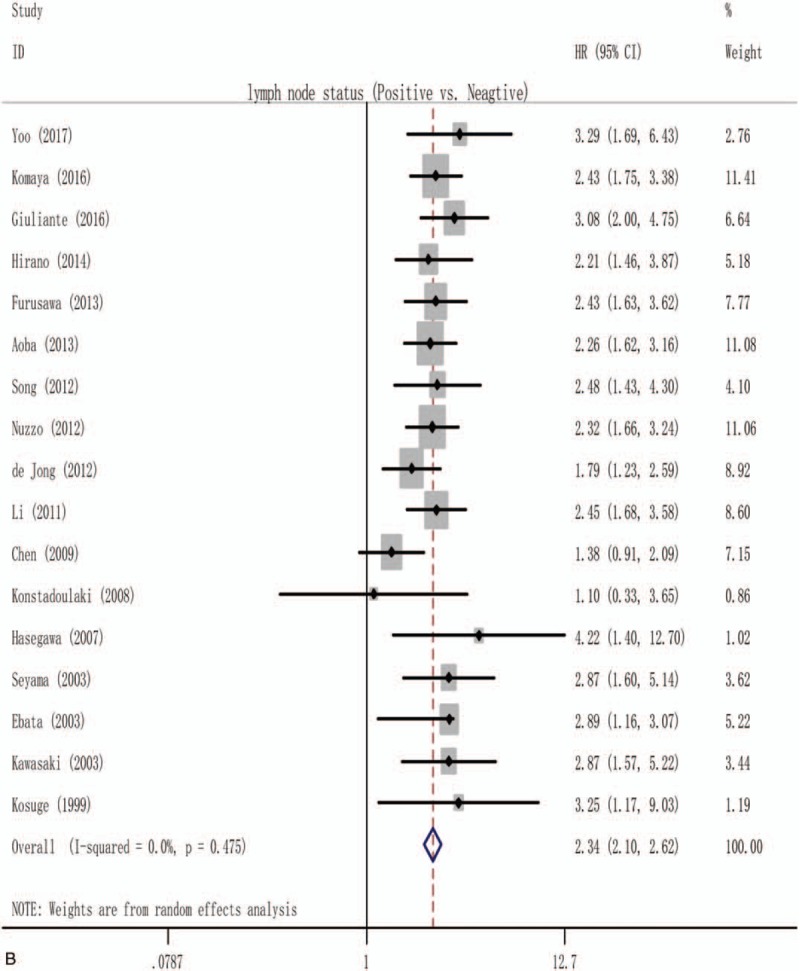
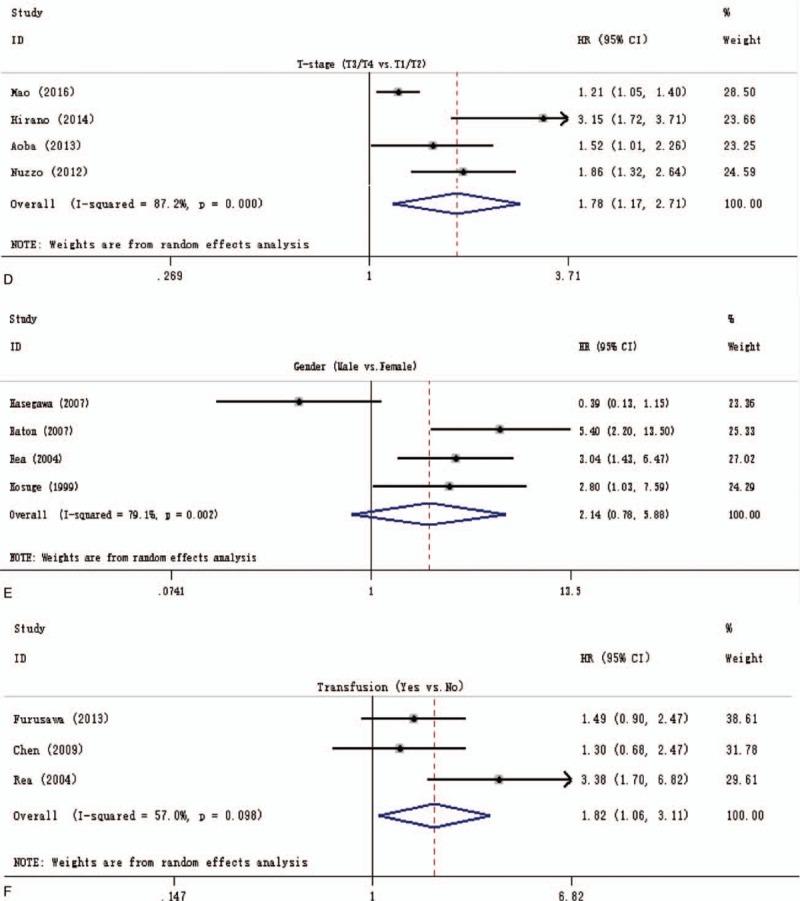
The surgical resection margins and lymph node involvement were evaluated for their association with OS in more than 15 studies, allowing for pooled analysis, as shown in Supplementary Figs. 1A and 2. Owing to the significant publication bias and/or heterogeneity within those included studies, only 14 studies[6,15,20–23,25,28–30,37,38,40,44] including 1634 resectable pCCA were eligible for the final pooled analysis of the prognostic role of positive surgical resection margins, with a pooled HR of 2.04 (1.73–2.41), and no heterogeneity among these studies was found (I2 = 0.0%; P = .604) as Fig. 2 A shows. Similarly, only 17 studies[4,6,8,9,12,13,16,17,20,21,23,25,28,31,41,44,45] including 2971 resectable pCCA allowed for a pooled analysis of the prognostic role of lymph node involvement (Fig. 2 B), and the pooled HR was 2.34 (2.10–2.62), with a heterogeneity of 0.0% (P = .475).
Figure 2.
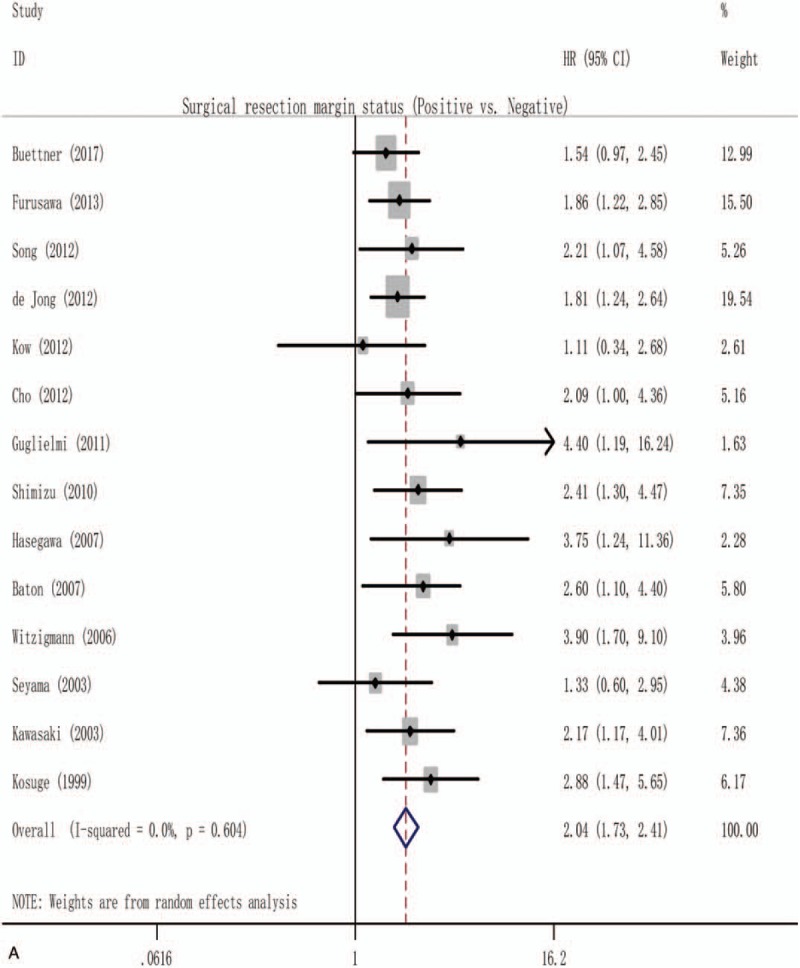
Forest plot for the pooled analysis the association of clinicopathological factors with overall survival of resectable perihilar cholangiocarcinoma. (A) Forest plot for the pooled analysis of surgical resection margins status. (B) Forest plot for the pooled analysis of lymph node status (positive vs negative). (C) Forest plot for the A pooled analysis of histological grade (poor or moderate vs well). (D) Forest plot for the pooled analysis of T-stage (T3 and/or T4 vs T1 and/or T2). (E) Forest plot for the pooled analysis of gender of participation (male vs female). (F) Forest plot for the pooled analysis of operative transfusion (yes vs no).
In the pooled analysis of the prognostic role of histological grade of pCCA, due to the indication of significant inconsistency if all 11 studies were included (Supplementary Fig. 1B). Only 8 studies[3,13,21,25,31,37,41,45] with 985 resectable pCCA were included for the quantitative analysis (Fig. 2 C), and the pooled HR was 2.71 (1.80–4.07), with a low heterogeneity (I2 = 34.2%; P = .155).
In addition, a pooled analysis of T-stage (Fig. 2 D), male gender (Fig. 2 E), and operative transfusion (Fig. 2 F) was also conducted to evaluate the prognostic significance of those 3 clinicopathological factors, with a pooled HR of 1.78 (1.17–2.71), 2.14 (0.78–5.88), and 1.82 (1.06–3.11), respectively, and with a significant heterogeneity among included studies (I2 = 87.2%; P = .000, I2 = 79.1%; P = .002, and I2 = 57.0%; P = .098 respectively).
4.2. The prognostic clinicopathological factors associated with DSS and DFS
Lymph node status and histological grade were also assessed for the association with DSS in 4 studies, and a quantitative analysis was conducted to assess the prognostic significance of these 2 clinicopathological factors, with a pooled HR of 1.80 (1.39–2.34) and 1.74 (1.25–2.44), respectively, and the heterogeneity revealed that I2 = 64.4% (P = .038) and I2 = 73.6% (P = .010), as described in Fig. 3A and B, respectively.
Figure 2 (Continued).
Forest plot for the pooled analysis the association of clinicopathological factors with overall survival of resectable perihilar cholangiocarcinoma. (A) Forest plot for the pooled analysis of surgical resection margins status. (B) Forest plot for the pooled analysis of lymph node status (positive vs negative). (C) Forest plot for the A pooled analysis of histological grade (poor or moderate vs well). (D) Forest plot for the pooled analysis of T-stage (T3 and/or T4 vs T1 and/or T2). (E) Forest plot for the pooled analysis of gender of participation (male vs female). (F) Forest plot for the pooled analysis of operative transfusion (yes vs no).
Furthermore, another pooled analysis was performed to assess the association of lymph node status with DFS within 3 studies.[8,22,29] As presented in Fig. 4, the pooled HR was 4.38 (1.89–10.14), heterogeneity testing revealed that I2 was 69.3% (P = .039).
Figure 2 (Continued).
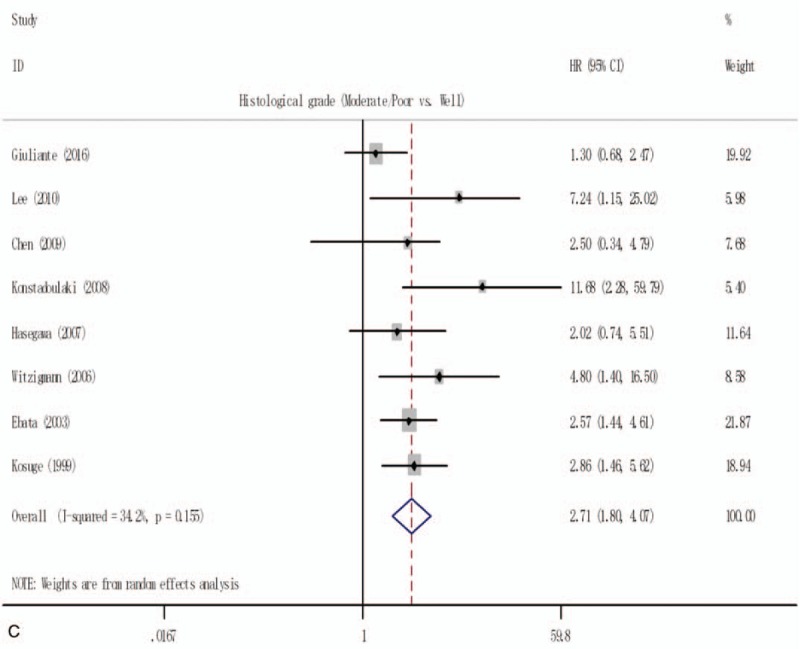
Forest plot for the pooled analysis the association of clinicopathological factors with overall survival of resectable perihilar cholangiocarcinoma. (A) Forest plot for the pooled analysis of surgical resection margins status. (B) Forest plot for the pooled analysis of lymph node status (positive vs negative). (C) Forest plot for the A pooled analysis of histological grade (poor or moderate vs well). (D) Forest plot for the pooled analysis of T-stage (T3 and/or T4 vs T1 and/or T2). (E) Forest plot for the pooled analysis of gender of participation (male vs female). (F) Forest plot for the pooled analysis of operative transfusion (yes vs no).
4.3. Risk of bias within studies
Assessment of risk of bias for each study included in this meta-analysis showed that 19 studies with an unclear risk,[2–7,24,26,28,31,37–43] 15 with a low risk,[8,9,12,16,17,20–23,25,27,29,30,44,46] and 4 with a high risk of bias,[10,14,15,45] as depicted in Supplementary Table 4. Begg funnel plots of the results of surgical resection margins (Fig. 5A) and lymph node status (Fig. 5B) and histological grade (Fig. 5C) presented no evidence of noticeable asymmetry. Furthermore, Egger test also showed no publication bias in the pooled analysis of the prognostic significance of surgical resection margins (Egger t value = 1.89, P = .083), lymph node status (Egger t value = 1.00, P = .332), and histological grade (Egger t value = 2.25, P = .066).
Figure 2 (Continued).
Forest plot for the pooled analysis the association of clinicopathological factors with overall survival of resectable perihilar cholangiocarcinoma. (A) Forest plot for the pooled analysis of surgical resection margins status. (B) Forest plot for the pooled analysis of lymph node status (positive vs negative). (C) Forest plot for the A pooled analysis of histological grade (poor or moderate vs well). (D) Forest plot for the pooled analysis of T-stage (T3 and/or T4 vs T1 and/or T2). (E) Forest plot for the pooled analysis of gender of participation (male vs female). (F) Forest plot for the pooled analysis of operative transfusion (yes vs no).
Figure 5.
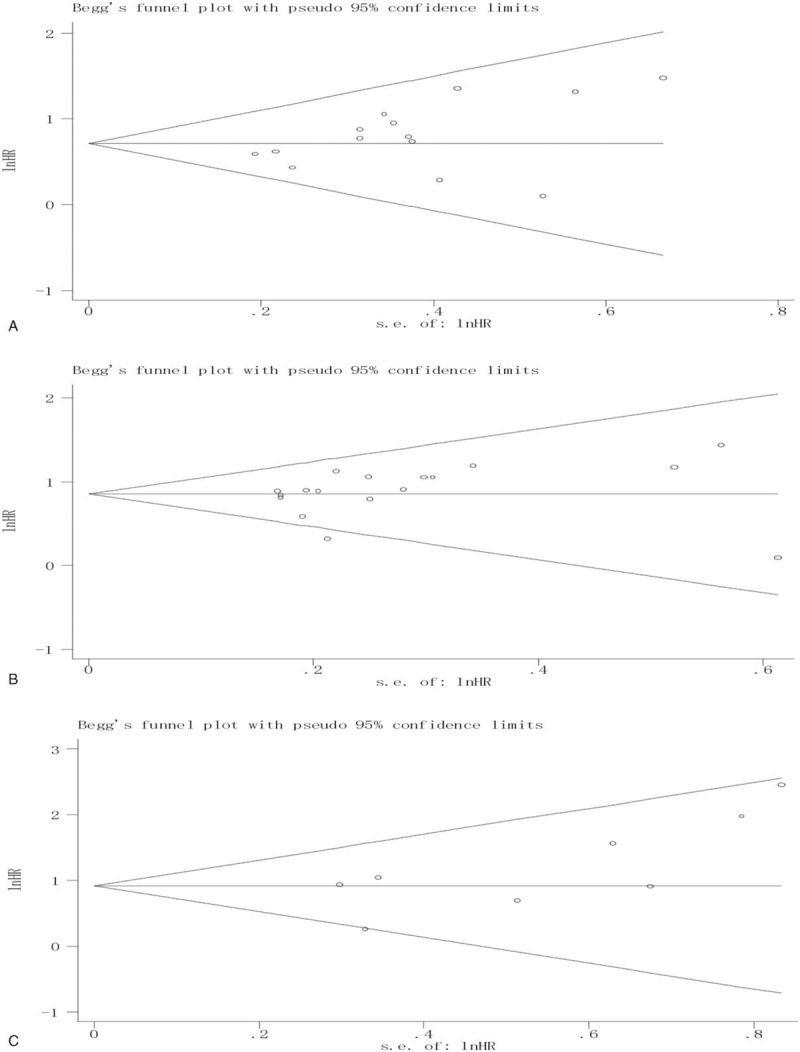
Begg funnel plot for the pooled analysis of the prognostic role of surgical resection margins (A), lymph node status (B), and histological grade (C).
4.4. Additional analyses
In the initial pooled analysis of the prognostic role of surgical resection margins, although the heterogeneity testing showed a lower inconsistency (I2 = 1.9%; P = .433) as all 18 studies[3,6,9,15,16,20–25,28–30,37,38,40,44] were included (Supplementary Fig. 1A: the pooled HR = 1.82 [1.60–2.06]), however, Egger test showed a significant risk of publication bias (Egger t value = 3.70, P = .002). To reduce risk of bias, 4 studies (2 studies[16,24] were from Japan, 1 study[3] from South Korea, and another one[6] from USA, Italy, Portugal, and Switzerland) were excluded from the analysis of the association of surgical resection margins with OS, the pooled analysis of remaining 14 studies also showed a significant result (Fig. 2 A), with the HR of 2.04 (1.73–2.41), heterogeneity testing showed that I2 = 0.0% (P = .604). In addition, Egger test showed no significant publication bias (P = .083). The exact cause of this difference is unknown, but mainly because of the differences in the population of participants, among these 18 studies, a half of them were from Japan, and the ratio of Japanese to the whole population was 1300/2669 (49%).
The initial pooled analysis of the prognostic role of lymph node status, including 22 studies[3,4,6,8,9,12–14,16,17,20–25,28,29,31,41,44,45] (Supplementary Fig. 2), showed a significant result with an HR of 2.41 (2.10–2.77). However, due to the indication of significant heterogeneity (I2 = 50.0%; P = .004) and a significant publication bias (Egger t value = 3.39, P = .03). After 5 studies[3,14,22,24,29] removed from the initial pooled analysis (Fig. 2 B), we got a significant result (pooled HR = 2.34 [2.10–2.62]), and no heterogeneity (I2 = 0.0%; P = .475) and no significant publication bias was found (Egger t value = 1.00, P = .332). This significant difference may be caused by we included 2 studies[14,24] with no data on the lymphadenectomy and 3 studies[3,22,30] with a various extent of lymphadenectomy in the initial pooled analysis.
Furthermore, in the initial pooled analysis of the prognostic role of histological grade, a pooled HR of 1.88 (1.40–2.52) showed a significant result (Supplementary Fig. 1B), but heterogeneity testing showed that there was a high inconsistency (I2 = 63.1%; P = .002). To reduce the heterogeneity, after 3 studies[9,14,20] removed, the pooled HR of 2.71 (1.80–4.07) similarly showed a significant result and heterogeneity among remaining 8 studies decreased obviously (I2 = 34.2%; P = .155). Besides 1 study with high-risk bias reported by Mao et al[14] had no data on the mean age of subjects, and the mean age of subjects in another 2 studies[9,20] were more than 65 year may account for this difference.
4.5. Sensitivity analysis
To test the stability of meta-analysis of the prognostic role of surgical resection margins, lymph node status, and histological grade in OS, we performed a sensitivity analysis by sequentially removing each eligible study. As shown in Fig. 6A–C, the present meta-sensitivity analysis did not suggest an undue influence of any single study.
Figure 3.
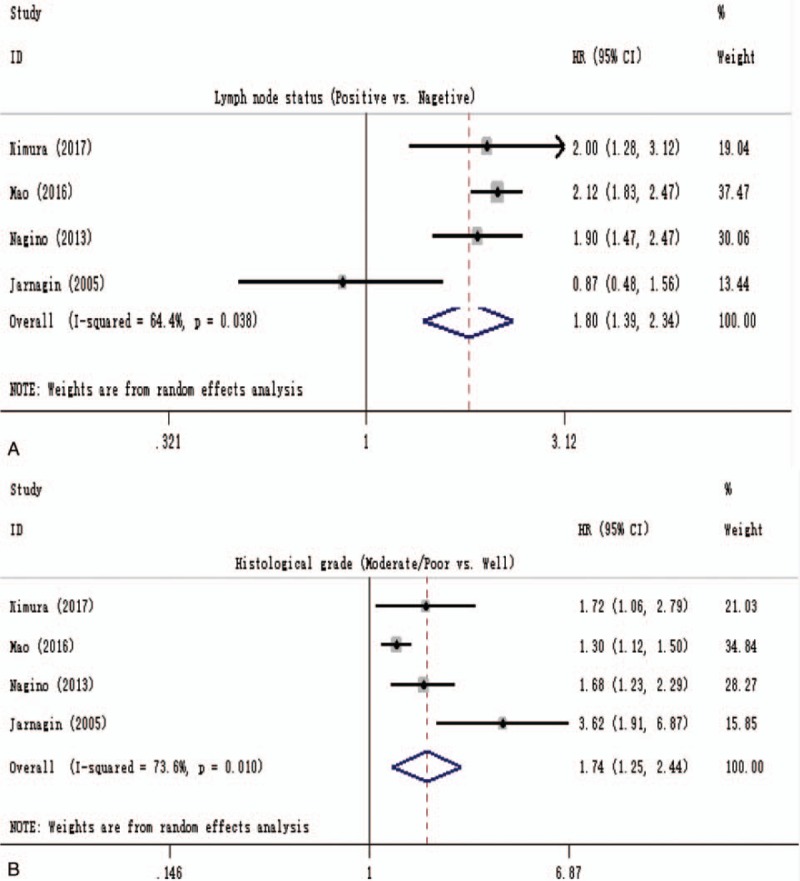
Forest plot for the pooled analysis the association of lymph node status (A) and histological grade (B) with DSS of resectable pCCA. DSS = disease-specific survival, pCCA = perihilar cholangiocarcinoma.
Figure 6.
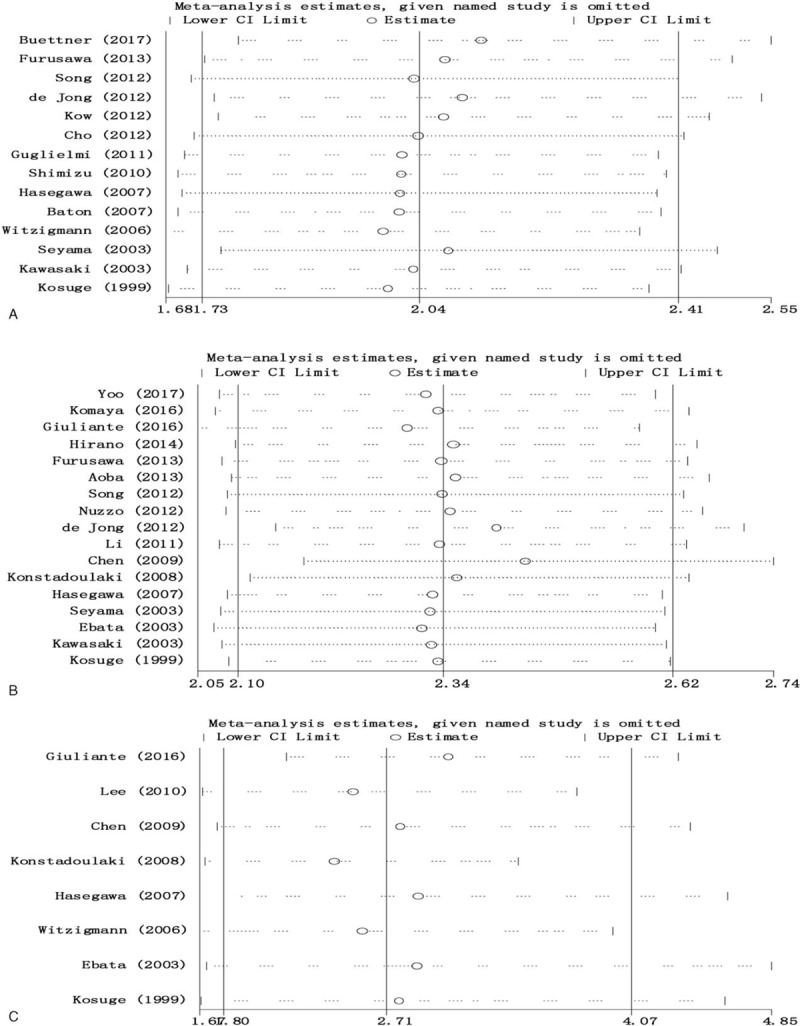
Sensitivity analysis of the results of pooled analysis of surgical resection margins (A), lymph node status (B), and histological grade (C).
4.6. The correlation between the age of resectable pCCA patients and 5-year OS rate, and the difference in resectablity rate, R0 resection, and 5-year OS between Eastern and Western countries
As shown in Fig. 7A, we used Pearson correlation coefficient analysis to determine the effect of older age on OS. However, no significant correlation (r = 0.2244; P = .2020) was found between these 2 parameters. Figure 7B is presenting the overall median resectability (95CI%) rate in Eastern and Western countries were 74.9 (66.4–78.4) % and 41.3 (32.6–80.8) %, respectively. Furthermore, Mann–Whitney U test result showed there was a significant difference (P = .0254) between these 2 groups.
Figure 4.
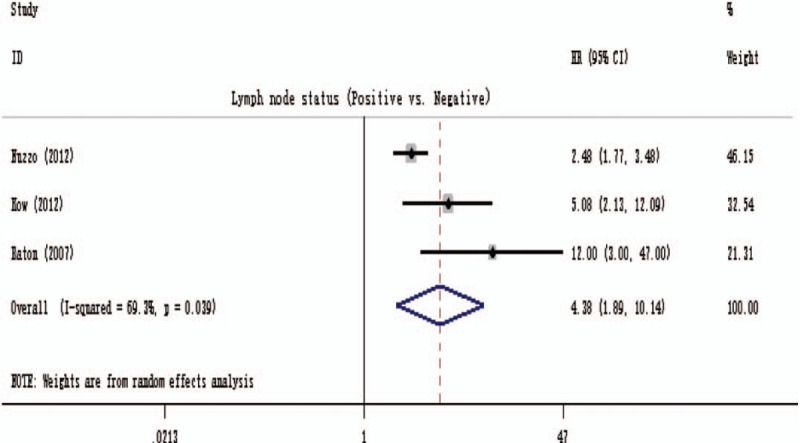
Forest plot for a pooled analysis of the association of lymph node status with disease-free survival.
No significant difference (P = .9810) was found in the R0 resection rate between Eastern and Western countries, as Fig. 7C showed, the overall median R0 resection rate (95CI%) rate in Eastern and Western countries were 70.7 (65.6–80.8) % and 75.9 (64.0–80.4) %, respectively.
As Fig. 7D showed, the overall 5-year OS (95CI%) rate in Eastern and Western countries were 33.0 (29.7–39.7) % and 25.5 (20.0–31.6) %, respectively. Moreover, Mann–Whitney U test result showed there was a significant difference (P = .001) between these 2 groups.
5. Discussion
Due to the initial progress was executed by using the preoperative management and surgical resection techniques, a progressive enhancement of resectability rate of pCCA ranging from 80% to 87% has been achieved.[3,16,17,41] However, in the light of recent events of this devastating cancer, there is now considerable concern about the OS even with using of major hepatectomy combined with en bloc resection of caudate lobe and extrahepatic bile duct, lymph node dissection, and with or without resection of the portal vein and hepatic artery.[5,6,11,14,15,17,22,37] Moreover, the postoperative mortality rate after major hepatectomy, ranging from 0% to 11%, varied considerably as reported in many published studies,[2,9,11,12,15–17,20,24,25,27,31,44] however, in order to improve the survival of pCCA, most centers have adopted a gold criteria of R0 resection, in which significant hepatectomy combined with en bloc resection of the extrahepatic bile duct and caudate lobe and lymph node dissection with varied extent, and/or pancreatoduodenectomy was performed to ensure complete resection of positive proximal and/or distal bile ducts.[15,17]
In this systematic review, more than 20 clinicopathological factors associated with prognosis of resectable pCCA have been assessed, however, among those survival factors, only 6 factors were qualified for quantitative analysis. Moreover, the results of the pooled analysis revealed that the positive surgical resection margins (HR = 2.04; 95%CI: 1.73–2.41), lymph node involvement (HR = 2.34; 95%CI: 2.10–2.62), and poor or moderate histological grade (HR = 2.71; 95%CI: 1.80–4.07) were the most influential adverse prognostic factors in resectable pCCA. In addition, the pooled analysis of the T-stage (Fig. 2 D) based on the protocols of American Joint Committee on Cancer 6th[8,14] or 7th edition[9,12] and operative transfusion (Fig. 2 F) also showed a significant result, inversely no association of the gender of participants (male vs female) with OS resectable pCCA (Fig. 2 E, HR = 2.14; 95%CI: 0.78–5.88), however, a high inconsistency among included studies in the pooled analysis of the prognostic role of T-stage (T3 or T4), male gender and operative transfusion may introduce some risk bias that cannot be underestimated.
Besides 6 clinicopathological factors associated with the survival of resectable pCCA as described above, allowing a pooled analysis, there were more than 10 clinicopathological factors, such as the age of patients (≥70 or ≥69), caudate lobe invasion, papilliary tumor, adjuvant chemotherapy, symptomatic at presentation, history of hepatitis, transmural extension of gall bladder, surgical margins of bile duct <5 mm, the preoperative serum total bilirubin (>3.0 mg/dL) and CA19–9 level (>196 U/L), no-hepatic resection, portal vein and/ or hepatic artery resection, PTBD, and the ratio of lymph nodes involved to lymph nodes retrieved (>0.2 or >0.25), were demonstrated as poor prognostic factors of resectable pCCA by many published studies.[5,12–14,16,26–28,30,38,42] Nevertheless, due to the limitation of sample size, the quantitative analysis was not able to be performed, but we should be cautious in interpreting those results since there may have some overlap or some unclear risk bias in terms of the significant result, for example, although 2 retrospective studies reported by Komaya et al[16] and Hirano et al[12] found that PTBD was associated with a reduced OS in resectable pCCA, but there was apparently some overlap in dividing participation into PTBD group and EBD group: 62 out of 168 (37%) patients from Komaya cohort and 10 out of 67 (15%) patients from Hirano cohort in PTBD groups had undergone EBD prior to undergoing PTBD, but for data analysis, those patients with a history of EBD prior to PTBD were included in PTBD group versus subjects with EBD alone palliating jaundice successfully.
We compared the difference in resectability rate, R0 resection, and 5-year OS between Eastern and Western countries. As Fig. 7B showed, the resectability rate of Eastern countries was significantly higher (P = .0254) than that of Western, but only 5 out of 15 included studies from Western countries reported the resectability related dataset, therefore, the false positive results caused by the limited sample size from Western Countries cannot be underestimated. Furthermore, although no significant difference in R0 resection rate was found between these 2 groups (Fig. 7C), the 5-year OS rate (Fig. 7D) of resectable pCCA patients from Eastern countries was significantly higher than (P = .001) that of patients from Western countries. The exact cause of this difference is unclear, the main reason may be considered as the ratio of patients with lymph node metastases (LN+) in Western Countries was greater than that of patients from Eastern countries. This hypothesis can be supported by Buettner et al's report,[15] in which the 5-year OS rate of LN+ pCCA patients reported by only was 13%.
Figure 7.
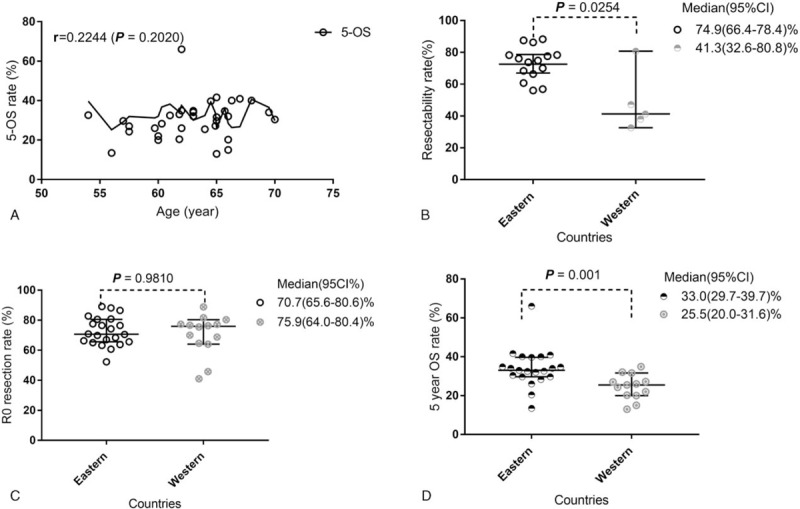
The correlation between the age of resectable pCCA patients and 5-year OS rate (A), and the difference in resectablity rate (B), R0 resection (C), and 5-year OS (d) between Eastern and Western countries. OS = overall survival, pCCA = perihilar cholangiocarcinoma.
To our knowledge, the present meta-analysis is the first paper to comprehensively evaluate the association of clinicopathological factors with the prognosis of resectable pCCA. Moreover, the pooled analysis with a total of 38 studies including 5681 resectable pCCA showed significant results, which indicates that the prognosis of resectable pCCA affected by multifactors (positive surgical resection margins, lymph node involvement, poor or moderate histological grade, operative transfusion, and T3 or T4 stage). In addition, a low heterogeneity and no evidence of publication bias in the analysis of the prognostic value of surgical resection margins, lymph node status, and histological grade was found, and the solid results of sensitivity analysis made the results of present meta-analysis more credible.
However, there were some limitations that cannot be underestimated. Firstly, this is a meta-analysis based on observational studies, therefore, the differences in the capacity of institution (the total of patients admitted for pCCA yearly), the level of proficiency of operators, operative time, and the diagnostic accuracy of frozen section examination of surgical margins among different medical centers still should not be underestimated. Secondly, the rate of major hepatectomy, ranging from 47% to 100%, varied considerably in these included studies.[2–17,20–31,37–44] Furthermore, a high heterogeneity was found in the pooled analysis the association of operative transfusion, the gender of participants, and T-stage with OS of resectable pCCA, due to the limitation of small sample size, we did not perform sensitivity analysis to reduce the inconsistency among included studies. These inconsistencies were considered to have affected the level of evidence. Moreover, these limitations mandate caution when interpreting the present results of pooled analysis of the operative transfusion, gender of participants, and T-stage (T3 or T4).
Currently, R0 resection has becoming a gold standard of surgical treatment of pCCA. Nevertheless, the extent of lymphadenectomy and the number of lymph node retrieved routinely still under debate. As described previously, the prognosis of pCCA is associated with multifactors. To improve the survival rate of this typical silent cancer postoperatively, each clinicopathological factors that can be controlled, associated with prognosis, should be miniaturized. Such as using advanced surgical equipment not merely using a Cavitron ultrasonic operative aspirator to reduce the blood loss during hepatectomy. Furthermore, advocating the autologous blood transfusion during operation and preoperative autologous blood donation in selected subjects.
In conclusion, the current meta-analysis provides evidence that positive surgical resection margins, lymph node involvement, and poor/moderate histological grade were the most influential adverse prognostic factors in resectable pCCA. Furthermore, operative transfusion and T3/T4-stage may be associated with a reduced OS in resectable pCCA that should be validated by a future prospective study with a well-design.
Author contributions
Wenbo Meng and Xun Li – revised the manuscript for significant intellectual content and obtained funding and study supervision. Yuan Yang – collection and extraction of data, development of methodology, statistical analysis, and interpretation of data. Zengwei Tang– study concept and design; collection and extraction of data, development of methodology, statistical analysis and interpretation of data, and drafting of the manuscript; and critical revision of the manuscript for significant intellectual content. Zhonghong Zhao and Kongyuan Wei – collection and extraction of data.
Conceptualization: Zengwei Tang.
Data curation: Zengwei Tang, Yuan Yang, Zhonghong Zhao, Kongyuan Wei.
Formal analysis: Zengwei Tang, Yuan Yang, Zhonghong Zhao.
Funding acquisition: Wenbo Meng, Xun Li.
Investigation: Zengwei Tang, Yuan Yang, Kongyuan Wei.
Methodology: Zengwei Tang.
Project administration: Wenbo Meng.
Resources: Zengwei Tang, Yuan Yang, Zhonghong Zhao, Kongyuan Wei.
Software: Zengwei Tang, Yuan Yang, Zhonghong Zhao.
Supervision: Wenbo Meng, Xun Li.
Writing – original draft: Zengwei Tang.
Writing – review & editing: Zengwei Tang, Wenbo Meng, Xun Li.
Supplementary Material
Footnotes
Abbreviations: DFS = disease-free survival, DSS = disease-specific survival, EBD = endoscopic biliary drainage, HR = hazard ratio, 95%CI = 95% confidence interval, OS = overall survival, PBD = preoperative biliary drainage, pCCA = perihilar cholangiocarcinoma, PTBD = percutaneous transhepantic biliary drainage, PVE = portal vein embolization.
Funding/support: The study was funded by “Light of West China” program of Chinese Academy of Science [2015 (90)]. The funding sources had no role in the design conduct of the study, collection, analysis, management and interpretation of the data, or preparation, review, or approval of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kimura N, Young AL, Toyoki Y, et al. Radical operation for hilar cholangiocarcinoma in comparable Eastern and Western centers: outcome analysis and prognostic factors. Surgery 2017;162:500–14. [DOI] [PubMed] [Google Scholar]
- [3].Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 2010;17:476–89. [DOI] [PubMed] [Google Scholar]
- [4].Li H, Qin Y, Cui Y, et al. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg 2011;28:226–31. [DOI] [PubMed] [Google Scholar]
- [5].Cheng QB, Yi B, Wang JH, et al. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol 2012;38:1197–203. [DOI] [PubMed] [Google Scholar]
- [6].de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer 2012;118:4737–47. [DOI] [PubMed] [Google Scholar]
- [7].Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343–55. [DOI] [PubMed] [Google Scholar]
- [8].Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012;147:26–34. [DOI] [PubMed] [Google Scholar]
- [9].Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg 2013;257:718–25. [DOI] [PubMed] [Google Scholar]
- [10].Dumitrascu T, Chirita D, Ionescu M, et al. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg 2013;17:913–24. [DOI] [PubMed] [Google Scholar]
- [11].Hakeem AR, Marangoni G, Chapman SJ, et al. Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil-lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma? Eur J Gastroenterol Hepatol 2014;26:1047–54. [DOI] [PubMed] [Google Scholar]
- [12].Hirano S, Tanaka E, Tsuchikawa T, et al. Oncological benefit of preoperative endoscopic biliary drainage in patients with hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:533–40. [DOI] [PubMed] [Google Scholar]
- [13].Giuliante F, Ardito F, Guglielmi A, et al. Association of lymph node status with survival in patients after liver resection for hilar cholangiocarcinoma in an Italian multicenter analysis. JAMA Surg 2016;151:916–22. [DOI] [PubMed] [Google Scholar]
- [14].Mao K, Liu J, Sun J, et al. Patterns and prognostic value of lymph node dissection for resected perihilar cholangiocarcinoma. J Gastroenterol Hepatol 2016;31:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buettner S, van Vugt JLA, Gaspersz MP, et al. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB (Oxford) 2017;19:735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Komaya K, Ebata T, Yokoyama Y, et al. Verification of the oncologic inferiority of percutaneous biliary drainage to endoscopic drainage: a propensity score matching analysis of resectable perihilar cholangiocarcinoma. Surgery 2017;161:394–404. [DOI] [PubMed] [Google Scholar]
- [17].Yoo T, Park SJ, Han SS, et al. Proximal resection margins: more prognostic than distal resection margins in patients undergoing hilar cholangiocarcinoma resection. Cancer Res Treat 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang Z, Yang Y, Meng W, et al. Best option for preoperative biliary drainage in Klatskin tumor: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg 2015;221:1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Furusawa N, Kobayashi A, Yokoyama T, et al. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg 2014;38:1164–76. [DOI] [PubMed] [Google Scholar]
- [21].Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256–63. [DOI] [PubMed] [Google Scholar]
- [22].Baton O, Azoulay D, Adam DV, et al. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg 2007;204:250–60. [DOI] [PubMed] [Google Scholar]
- [23].Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg 2003;238:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miyazaki M, Ito H, Nakagawa K, et al. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg 1999;189:575–83. [DOI] [PubMed] [Google Scholar]
- [25].Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg 1999;230:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nari GA, Palacios OG, Lopez-Ben S, et al. Hilar cholangiocarcinoma: The number of positive nodes and positive node/total node ratio is a significant prognostic factor for survival. Cir Esp 2014;92:247–53. [DOI] [PubMed] [Google Scholar]
- [27].Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129–40. [DOI] [PubMed] [Google Scholar]
- [28].Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg 2013;83:268–74. [DOI] [PubMed] [Google Scholar]
- [29].Kow AW, Wook CD, Song SC, et al. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg 2012;36:1112–21. [DOI] [PubMed] [Google Scholar]
- [30].Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672–9. [DOI] [PubMed] [Google Scholar]
- [31].Chen XP, Lau WY, Huang ZY, et al. Extent of liver resection for hilar cholangiocarcinoma. Br J Surg 2009;96:1167–75. [DOI] [PubMed] [Google Scholar]
- [32].Higgins, JPT. Cochrane handbook for systematic reviews of interventions version 5.0. 2[M]// Cochrane handbook for systematic reviews of interventions /. Wiley-Blackwell, 2008:102–8. [Google Scholar]
- [33].Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 2010;138:1714–26. [DOI] [PubMed] [Google Scholar]
- [34].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [35].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006;244:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guglielmi A, Ruzzenente A, Campagnaro T, et al. Prognostic significance of lymph node ratio after resection of peri-hilar cholangiocarcinoma. HPB (Oxford) 2011;13:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2010;17:463–9. [DOI] [PubMed] [Google Scholar]
- [40].Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg 2010;251:281–6. [DOI] [PubMed] [Google Scholar]
- [41].Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Aggressive surgical resection for hilar cholangiocarcinoma: is it justified? Audit of a single center's experience. Am J Surg 2008;196:160–9. [DOI] [PubMed] [Google Scholar]
- [42].Jarnagin WR, Bowne W, Klimstra DS, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg 2005;241:703–12. discussion 712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg 2004;139:514–23. discussion 523–525. [DOI] [PubMed] [Google Scholar]
- [44].Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 2003;238:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ebata T, Nagino M, Kamiya J, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg 2003;238:720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Capussotti L, Muratore A, Polastri R, et al. Liver resection for hilar cholangiocarcinoma: in-hospital mortality and longterm survival. J Am Coll Surg 2002;195:641–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


