Abstract
To investigate the effect of neoadjuvant chemotherapy in patients with advanced vulvar cancer and to provide references for clinical treatment.
Clinical and pathological data of 12 patients with advanced vulvar carcinoma were collected. The response and operability rates, adverse effects, and prognosis of neoadjuvant chemotherapy were retrospectively analyzed.
The mean patient age was 45.8 (range 26–69) years. Among 12 patients, 9 underwent treatment with bleomycin and cisplatin with or without vincristine. The overall response rate was 67%. Five patients (56%) experienced grade 1 or 2 bone marrow suppression or gastrointestinal reactions. Seven patients (78%) underwent radical surgery. The mean overall survival time was 34.1 (range 3–69) months, the mean progression free survival time was 26 (range 3–69) months, and the 1-year survival rate was 83%. The other 3 patients received combined paclitaxel and cisplatin treatment. The overall response rate was 67%. All 3 patients (100%) experienced grade 2 hair loss or anemia and 2 of them (67%) underwent radical vulvectomy. The mean overall survival time was 11.7 (range 5–15) months, the mean progression free survival time was 7.7 (range 3–15) months and the 1-year survival rate was 100%. Time to overall survival and progression free survival were not significantly different between the 2 groups (P = .46 and P = .39).
Owing to their high overall response rate and tolerable adverse effects, either bleomycin–cisplatin-based or paclitaxel-based neoadjuvant chemotherapy regimen can be considered a therapeutic option for advanced vulvar cancer.
Keywords: efficacy, neoadjuvant chemotherapy, prognosis, surgery, vulvar cancer
1. Introduction
Vulvar cancer is a rare disease that accounts for about 5.6% of all gynecological malignancies. In the United States, an estimated 6020 new cases and 1150 deaths are projected to occur in 2017.[1] The average age at diagnosis is 65 to 70 years.[2,3] However, the incidence of vulvar cancer in young women has recently increased significantly, probably owing to the progression of human papillomavirus (HPV) infection to vulvar intraepithelial neoplasia.[4,5] Vulvar cancer is commonly asymptomatic and diagnosed at stage III to IV (International Federation of Gynecology and Obstetrics staging system) in about 30% to 35% of patients. Approximately 90% of the cases are likely to be squamous cell carcinoma.[6]
Advanced carcinoma of the vulva is defined as cancer that commonly invades or crosses the borders of surrounding structures such as the urethra, anus, bladder, and other adjacent organs.[7] It presents initially in an inoperable form, that is, primary radical surgery does not remove the tumor with adequate margins. Therefore, primary exenterative surgery is needed in this patient population, which can lead to severe postoperative comorbidities and poorer quality of life. Although effective, radiotherapy is associated with considerable toxicity, including frequent skin damage. Thus, finding a more optimal treatment program is imperative.
Studies have shown that advanced vulvar cancer is sensitive to chemotherapy and that neoadjuvant chemotherapy (NACT) can minimize the size of the tumor and the scope of surgery, thus improving the resection rate and quality of life.[8] In this study, we analyzed the efficacy of NACT in patients with advanced vulvar squamous cell carcinoma at our hospital and attempted to provide some references for clinical treatment.
2. Material and methods
2.1. Basic information
In the Department of Gynecology of West China Second University Hospital, we identified 12 patients diagnosed with advanced vulvar squamous cell cancer between January 2011 and December 2016. Of them, 9 patients with urethra involvement needed urinary diversion surgery, 1 patient with bladder involvement needed a urostomy, and 1 patient with rectal involvement needed a colostomy. Surgery was difficult for the remaining 1 patient with fixed regional lymph node involvement. The treatment protocol was approved by local ethics committee. Before treatment, all patients were informed about alternative treatment modalities (primary exenterative surgery, neoadjuvant chemoradiation, or neoadjuvant chemotherapy). The patients agreed to receive NACT after a thorough discussion with their attending gynecologist. The choice to use bleomycin–cisplatin (BC), bleomycin–vincristine–cisplatin (BVC), or cisplatin–paclitaxel (CP) regimen was made by the patient's gynecologist.
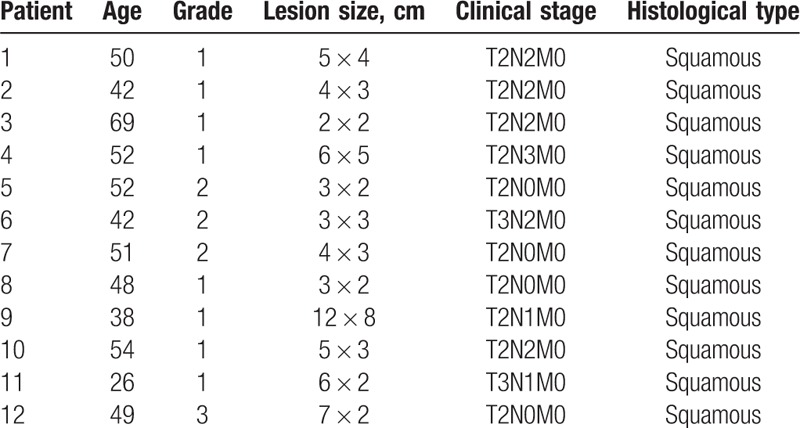
The mean age of the study population was 45.8 years (range 26–69 years). The tumor grade, lesion size, clinical stage, and histological type are shown in Table 1. Ten patients underwent HPV typing: 5 patients were positive for HPV-16, 1 patient was positive for HPV-11, and 4 patients were HPV-negative.
Table 1.
Patient characteristics.
2.2. Chemotherapy regimens
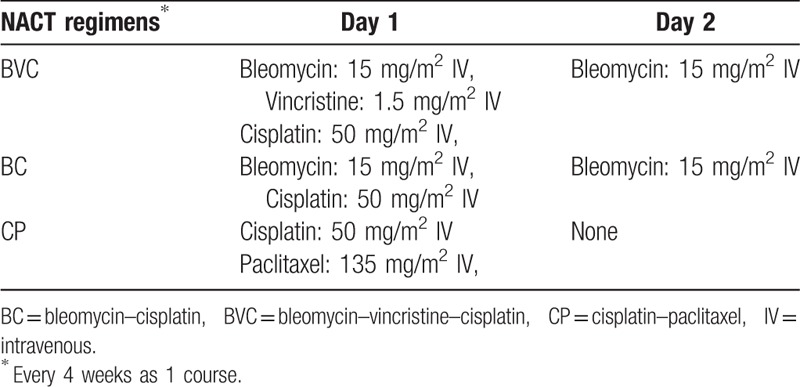
The NACT regimens included in this study were bleomycin-based (BC or BVC regimen) and paclitaxel-based (CP). The specific regimens used are shown in Table 2.
Table 2.
Neoadjuvant chemotherapy regimens used.
2.3. Response and toxicity assessment
Response assessment was performed according to the Response Evaluation Criteria in Solid Tumors, version 1.1.[9] Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 4.0.[10]
In addition, we assessed the operability rate, intraoperative blood loss, postoperative complications, pathology, and survival rates.
2.4. Follow-up and prognosis
Patients were followed through either outpatient visits or telephone interviews. Follow-up was started from the time of diagnosis and ended at the time of the last follow-up (May 31, 2017). Survival time was calculated from the date of diagnosis until death of any cause, and surviving patients were censored at the time of the last follow-up (May 31, 2017).
2.5. Statistical analysis
The data were analyzed using SPSS version 21.0 with Kaplan–Meier method for the survival curve and the log-rank test for comparison of the survival rate of the 2 groups (BC and BVC groups vs CP group). P < .05 was considered statistically significant.
3. Results
3.1. Efficacy of NACT
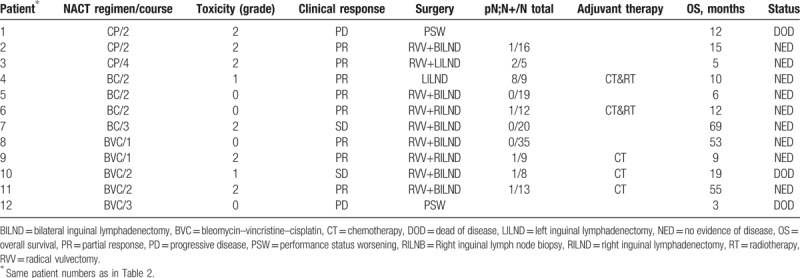
In this study, 12 patients received different NACT regimens, as follows: 9 patients were treated with a BC or BVC regimen, receiving an average of 2 courses of treatment (range 1–4). The overall response rate was 67% (6 patients had a partial response [PR]; 2 patients demonstrated stable disease [SD]; 1 patient demonstrated progressive disease [PD]). Five patients experienced grade 1 or 2 bone marrow suppression or gastrointestinal reactions. Three patients underwent an average of 3 courses (range 2–4) of a CP regimen. The overall response rate was 67% (2 cases with PR; the remaining 1 case demonstrated PD). Three patients experienced grade 2 hair loss or anemia (Table 3).
Table 3.
Treatment and outcomes.
3.2. Surgery, postoperative pathology, and adjuvant therapy
In the BC or BVC group, 7 patients (78%, 7/9) underwent radical vulvectomy (Table 3). Of these 7 patients, 3 underwent partial urethral resection and 1 underwent flap transplantation. For 1 patient (11%) with SD, the surgeon could not completely remove the wide-ranging lesions and the left inguinal lymph node was becoming ruptured; thus, only left inguinal lymph node dissection was performed. The other 1 patient (11%) did not undergo surgery because of PD. The average intraoperative blood loss was 368 mL (range 20–1600 mL), and 1 patient experienced delayed postoperative wound healing owing to local infection. Furthermore, 1 patient had a lymphatic intravascular tumor embolism and 5 patients were diagnosed as having pathologically positive lymph nodes. Five patients received postoperative adjuvant chemotherapy (the same as the NACT regimen) at our hospital; of these, 2 received concurrent radiotherapy (the specifics of which were unknown) at another hospital.
In the CP group, 2 patients (67%, 2/3) underwent radical vulvectomy (Table 3). One patient did not undergo surgery owing to PD. There was an average 900 mL (range 300–1500 mL) intraoperative blood loss and the patients experienced no complications. Both the surgical patients had pathologically positive lymph nodes. And they did not receive postoperative adjuvant chemotherapy or radiotherapy due to economic reasons.
3.3. Follow-up and prognosis
The mean follow-up duration was 28.6 months (range 4–55 months). Two patients died after an ineffective chemotherapy and 1 patient died of disease recurrence after 19 months (Table 2). In the BC and BVC groups, the mean overall survival was 34.1 months (range 3–69 months), the mean progression free survival time was 26 (range 3–69) months and the 1-year survival rate was 83%. In the CP group, the mean overall survival was 11.7 months (range 5–15 months), the mean progression free survival time was 7.7 (range 3–15) months and the 1-year survival rate was 100%.
The overall survival and progression free survival distributions of the BC/BVC groups and the CP group are shown in Figures 1 and 2, respectively. Time to overall survival and progression free survival were not significantly different between the 2 groups (P = .46 and P = .39).
Figure 1.
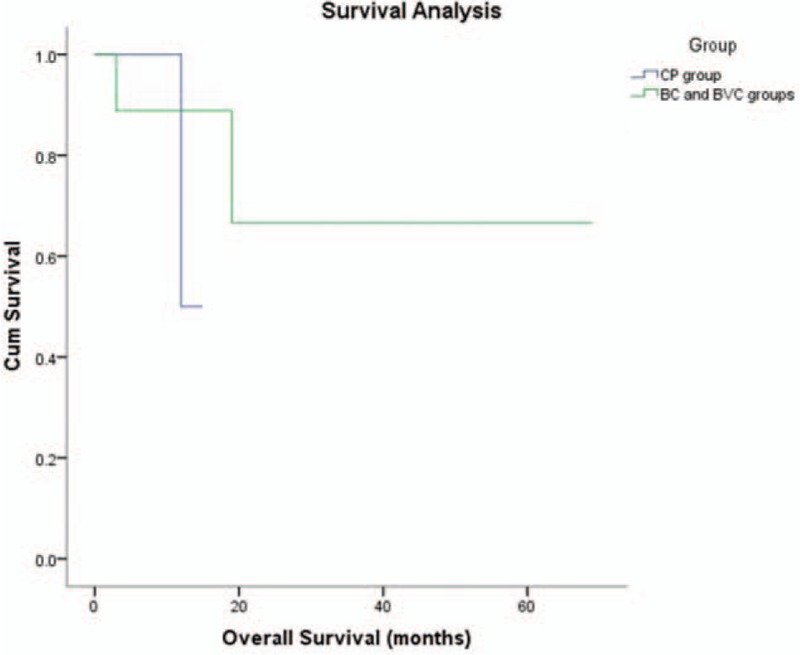
Kaplan–Meier analysis of overall survival for the CP group and the BC and BVC groups. BC = bleomycin–cisplatin, BVC = bleomycin–vincristine–cisplatin, CP = cisplatin–paclitaxel.
Figure 2.
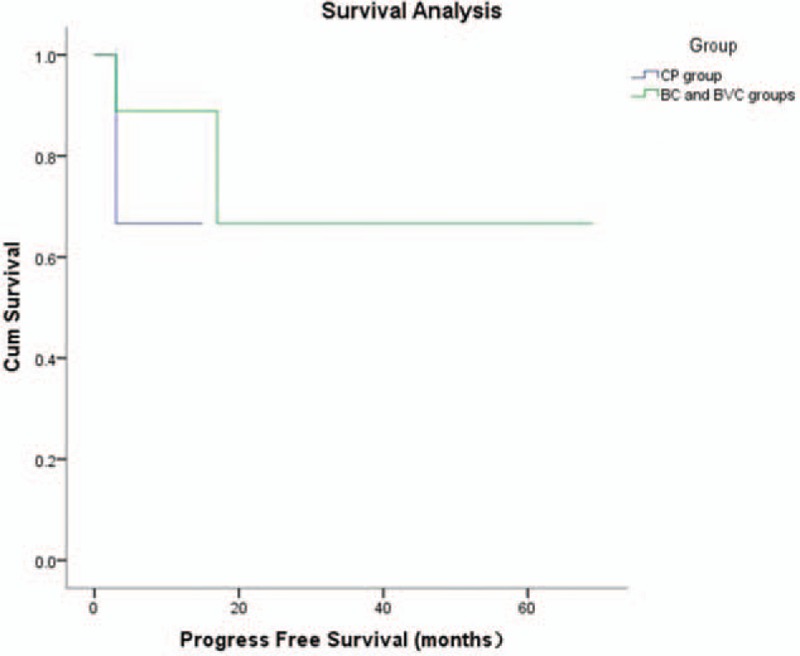
Kaplan–Meier analysis of progression free survival for the CP group and the BC and BVC groups. BC = bleomycin–cisplatin, BVC = bleomycin–vincristine–cisplatin, CP = cisplatin–paclitaxel.
4. Discussion
Exenterative surgery is one of the main treatments for advanced vulvar cancer. However, perioperative mortality rate associated with this surgery can be up to 10% and the incidence rate of complications is > 66.6%. Moreover, it carries a risk of procedure-related morbidity, physical disfigurement, sexual dysfunction, and a largely unknown influence on the overall quality of life. Its 5-year survival rate is less than 50%.[11–14] Another alternative treatment approach for vulvar cancer is chemoradiation. Thomas et al[15] reported that compared with pelvic exenterative surgery, chemoradiotherapy has a good prognosis; however, it causes severe skin toxicity of the vulvar perineum, which is considered unacceptable. In 1987, Boronow et al[16] were the first to report on 37 patients with advanced vulvar squamous cell carcinoma treated with neoadjuvant radiotherapy followed by surgery, demonstrating that the procedure has a good efficacy. In 80% of patients with involvement of adjacent organs, exenterative surgery was avoided; yet, toxicity was still intolerable. In 1990, the Japanese scholars Shimizu et al[17] reported on a 57-year-old patient with stage IV vulvar squamous cell carcinoma who underwent NACT followed by surgery. The patient experienced a complete response with acceptable toxicity. One month later, the patient underwent radical vulvectomy and lived without disease for 20 months. Subsequently, gynecologists increasingly began to adopt NACT for the treatment of patients with advanced vulvar cancer.
Currently, NACT is in the exploratory stage and the treatment regimen varies among clinicians, with no uniform standard. Bleomycin-based combination chemotherapy regimens are applied extensively. Durrant et al[18] conducted a phase II clinical trial that included 31 patients with inoperable primary or recurrent vulvar squamous cell carcinoma who received 3 courses of bleomycin (B), methotrexate (M), and CCNU (C), followed by surgery. The overall response rate was 58%; 9 patients did not complete the total courses of NACT owing to excessive toxicity and 2 patients died of myelosuppression and pulmonary fibrosis. The operability rate was only 26%. Although this study reported a high overall response rate, the severe adverse effects of the BMC regimen cannot be ignored. To reduce toxicity, Wagenaar et al[19] adjusted the BMC regimen by reducing the accumulative dose of bleomycin and CCNU by 25% and decreasing methotrexate by 50%. The overall response rate was 56% (14/25). Approximately 40% of patients with advanced or recurrent vulvar cancer had at least one type of grade 3 or 4 chemotherapy toxicity; 3 patients terminated their study participation because of grade 4 mucositis and 1 patient died of chemotherapy toxicity. Consequently, 57% (8/14) of the responding patients accepted surgery. At a median follow-up of 8 months, 3 patients remained alive, 18 died of vulvar malignancies, 2 died of toxicity, 1 died of unknown cause, and the remaining 1 patient died of intercurrent disease. The 1-year survival rate was only 32%. The authors concluded that the BMC regimen is not a good choice for patients with advanced or recurrent vulvar cancer because of its unavoidable adverse effects. Benedetti-Panici et al[20] reported on 21 patients with stage IV vulvar cancer treated with 2 or 3 courses of a cisplatin, bleomycin, and methotrexate (PBM) regimen before surgery. Of these patients, 2 (10%) had tumor response, 14 (67%) had lymph node response, and the overall response rate was 79%. The toxicity of this regimen was acceptable. However, despite its high response rate, the prognosis was undesirable. The 3-year survival rate was 24%, and 60% of the patients had tumor recurrence at 3 to 17 months postoperative follow-up. In our study, 9 patients received a BC or BVC regimen and the overall response rate was 67%. Five patients (56%) experienced grade 1 or 2 myelosuppression or gastrointestinal reactions and the operability rate was 78%. The average duration of survival was 30.9 months (3–69 months) and the 1-year survival rate was 83%. The authors believe that compared with the BMC and PBM regimens, a BC-based chemotherapeutic regimen has high response and operability rates with an acceptable adverse effect profile. However, the included number of patients is inadequate and large-scale studies associated with this regimen are needed.
Paclitaxel-based chemotherapy treatment is reported to also have a good response in patients with vulvar cancer. Domingues et al[21] reported promising results with single-agent paclitaxel followed by surgery, with an overall response rate of 40%, overall operability rate of 40%, and a 1-year survival rate of 60%. Later, Aragona et al[8] retrospectively analyzed 12 patients with advanced vulvar cancer who underwent 3 cycles of a TC (paclitaxel 175 mg/m2 intravenous [IV] on day 1, cisplatin 50 mg/m2 per day IV on days 2–3) or paclitaxel–cisplatin–5-fluorouracil (paclitaxel 175 mg/m2 IV on day 1, cisplatin 50 mg/m2 per day IV on days 2–3, and 5-fluorouracil 800 mg/m2 per day IV on days 1–4) regimen every 3 weeks before surgery. The overall response and operability rates were both 83% and 33% of the patients experienced grade 3 or 4 hematologic or gastrointestinal toxicity. The efficacy of this regimen was better than that of a single-agent paclitaxel regimen but the toxicity was considerable. The authors considered that it may be related to 5-fluorouracil or the accumulative dose of cisplatin. Besides, Raspagliesi et al[22] conducted a prospective study in which 10 patients with stage III-IV vulvar squamous cell cancer received 3 courses of NACT with a CP (the accumulative dose of cisplatin was reduced to 70 mg/m2 IV for only 1 day and the dose of paclitaxel was not changed) or a paclitaxel–ifosfamide–cisplatin (cisplatin 50 mg/m2 IV was given only on day 1, ifosfamide was added at 5 g/m2 IV on day 2, and the dose of paclitaxel was not changed) regimen. The overall response rate (80%) was similar to that reported in a previous study. Although 40% of patients also experienced grade 3 or 4 bone marrow toxicity, it was tolerable owing to treatment with granulocyte-colony stimulating factor. The operability rate was 90% and the 1-year survival rate was 80%. In our study, 3 patients received a CP regimen, in which the dose of paclitaxel was adjusted to 135 mg/m2 and that of cisplatin was 50 mg/m2, both on day 1. The results were favorable as the overall response and operability rates were both 67% and the toxicity was manageable. The 1-year survival rate was 100%. Concerning the adjustment of paclitaxel and cisplatin, the adverse effect profile was obviously improved while the efficacy remained promising. Furthermore, the authors assumed that the application of the same regimen must take into consideration geographical regions and ethnic variations. Asian patients may not tolerate the large accumulative dose of antitumor chemotherapeutics that is possible in Western patients.
In summary, NACT may be a reliable and promising therapeutic strategy for locally advanced vulvar cancer. BC-based or paclitaxel-based neoadjuvant chemotherapy has a high response rate and tolerable adverse effects. Additionally, it has a unique ability to reduce the size of tumor lesions and improve the operability rate, prognosis, and quality of life.
To the best of our knowledge, we are the first to retrospectively analyze neoadjuvant chemotherapy of vulvar cancer in Southwestern China. Most patients in our study achieved good responses and adverse effects were tolerated. However, the number of patients included in this study (BC and BVC groups of 9 and CP group of 3 patients) was extremely small, preventing the determination of a superior treatment strategy to those elsewhere. Therefore, a multicenter, large, double-blind randomized clinical trial is needed for further investigation.
Author contributions
Analysis: Danqing Wang, Yizhen Niu.
Collection: Meimei Huang, Xiu Gao.
Design: Yin Rutie, Qingli Li.
Investigation: Xiu Gao.
Methodology: Qingli Li.
Software: Meimei Huang.
Supervision: Danqing Wang.
Writing: Yizhen Niu.
Writing – original draft: Yizhen Niu.
Writing – review & editing: Rutie Yin.
Yizen Niu: 0000-0002-9221-6727
Footnotes
Abbreviations: BC = bleomycin–cisplatin, BILND = bilateral inguinal lymphadenectomy, BMC = bleomycin (B), methotrexate (M), and CCNU (C), BVC = bleomycin–vincristine–cisplatin, CP = cisplatin–paclitaxel, CT = chemotherapy, DOD = dead of disease, HPV = human papillomavirus, IV = intravenous, LILND = left inguinal lymphadenectomy, NACT = neoadjuvant chemotherapy, NED = no evidence of disease, OS = overall survival, PD = progressive disease, PR = partial response, PSW = performance status worsening, RILNB = right inguinal lymph node biopsy, RILND = right inguinal lymphadenectomy, RT = radiotherapy, RVV = radical vulvectomy, SD = stable disease.
Statement of nonduplication: Our manuscript is a unique submission and is not being considered for publication by any other source in any medium. Moreover, the manuscript has not been published, in part or in full, in any form.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Office for National Statistics (ONS). Cancer Registration Statistics. England, UK: Office for National Statistics and Public Health England; 2015. [Google Scholar]
- [3].Dellinger TH, Hakim AA, Lee SJ, et al. Surgical management of vulvar cancer. J Natl Compr Canc Netw 2017;15:121–8. Epub 2017/01/04. PubMed PMID: 28040722. [DOI] [PubMed] [Google Scholar]
- [4].Judson PL, Habermann EB, Baxter NN, et al. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol 2006;107:1018–22. [DOI] [PubMed] [Google Scholar]
- [5].Lai J, Elleray R, Nordin A, et al. Vulval cancer incidence, mortality and survival in England: age-related trends. BJOG 2014;121:728–38. [DOI] [PubMed] [Google Scholar]
- [6].Wakeham K, Kavanagh K, Cuschieri K, et al. HPV status and favourable outcome in vulvar squamous cancer. Int J Cancer 2017;140:1134–46. [DOI] [PubMed] [Google Scholar]
- [7].O’Donnell RL, Verleye L, Ratnavelu N, et al. Locally advanced vulva cancer: a single centre review of anovulvectomy and a systematic review of surgical, chemotherapy and radiotherapy alternatives. Is an international collaborative RCT destined for the “too difficult to do” box? Gynecol Oncol 2017;144:438–47. [DOI] [PubMed] [Google Scholar]
- [8].Aragona AM, Cuneo N, Soderini AH, et al. Tailoring the treatment of locally advanced squamous cell carcinoma of the vulva: neoadjuvant chemotherapy followed by radical surgery: results from a multicenter study. Int J Gynecol Cancer 2012;22:1258–63. [DOI] [PubMed] [Google Scholar]
- [9].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990) 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [10].National Institutes of Health National Cancer Institute, Department of health and human services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06–14_QuickReference_5x7.pdf. [Google Scholar]
- [11].Carlson JW, Kauderer J, Walker JL, et al. A randomized phase III trial of VH fibrin sealant to reduce lymphedema after inguinal lymph node dissection: a Gynecologic Oncology Group study. Gynecol Oncol 2008;110:76–82. [DOI] [PubMed] [Google Scholar]
- [12].Graham K, Burton K. Unresectable” vulval cancers: is neoadjuvant chemotherapy the way forward? Curr Oncol Rep 2013;15:573–80. [DOI] [PubMed] [Google Scholar]
- [13].Hacker NF, Leuchter RS, Berek JS, et al. Radical vulvectomy and bilateral inguinal lymphadenectomy through separate groin incisions. Obstet Gynecol 1981;58:574–9. Epub 1981/11/01. PubMed PMID: 7301232. [PubMed] [Google Scholar]
- [14].Rajaram S, Gupta B. Management of vulvar cancer. Rev Recent Clin Trials 2015;10:282–8. Epub 2015/09/29. PubMed PMID: 26411953. [DOI] [PubMed] [Google Scholar]
- [15].Thomas GM, Dembo AJ, Bryson SC, et al. Changing concepts in the management of vulvar cancer. Gynecol Oncol 1991;42:9–21. Epub 1991/07/01. PubMed PMID: 1916517. [DOI] [PubMed] [Google Scholar]
- [16].Boronow RC, Hickman BT, Reagan MT, et al. Combined therapy as an alternative to exenteration for locally advanced vulvovaginal cancer. II. Results, complications, and dosimetric and surgical considerations. Am J Clin Oncol 1987;10:171–81. Epub 1987/04/01. PubMed PMID: 3565317. [DOI] [PubMed] [Google Scholar]
- [17].Shimizu Y, Hasumi K, Masubuchi K. Effective chemotherapy consisting of bleomycin, vincristine, mitomycin C, and cisplatin (BOMP) for a patient with inoperable vulvar cancer. Gynecol Oncol 1990;36:423–7. Epub 1990/03/01. PubMed PMID: 1690680. [DOI] [PubMed] [Google Scholar]
- [18].Durrant KR, Mangioni C, Lacave AJ, et al. Bleomycin, methotrexate, and CCNU in advanced inoperable squamous cell carcinoma of the vulva: a phase II study of the EORTC Gynaecological Cancer Cooperative Group (GCCG). Gynecol Oncol 1990;37:359–62. Epub 1990/06/01. PubMed PMID: 1693584. [DOI] [PubMed] [Google Scholar]
- [19].Wagenaar HC, Colombo N, Vergote I, et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol Oncol 2001;81:348–54. Epub 2001/05/24. doi: 10.1006/gyno.2001.6180. PubMed PMID: 11371121. [DOI] [PubMed] [Google Scholar]
- [20].Benedetti-Panici P, Greggi S, Scambia G, et al. Cisplatin (P), bleomycin (B), and methotrexate (M) preoperative chemotherapy in locally advanced vulvar carcinoma. Gynecol Oncol 1993;50:49–53. [DOI] [PubMed] [Google Scholar]
- [21].Domingues AP, Mota F, Durao M, et al. Neoadjuvant chemotherapy in advanced vulvar cancer. Int J Gynecol Cancer 2010;20:294–8. Epub 2010/02/23. PubMed PMID: 20169671. [DOI] [PubMed] [Google Scholar]
- [22].Raspagliesi F, Zanaboni F, Martinelli F, et al. Role of paclitaxel and cisplatin as the neoadjuvant treatment for locally advanced squamous cell carcinoma of the vulva. J Gynecol Oncol 2014;25:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


