Abstract
The aim of the study was to study the changes in brain structure and functional connectivity in primary insomnia (PI) patients, as well as to explore the biological characteristics of PI abnormality and the pathophysiological mechanism underlying the brain structure and the abnormal functional connectivity under depression.
Voxel-based morphometry (VBM) technique and resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) techniques were used to investigate the brain structure and rs-fc in PI and light-moderate primary insomnia with depression (PID) patients; healthy individuals were used as the normal control (NC) group. The differences between the 3 groups, the correlation between the brain network connection of the anterior cingulate cortex (ACC), and clinical information were compared.
Compared with the NC group, patients in PI and PID groups showed changes in brain structure and brain functional connectivity, which might be related to the pathophysiological mechanism of primary insomnia. PI patients had enhanced connections in the left anterior cingulate cortex/insula, left posterior cingulate, and the right limbic lobe/cingulate gyrus/paracingulate gyrus with ACC. Compared with PI patients, PID patients had weaker brain functional connectivity in the left corpus callosum/posterior cingulate with ACC and enhanced functional connectivity in the frontal and limbic lobes with ACC, suggesting that PI patients with depression had abnormal brain network connection.
Primary insomnia has abnormalities in intracephalic multisystem structure and neural network connection. The interaction and influence between depression and insomnia aggravate the cognitive function damage. This study provided the theoretical basis for exploring the neuropathology underlying the PID disorder and cognitive function.
Keywords: anterior cingulate cortex, cognitive function, depressive disorder, functional magnetic resonance, primary insomnia, resting-state functional connectivity, voxel-based morphometry
1. Introduction
Insomnia is a common risk factor for the attack of other mental diseases. It is divided into primary and secondary insomnia. Primary insomnia (PI) refers to the difficulty in falling asleep, maintaining sleep, or refreshing after sleep for at least 1 month, rather than secondary to other sleep disorders, excluding causatives such as drug or other mental disorders.[1] The morbidity rate is 3% to 5%,[2] accounting for 25% of chronic insomnia. PI can increase the risk of suffering from cardiovascular diseases and diabetes in the middle-aged and elderly individuals.[3] The slow disease course causes a decrease in functional activities during the daytime, severely influencing the normal physiological activities and the quality of life. Thus, the neurobiological investigations on PI can provide effective imaging evidence for diagnosing the disease and evaluating the therapy.
Chronic insomnia is one of the risk factors for the attack of cardiovascular diseases as well as death. This attack might be correlated with hyperarousal, a disorder of circadian rhythm, and endocrine disequilibrium.[3] PI possesses several characteristics, such as early and easy awakening, decreased sleep quality, and difficulty in sleep initiation and maintenance, accompanied by significant daytime functional injury. PI is often accompanied by hyperarousal status.[4] Such patients are under overreaction and stress with respect to mental state, physiology, emotion, and cognition; the level of hormone increases and the overall basal metabolic level also increases, along with the physiological arousal. In addition, they often present circadian dysregulation and abnormality in the sleep-awakening mechanism that causes emotional disorder and greatly influences the health. Clinically, PI patients are often accompanied by anxiety and depression disorder in different degrees, and also present habitual anxiety.[5] Insomnia is an independent risk factor for depression, with a complicated relationship. The quality of sleep interplays with an emotional disorder, and the disorder of sleep-awakening regulation aggravates the emotional symptoms. Long-term vicious circle leads to damage of the cognitive function, subjective sensation, or mental disorder. Shekleton et al[6] found that PI patients presented cognitive function damages in different degrees. Furthermore, only a few studies based on magnetic resonance were carried out on PI. Harper et al[7] reported that the pathogenesis of PI might be closely related to the arousal system (reticular structure ascending activating system and hypothalamus), emotional regulation system (hippocampus, amygdala, and anterior cingulate cortex), and cognitive system (prefrontal cortex), which provides the basis for magnetic resonance on the neuropathological mechanism of PI.
Recently, magnetic resonance has been developed rapidly in neurosciences, which is divided into structural and functional types, and widely applied in various investigations of nerve and mental disorders, such as schizophrenia,[8] Alzheimer's disease (AD),[9] and epilepsy.[10] Using these magnetic resonance techniques in PI is useful for further exploring the disease as well as clinical application. Voxel-based morphometry (VBM) indicates the morphological and biological characteristics of brain tissues, and hence we used this technique to analyze the morphological changes in the gray matter structure of the brain in PI patients and those with depression (PID). Functional magnetic resonance imaging (fMRI) can indicate the status of brain tissue and neural activity; it is divided into resting-state and task-state. Resting-state fMRI (rs-fMRI) is based on the blood oxygenation level-dependent (BOLD) signal that is generated to maintain the activity of the brain without any specific tasks or clear external/internal stimuli. The functional connectivity (fc) analysis based on rs-fMRI (rs-fcMRI) can analyze the network connections of brain function, in order to prospectively investigate the regulation of nerve function connection of PI. The anterior cingulate cortex (ACC) plays critical roles in the human brain function, such as cognitive function, automatic control, and emotion processing. Carter et al[11] found that with a prolonged sleep deprivation, the functional activities of ACC are decreased, thereby leading to reduced attention and executive function. Herein, the bilateral ACC has been considered as the seed point, and rs-fcMRI analysis is performed to explore the abnormality of ACC network connection in PI and PID patients. We also explored the influences of cognitive impairment and emotional disorder on the brain neural network in resting-state.
Moreover, whether the brain structure and brain function of PI would change? Whether the depressive disorder has influences on the brain structure and functional connectivity of PI? Whether the changed brain region would cause changes in the brain function? Whether the clinical scores Pittsburgh Sleep Quality Index (PSQI) and Hamilton Depression Scale (HAMD) have a correlation with the brain region under abnormal functional connectivity? Only a limited number of current studies are available of the above issues. Therefore, using normal control group as reference, we explored the brain structure and rs-fcMRI in PI and PID patients and compared and analyzed the differences among them, as well as the correlations between the ACC brain network connection and clinical information, which could aid in the early-stage diagnosis and treatment of PI patients.
2. Materials and methods
2.1. Study subjects
The present study consisted of 3 groups: normal control (NC), PI, and PID (light and moderate depression) groups. The study protocol was approved by the Ethics Committees of Henan Provincial People's Hospital. All patients signed the informed consent.
The inclusion criteria for participation in the study were as follows: neither physical and mental diseases nor family history of neuronal and mental disease; no history of alcohol and psychotropic drug abuse; education ≥6 years (above elementary school); age 20 to 50 years; Han nationality and right-handedness; no contraindications of magnetic resonance examination in the body, and no organic diseases found in the brain; all participation do not have MRI contraindications, such as metallic implants, claustrophobia, or devices in the body.
2.1.1. Patients group
All the patients were from the Department of Neurology (outpatient and inpatient). A total of 36 patients with PI from January 2013 to November 2014 were included, and finally, 30 patients complying with the inclusion criteria were enrolled. The cohort comprised of 15 patients with PI (6 males and 9 females), aged between 22 and 50 years (average, 37.13 ± 2.53 years), and education of 11.53 ± 1.125 years. Another 15 patients presented light and moderate depression (7 males and 8 females) were aged between 28 and 50 years (average, 40.53 ± 1.919 years), and education of 11.40 ± 0.755 years.
2.1.1.1. Inclusion criteria
-
1.
Complying with the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Diseases, Fourth Edition (DSM-IV) of PI: A duration of insomnia of ≥1 year with sleep difficulty occurring at least 3 nights per week.
-
2.
Patients who never received clinical intervention therapy;
-
3.
Inclusion criteria for PID: PSQI score ≥ 7, Hamilton anxiety scale (HAMA) score <14, HAMD in PI group <7, PID group 7 ≤ HAMD ≤ 24.
2.1.2. NC group
Fifteen healthy individuals (7 males and 8 females), age- and sex-matched were enrolled in the NC, aged 21 to 49 years (average, 32.60 ± 2.541 years), education of 32.60 ± 2.541 years, PSQI score <7, HAMD score <7, and HAMA<7 score. The control group neither presented any diseases in the past 2 weeks nor received any drugs. Smoking, drinking, and staying up late, as well as ingesting stimulating foods were not allowed within 3 days before scanning.
2.2. Study methods
2.2.1. Clinical evaluation
Two experienced neurological physicians graded the clinical scales and graphs, including PSQI, HAMD, HAMA, and Rey Complex Figure Test (RCFT). The parameters for the scales were as follows: PSWI: total score 0 to 21, high value indicates poor sleep quality, PSQI ≥7 is insomnia; HAMD: total score 52, <7 is normal, 7 to 16 is light depression, 17 to 24 is moderate depression, >24 is severe depression; HAMA: <7 is not anxiety, 14 is boundary value, and >14 is anxiety disorder. Intraclass correlation coefficient (ICC) was used to evaluate the consistency of PSQI scores (ICC = 0.98), and weighted Kappa value was used to assess the consistency of HAMD, HAMA, and RCFT scores from the 2 physicians. Kappa value >0.75 indicates a good consistency of the score as assessed by the 2 physicians.
2.2.2. Magnetic resonance examination
Siemens Trio Tim 3.0 T magnetic resonance imaging system (Siemens, Erlangen, Germany) was used, as well as 12 head channels phased the array coil. All the subjects underwent whole brain 3D high-resolution T1W1 structure imaging and rs-fcMRI scanning of the whole brain.
The whole brain structure imaging was conducted by 3D high-resolution magnetization for preparing fast gradient echo imaging (3D MPRAGE) sequence and sagittal encompassing the whole brain scanning. Scanning parameters: TR/TE = 2300/2.98 ms, reversing time TI = 900 ms, flip angle 9°, slice thickness 1.2 mm, visual field FOV 240 × 256 mm2, matrix 256 × 256, number of excitation NEX 1, voxel 1 × 1 × 1.2 mm3, and scanning time total 9′14″.
rs-fcMRI: The subjects should be told to keep quiet, relax, eyes closed, and placed down on the examination table. Gradient echo combined with single excitation EPI technique was used. Scanning parameters: TR/TE = 3000/30 ms, visual field FOV 1200 × 1200 mm2, matrix 64 × 64, slice thickness: 3 mm, slice gap 0.5 mm, totally 36 layers, scanning time 7′06″.
The criteria before scanning were as follows: do not drink stimulants, such as alcoholic beverage, strong tea, and coffee at the scanning day; women should not be in pregnancy or menstrual period; onlookers are forbidden; the scanning time should not be later than 9:00 pm; the patients should come to the waiting area before 30 minutes.
2.2.3. Data processing
The original DICOM (digital imaging and communications in medicine) data were transferred by MRIcro software, analyzed, and processed using SPM8 (statistical parametric maps) and VBM tools in MATLAB R2009b software. REST software of MATLAB r2009b was used to remove the concomitant variables, such as head motion parameter, whole brain signal, white matter signal, and cerebrospinal fluid signal (CSF).
2.2.4. rs-fcMRI images processing
SPM8 (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and REST1.8 (resting-state data analysis toolkit, http://www.restfmri.net/forum/REST) in MATLAB R2009b software were used to reprocess the fMRI imaging data of the subjects. To eliminate the interference of the surrounding environment and instability of the magnetic field, the images of the initial 10 time points were excluded. All functional runs were expressed relative to the first values in each run. We set a movement threshold of 1.5 mm and 1.5° for the 3 linear and 3 axial coordinates to eliminate subjects with excessive head movement. However, none of the subjects had head movements that exceeded threshold. All functional runs were normalized to Montreal Neurological Institute (MNI) space with voxel resampling to 3 × 3 × 3 mm3. After spatial normalization, we used REST to extract the linear changes over time within the 0.01 to 0.08 Hz bandwidth. The resulting time series were then spatially smoothed with a 4-mm full width at half maximum (FWHM) Gaussian kernel.
2.2.5. Functional connectivity of rs-fcMRI
WFU_pick Atlas software (http://www.ansir.wfubmc.edu) was used to select the bilateral ACC as the region of interest (ROI) in automatic anatomical labeling (AAL), generating seed points, and extracting the average reference time series of bilateral ACC. Voxels within the seed region were averaged to generate reference time series. Lastly, all the time series of the voxel of the whole brain were processed by correlation analysis to obtain a figure relevant to functional connectivity. The correlation coefficient “r” was transferred by Fisher “Z” to make the data comply with a normal distribution, followed by calculating the functional connectivity between bilateral ACC and whole brain. The T value indicated the correlation of functional connectivity; a higher T value indicated superior correlation.
2.2.6. Statistical analysis
Statistics for general information: SPSS17.0 was used to analyze the data. ANOVA was used to compare the differences in sex, age, and level of education among the 3 groups. P < .05 was termed as statistical significance.
VBM data analysis: Single sample t test was used to compare the differences in the gray matter volume in each brain region among NC, PI, and PID groups. P < .001 served as the test threshold, and cluster >50 voxels. The brain region images with a statistical difference were overlapped on the 3D structure of MNI provided by SPM8, localized by MIN coordinates, and Brodmann (BA) partition. The regions were observed and recorded. T value indicated the decrease in the degree of gray matter volume; the higher T value indicated the greater reduction in the degree.
Rs-fcMRI data analysis: Double sample t test in REST 1.8 software was used to compare the differences among the NC, PI, and PID groups. P < .01 served as the significant activation threshold, and activated voxels ranged >52 voxels (corrected by AlphaSim). The 3D images were generated using BrainNet Viewer, and xjView software was used to automatically identify the abnormal brain region, such that the functional connectivity between each brain region and ACC of each patient could be well understood, as along with the connection changes under resting-state.
Correlation analysis: REST1.8 software was used for extracting the functional connectivity strength, that is, the Z value of each ROI from the brain regions with abnormal functional connectivity in the 3 groups. Pearson's correlation in the SPSS17.0 software was used to analyze the correlation between Z values and scores in each scale. P < .05 was considered as statistical significance.
3. Results
3.1. Baseline data
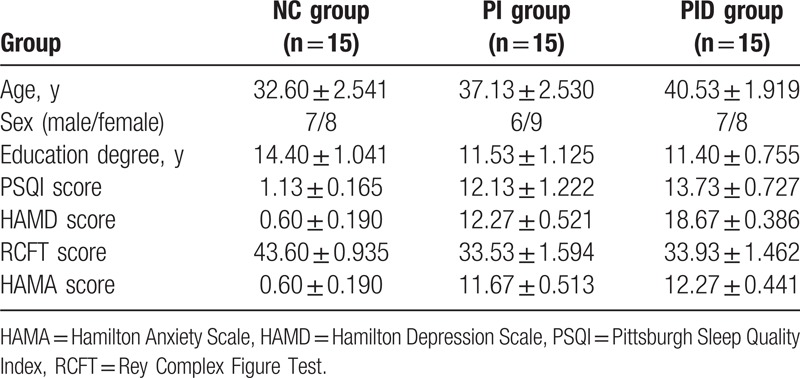
No statistically significant differences were observed with respect to sex, age, and the level of education among the 3 groups (P > .05) (Table 1).
Table 1.
Baseline clinical data of the subjects in the 3 groups.
3.2. VBM
Compared with the NC group, the volumes of brain structure in multiple sites decreased in PI patients; however, the volume of the left middle temporal gyrus increased (P < .001, cluster size >50 voxels) (Tables 2 and 3; Figs. 1 and 2).
Table 2.
Brain region with decreased gray matter volume in PI patients as compared with the NC group.
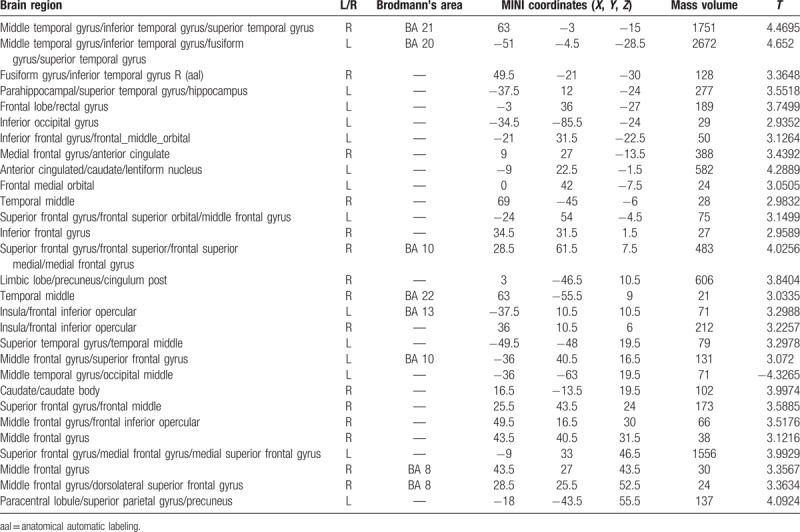
Table 3.
Brain region with increased gray matter volume in PI patients as compared with the NC group.
![]()
Figure 1.
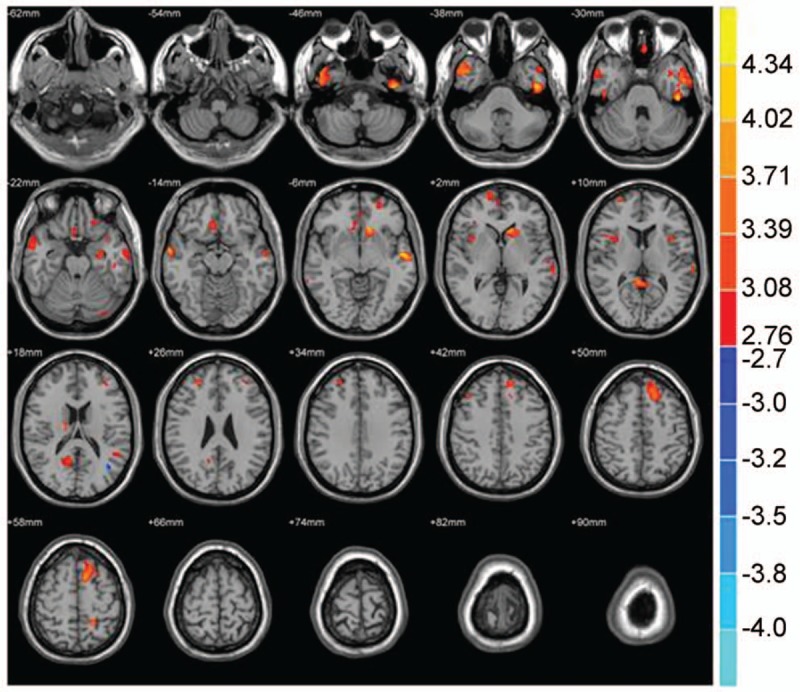
Compared with the NC group, brain region showed decreased gray matter volume in the PI group; P < .001, cluster >50 voxels.
Figure 2.
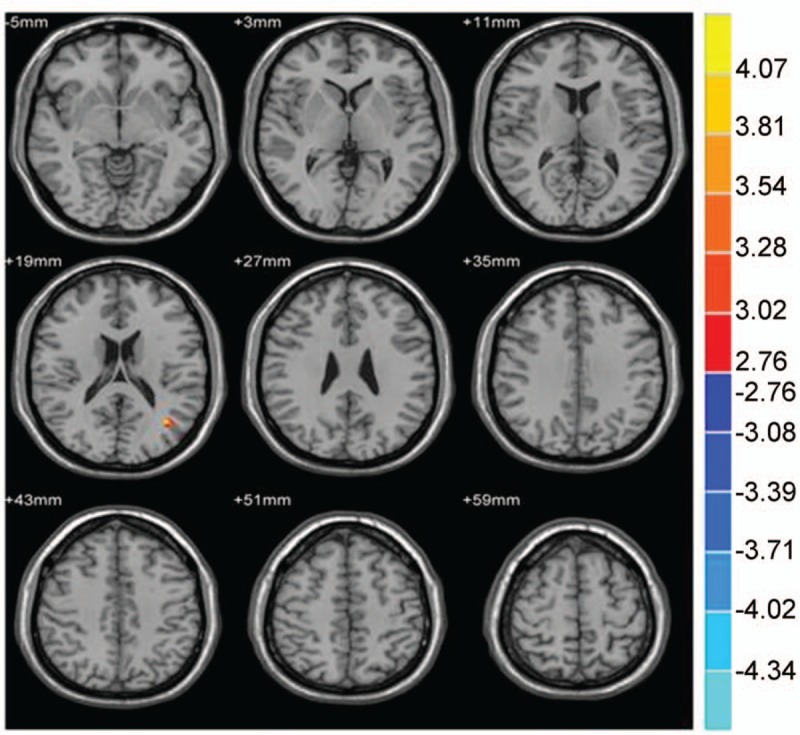
Compared with the NC group, brain region showed increased gray matter volume in the PI group; P < .001, cluster >50 voxels.
Compared with the NC group, the volumes of multiple sites in the PID group decreased, and the volumes of multiple sites increased (P < .001, cluster >50 voxels) (Tables 4 and 5, Figs. 3 and 4).
Table 4.
Brain region with decreased gray matter volume in PI patients as compared with the PID group.
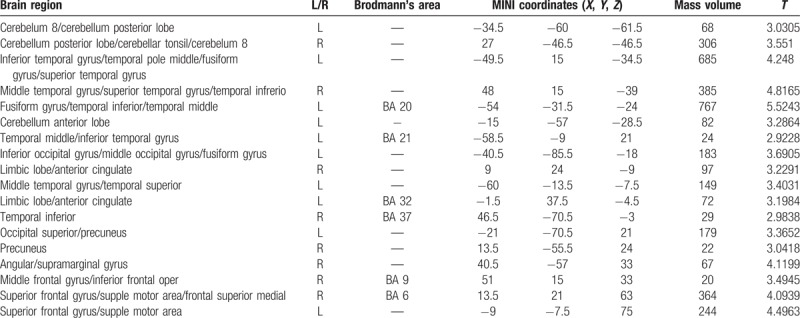
Table 5.
Brain region with increased gray matter volume in PID patients as compared with the NC group.
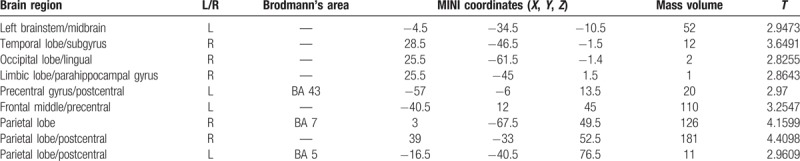
Figure 3.
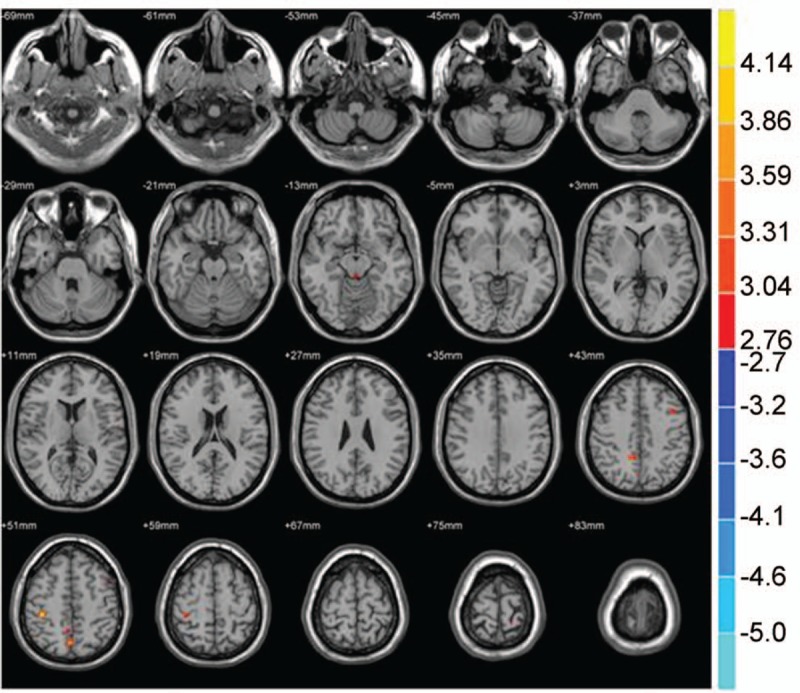
Compared with the NC group, brain region showed decreased gray matter volume in the PID group; P < .001, cluster>50 voxels.
Figure 4.
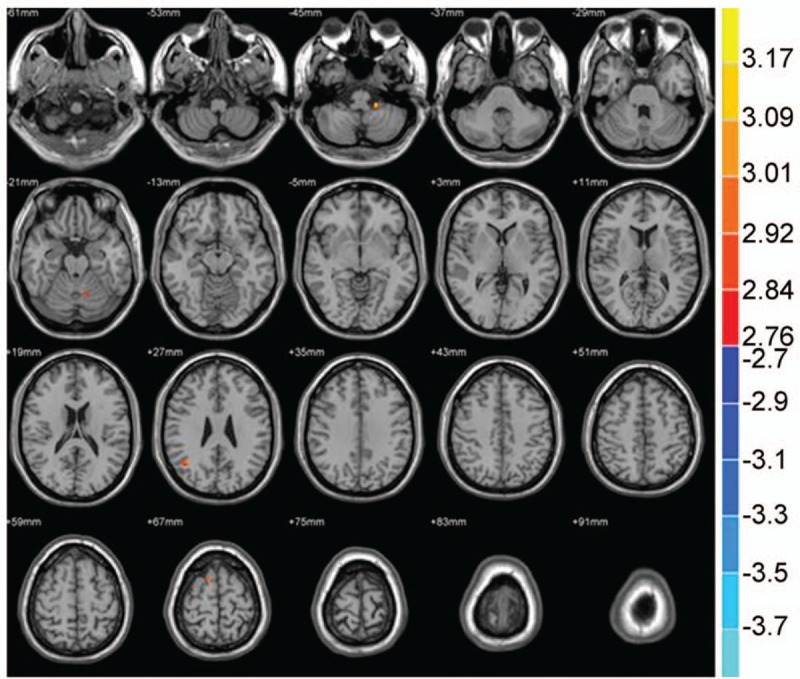
Compared with the NC group, brain region showed increased gray matter volume in the PID group; P < .001, cluster >50 voxels.
Compared with the PI group, the volumes of multiple sites in the PID group decreased, and the volumes of multiple sites increased (P < .001, cluster >50 voxels) (Tables 6 and 7; Figs. 5 and 6).
Table 6.
Brain region with decreased gray matter volume in PID patients as compared with the PI patients.

Table 7.
Brain region with increased gray matter volume in PID patients as compared with the PI patients.
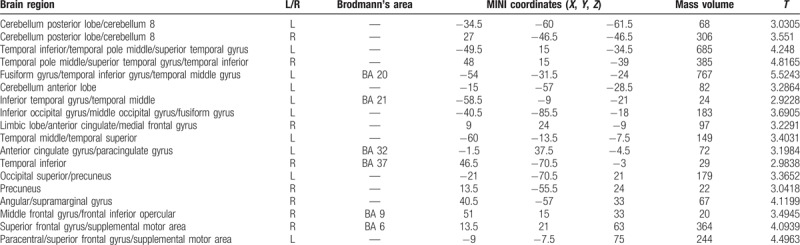
Figure 5.
Compared with the PI group, brain region showed decreased gray matter volume in the PID group; P < .001, cluster >50 voxels.
Figure 6.
Compared with the PI group, brain region showed decreased gray matter volume in the PID group; P < .001, cluster >50 voxels.
3.3. Rs-fcMRI
3.3.1. Brain functional connectivity analysis considers bilateral ACC as a seed point
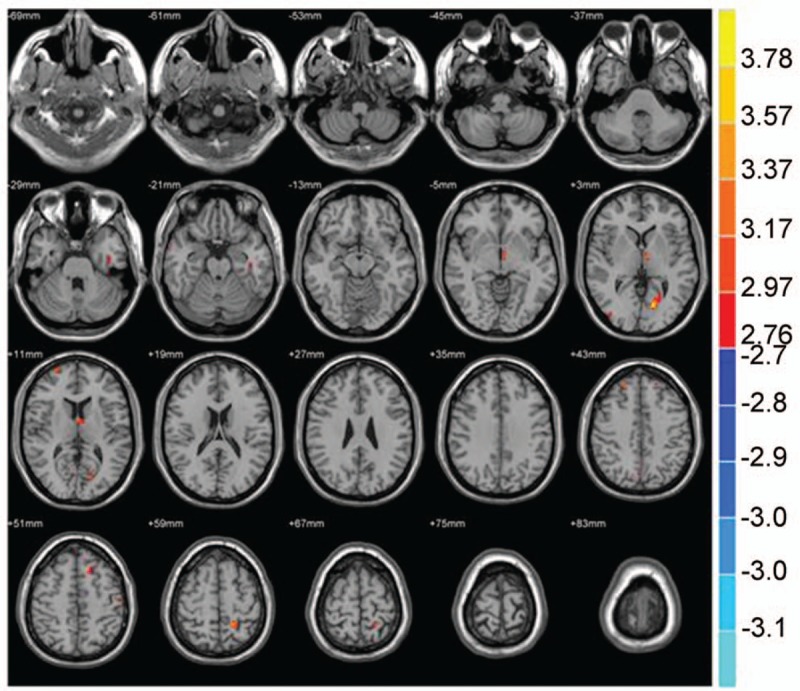
In the brain functional connectivity neural networks of the NC group, multiple sites showed a positive correlation with ACC, and multiple sites exhibited a negative correlation with the anterior cingulate cortex (P < .01, cluster >20 voxels) (Table 8 and Fig. 7).
Table 8.
Brain functional connectivity in NC group considered as bilateral ACC (seed point).
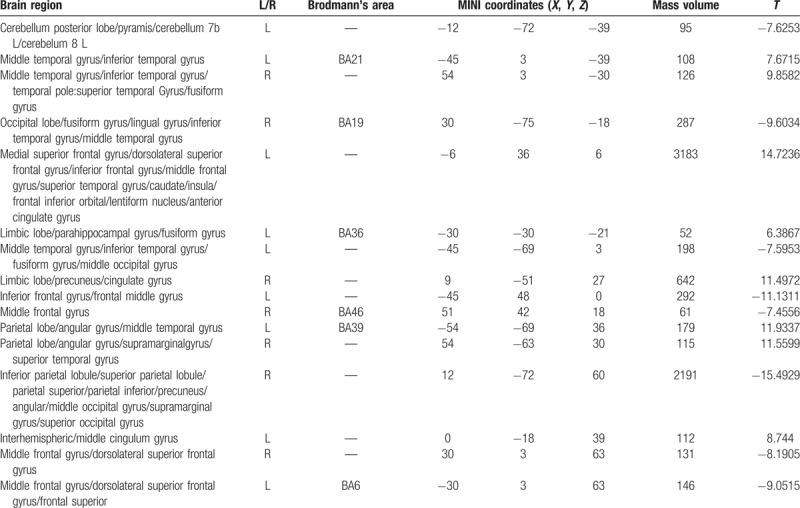
Figure 7.
Brain functional connectivity in NC considering bilateral ACC as a seed point. The significant threshold was set at P < .01, cluster >20 voxels. Red indicates the area with the enhanced connection; blue indicates the area with the weakened connection.
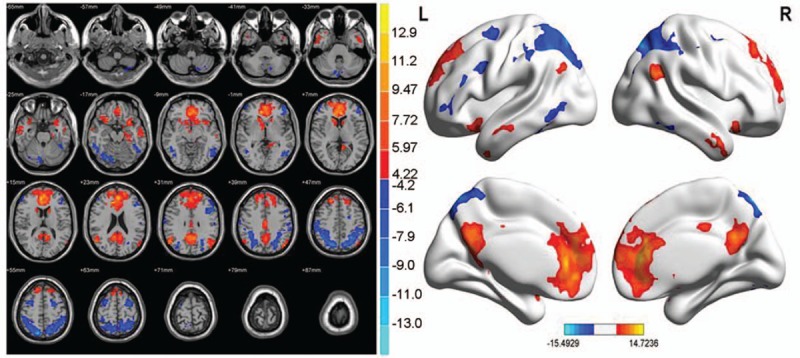
In the neural networks of the abnormal brain functional connectivity of PI patients, multiple sites showed a negative correlation with ACC, and multiple sites were positively correlated with ACC (P < .01, cluster >20 voxels) (Table 9 and Fig. 8).
Table 9.
Abnormal brain functional connectivity in PI group considered as the bilateral ACC (seed point).
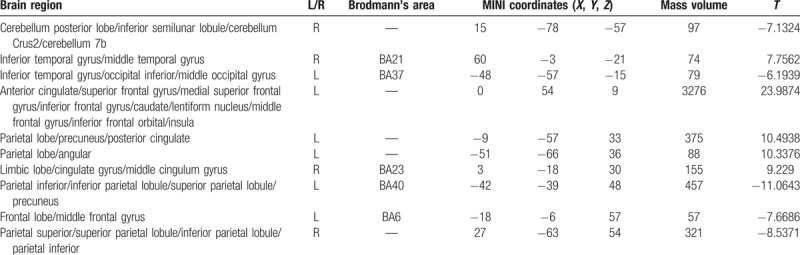
Figure 8.
Abnormal brain functional connectivity in PI patients, considering bilateral ACC as a seed point. The significant threshold was set at P < .01, cluster >20 voxels. Red indicates the area with the enhanced connection; blue indicates the area with the weakened connection.
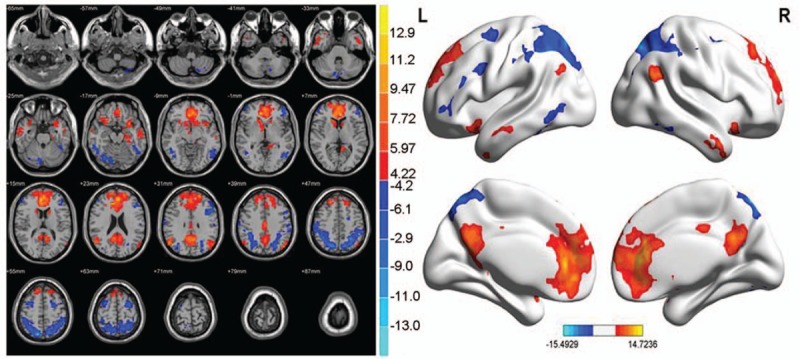
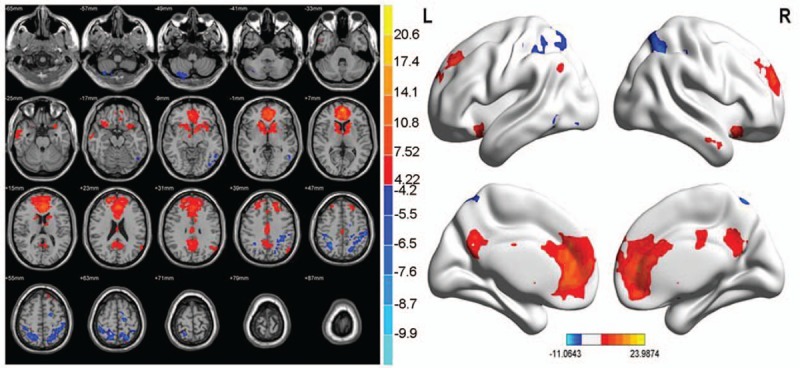
In the abnormal brain functional connectivity neural networks in PID patients, multiple sites were negatively correlated with ACC, in addition multiple sites showed a positive correlation with ACC (P < .01, cluster >20 voxels) (Table 10 and Fig. 9).
Table 10.
Abnormal brain functional connectivity in PID group, bilateral ACC considered as a seed point.
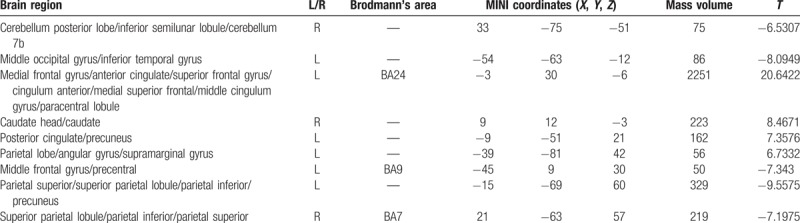
Figure 9.
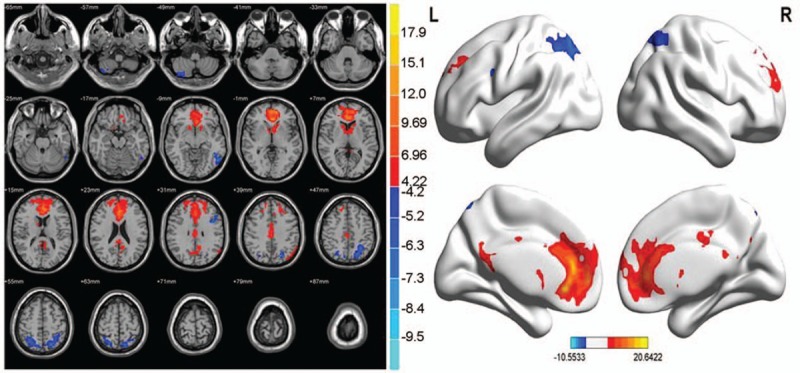
Abnormal brain functional connectivity in PID patients, considering bilateral ACC as a seed point. The significant threshold was set at P < 0.01, cluster >20 voxels. Red indicates the area with the enhanced connection; blue indicates the area with the weakened connection.
Compared with the NC, the brain region was negatively correlated with the ACC functional connectivity in PI patients, including the right inferior temporal gyrus, right superior parietal gyrus/superior parietal lobule/inferior parietal lobule, and left limbic lobe/cingulate gyrus. The region that was positively correlated with ACC functional connectivity included the left cerebellum anterior lobe/culmen/lingual gyrus (P < .01, cluster >20 voxels) (Table 11 and Fig. 10).
Table 11.
Brain functional connectivity analysis, bilateral ACC considered as a seed point for comparison between the PI and NC groups.

Figure 10.
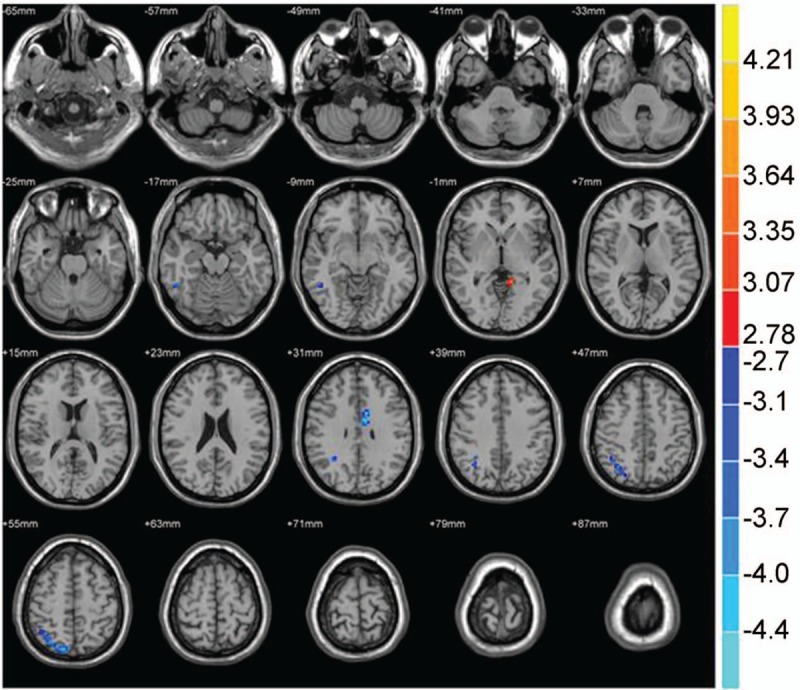
The brain functional connectivity was analyzed between the PI patients and NC group, considering bilateral ACC as a seed point. The significant threshold was set at P < .01, cluster >20 voxels. Red indicates the area with the enhanced connection; blue indicates the area with the weakened connection.
Compared with the NC group, the brain region negatively correlated with the ACC functional connectivity of PID patients, including brainstem/midbrain, left middle temporal gyrus, and left posterior cerebellar lobe/cerebellum Crus1 area. The region positively correlated with the ACC functional connectivity included the left parietal lobe/subgyrus/Rolandic operculum, left parietal lobe/subgyrus/pars triangularis inferior frontal gyrus, right parietal lobe/middle occipital gyrus, and right superior parietal gyrus (P < .01, cluster >20 voxels) (Table 12 and Fig. 11).
Table 12.
Brain functional connectivity analysis, bilateral ACC considered as a seed point for comparison between the PID and NC groups.

Figure 11.
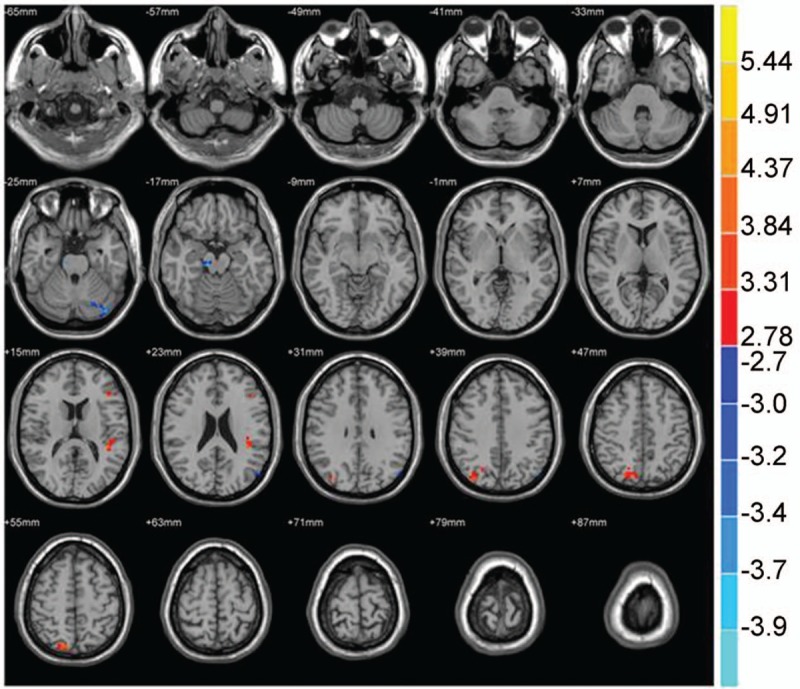
The brain functional connectivity was analyzed between the PID patients and NC group, considering bilateral ACC as a seed point. The significant threshold was set at P < .01, cluster >20 voxels. Red indicates the area with the enhanced connection; blue indicates the area with the weakened connection.
Compared with the PI patients, the left corpus callosum/posterior cingulate in the PI patients was negatively correlated with ACC functional connectivity, whereas the midbrain was positively correlated (P < .01, cluster >20 voxels) (Table 13 and Fig. 12).
Table 13.
Brain functional connectivity analysis, bilateral ACC considered as a seed point for comparison between the PID and PI groups.

Figure 12.
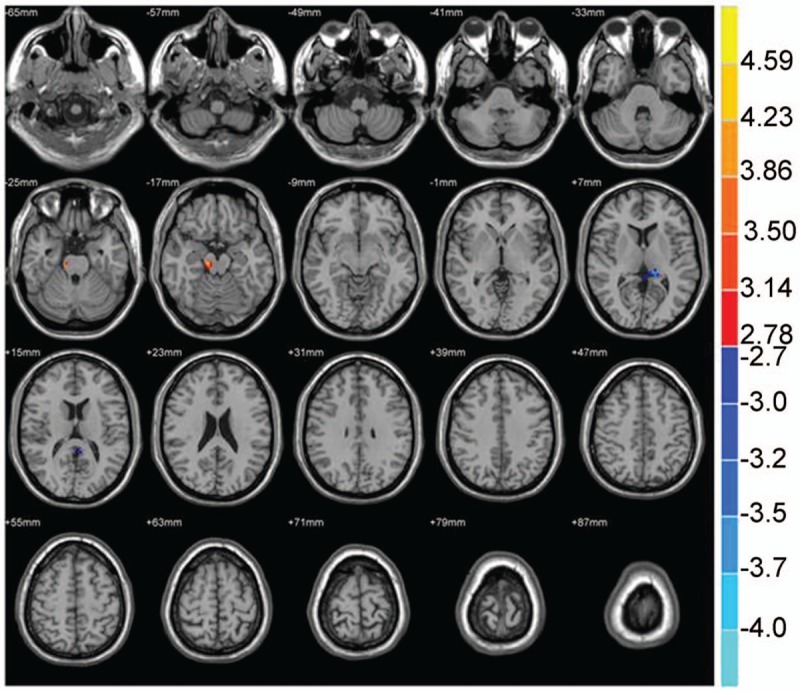
The brain functional connectivity was analyzed between the PI patients and PID patients, considering bilateral ACC as a seed point. The significant threshold was set at P < .01, cluster >20 voxels. Red indicates the area with the enhanced connection; blue indicates the area with the weakened connection.
3.3.2. Correlation analysis
Abnormal areas in brain functional connectivity in PI patients and NC group: left cingulate gyrus, left lingual, right inferior temporal gyrus, and right superior parietal gyrus were not significantly associated with the PSQI score (P > .05) (Fig. 13).
Figure 13.
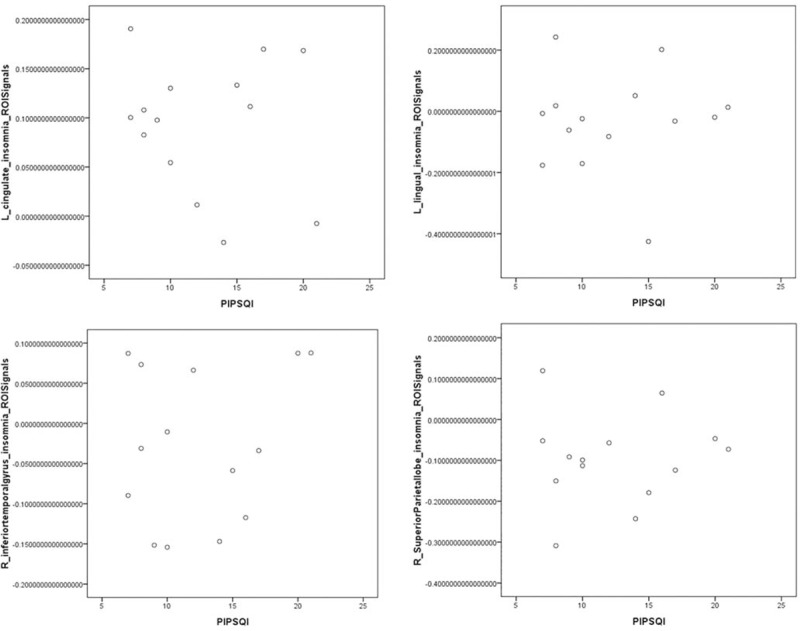
The abnormal areas of brain functional connectivity in PI patients and NC group: left cingulate gyrus, left lingual gyrus, right inferior temporal gyrus, and right superior parietal gyrus were not significantly associated with the PSQI score (P >.05).
Abnormal areas in brain functional connectivity in PID patients and NC group: midbrain, left middle temporal gyrus, and left cerebellum Crus1 area were negatively correlated with the PSQI score (P < .05), and the left Rolandic operculum, left pars triangularis inferior frontal, right middle occipital gyrus, and right superior parietal gyrus were not significantly associated with the PSQI score (P > .05). The other abnormal areas of brain functional connectivity were not significantly associated with the HAMD score (P > .05), except the right pars triangularis inferior frontal region that was positively correlation with the HAMD score (P < .05) (Figs. 14–17).
Figure 14.
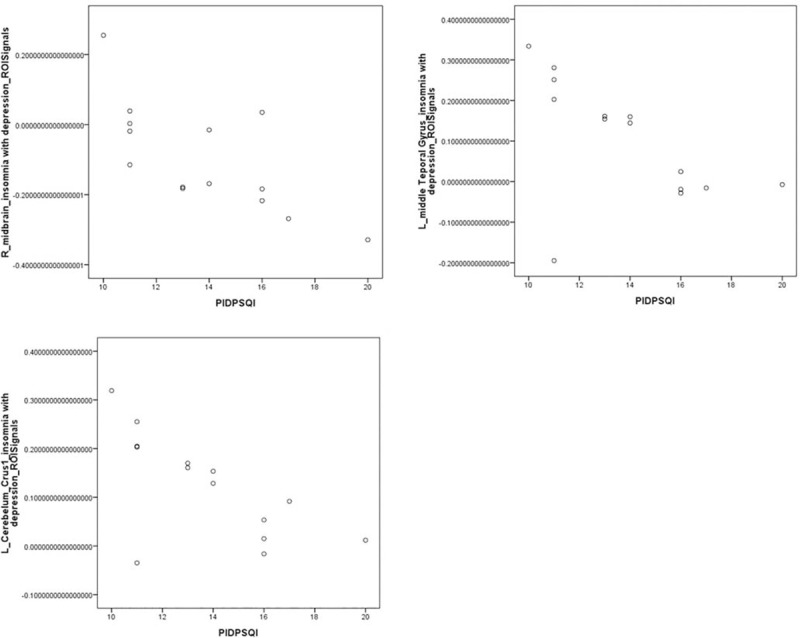
The abnormal areas of brain functional connectivity in PID patients and NC group: midbrain, left middle temporal gyrus, and left cerebellum Crus1 area showed significant negative correlations with the PSQI score (r = −0.718, −0.556, −0.662, respectively; P < .05).
Figure 17.

The abnormal areas of brain functional connectivity in PID patients and NC group: left middle temporal gyrus, left Rolandic operculum, right cerebellum Crus1 area, midbrain, right middle occipital gyrus, and right superior parietal gyrus were not significantly associated with the HAMD score (P > .05).
Figure 15.
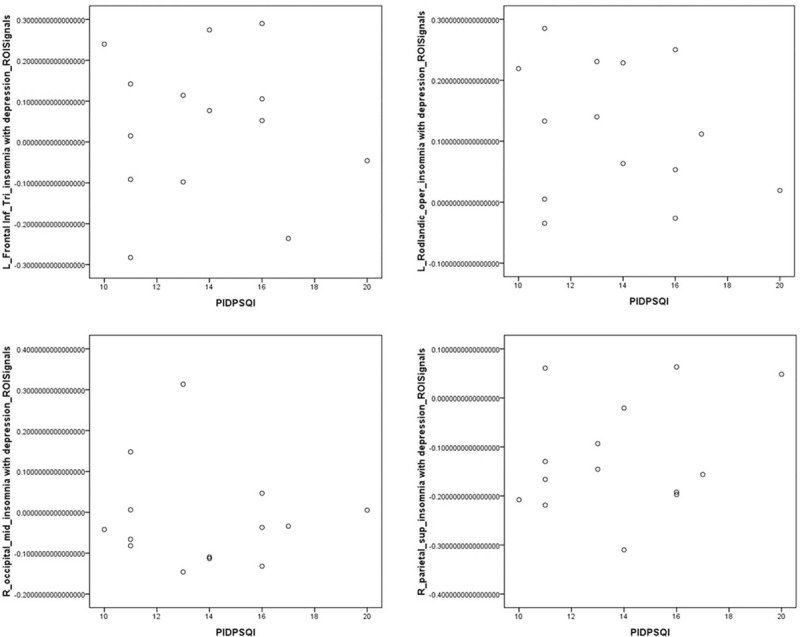
The abnormal areas of brain functional connectivity in PID patients and NC group: left Rolandic operculum, left pars triangularis inferior frontal gyrus, right middle occipital gyrus, and right superior parietal gyrus were not significantly associated with the PSQI score (P > .05).
Figure 16.
The abnormal areas in brain functional connectivity in PID patients and NC group. The functional connectivity degree of left pars triangularis inferior frontal gyrus showed a significant positive correlation with the HAMD score (r = 0.720, P < .05).
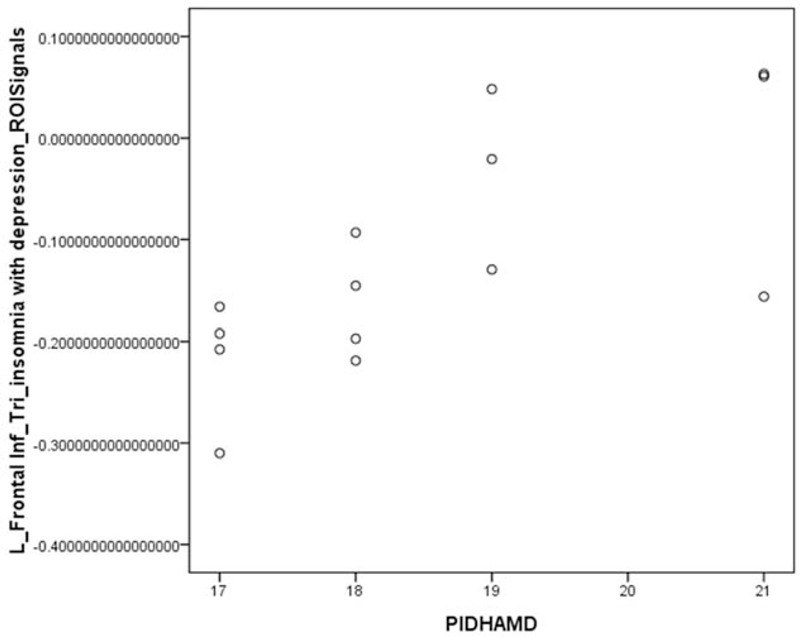
The abnormal areas in brain functional connectivity in PID and PI patients: left posterior cingulate showed a positive correlation with the HAMD score (P < .05), whereas the midbrain was not associated similarly (P > .05) (Fig. 18).
Figure 18.
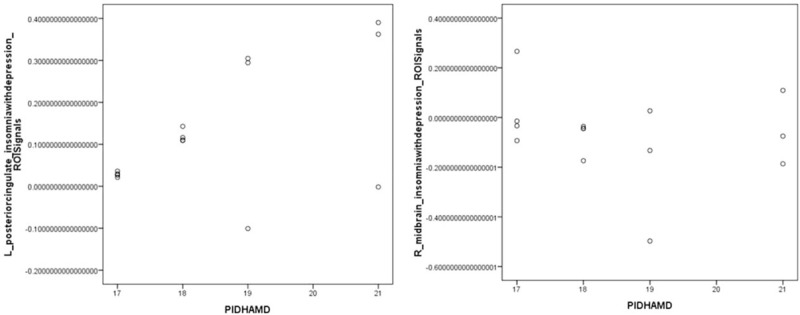
The abnormal areas of brain functional connectivity in PID and PI patients. The left posterior cingulate was positively correlated with the HAMD score (r = 541, P < .05), whereas the midbrain was related significantly (P > .05).
4. Discussion
4.1. Investigation of VBM
The concept of magnetic resonance of brain structure based on voxel was proposed by Wright et al[12] in 1995. VBM has been widely applied in the diseases of the central nervous system, such as AD,[13] epilepsy,[14] schizophrenia,[15] and depression.[16] Only a few studies are available in the brain structure of PI patients, and the influence of depressive disorder on the changes in PI brain morphology is a prospective investigation. Assuming that the depression negatively influences the development of the disease in PI patients, an abnormal connection in the brain functional network is observed, and the brain structure will exhibit morphological changes. Therefore, PI patients were divided into 2 groups and compared with the NC group to evaluate the changes in brain structure in PI and PID patients.
The results were deduced as follows:
-
1.
Compared with the NC group, the volumes of brain structure in multiple sites decreased in PI patients; however, the volume of the left middle temporal gyrus increased. This phenomenon was consistent with most of the results from the study by Joo et al,[17] except the increased volume of the left middle temporal gyrus. Recently, the volume of rostral cingulate zone increased as compared with the control group, and the severity of insomnia was relevant to the rostral cingulate zone volume, which might be caused by the compensatory reaction of the brain chronic insomnia.[18] Thus, we deduced that the increase in the volume of middle temporal gyrus in insomnia patients was an adaptation to the long-term excessive brain response and remodeling of the brain structure. The middle temporal gyrus is time-efficient in the extraction of new memories and consolidation of the long-term memory. The activity of middle temporal gyrus significantly decreases with age under the memory task status.[19] The abnormality in the occipital cortex area is correlated with the loss of cognitive function (such as visual attention and memory) during chronic persistent disease course. The structural abnormality of the frontal and temporal lobes leads to a decrease in cognitive level, thereby severely influencing the quality of life in PI patients. The decrease in the volume of anterior prefrontal cortex area influences the emotional and social function, thereby leading to a damage in cognitive function. The decrease in the volume of visual association cortex area is closely related to the cognitive function disorder in PI patients. The decrease in the volume of the right fusiform gyrus was correlated with the abnormality in self-awareness. The morphological abnormality of the paracentral lobule may influence the attention and the ability to problem solving, working memory, and self-cognition capacity. Cingulate gyrus plays a pivotal role in cognitive function and emotional memory. The high RCFT and HAMD values in PI patients were in agreement with our results. Thus, it was deduced that the decrease in the volume of brain region formed the basis for the cognitive pathological changes, thereby becoming a risk condition accompanied by emotional disorder in insomnia patients. Pillay et al[20] reported that the volumes of caudate nucleus and lenticular nucleus were negatively correlated with the degree of depression. The results of our study also indicated that the abnormality in this area could increase the morbidity of depression. The structural abnormality in the left cerebellum will cause damage to the cognitive function in different degrees, especially spatial abstraction generalization and concept formation ability. Moreover, the long-term hyperarousal status is related to the excessive activities of ascending reticular activation pathway, hypothalamus–pituitary gland–adrenal cortex, and sympathy–adrenal medulla system.[2,4] Therefore, the glucocorticoids and adrenaline increase, melatonin decreases, the brain regions with distribution of hormone receptor are influenced, the neuron is inhibited and missing, and the brain structure is altered. Hippocampus, prefrontal lobe, interior orbital lobe, ACC, and amygdala are all located in the regions widely distributed with glucocorticoids receptors.[21] Therefore, the missing regional neuron might be related to the increased adrenal cortisol in patients with insomnia.
-
2.
Compared with NC, the brain structure volumes in multiple sites of PID patients were modified. A network connection is formed during the whole sleeping period, involving the frontal lobe, parietal lobe, temporal lobe, occipital lobe, limbic system and brainstem, and cerebellum, harboring a wide functional connectivity. The sleep mechanism of PID patients is not harmonious with awakening mechanisms. The PSQI of PID patients is relatively high, and the whole sleeping network structure is damaged. The result indicated that owing to the plasticity of brain, the volume of brain region is decreased; however, the adaptive volume of a part of the region is increased, which provided the basis for investigating the underlying regulatory mechanism. The structural changes in the frontal lobe, parietal lobe, temporal occipital lobe, and limbic system are related to the disorders of the cognitive function (e.g., spatial memory) in PI patients. The decrease in the volume of supplementary motor area can influence the subjective consciousness and cognitive function in patients. The brain region volumes of cerebellum, prefrontal cortex, and anterior cingulate cortex decrease in the PI patients with light and moderate depression, which might be associated with emotional factors. Peng et al[22] also found that the gray matter densities in the dorsolateral prefrontal lobe, dorsal medial prefrontal lobe, bilateral temporal pole, right superior temporal gyrus, bilateral insular lobe, left parahippocampalgyrus, and cerebellar cortex were decreased; this phenomenon was similar to the findings of the present study. Thus, the depression might have influences on the structural changes in the brain of PI patients.
-
3.
Compared with PI patients, the changes in the brain structure occurred in multiple sites in PID patients. The HAMD score in PID patients was higher than that of PI patients. According to the neurobiology, the functional disorder of sleep-awakening neural regulation mechanism may cause emotional reaction, which might disrupt the equilibrium between homeostasis and circadian rhythm and interact with brain functional area associated with sleep. Thus, we speculated that for PID patients with decreased brain gray matter volume, depression played a major role in the vicissitudes. The insomnia disorder played a secondary role and was also related to the neuropathological mechanism in PID patients. The neuroendocrine mechanism in depression patients is disordered, characterized by the hypothalamic–pituitary–adrenal hyperfunction. The neuron will degenerate and shrink by hormonal activities, neurotransmitters, or receptors, and the brain structure in depression patients will also change. The prefrontal cortex, hippocampus, anterior cingulate cortex, and basal nuclei have rich neurotransmitters and receptors that are the main regions for hormonal effects. Taki et al[23] found that the volumes of brain gray matter in the frontal lobe, temporal lobe, precuneus, cingulate gyrus, amygdala, hippocampus, and parahippocampalgyrus decreased. This phenomenon was similar to our results, which proved that the area with decreased gray matter volume was associated with depression. The volume decrease in the bilateral inferior frontal gyrus and the superior frontal gyrus/supplementary motor area might form the structural basis for the depression symptom and cognitive impairment in PID patients. The missing volume of the temporal lobe gyrus is related to the damage in the short-term memory of PID patients. Cerebellum might be a crucial component associated with the neural circuits during mood disorders.[24] The decrease in the left cerebellum 8 and 10 area suggested an abnormality in the depression-related brain region in PI patients. Nevertheless, compared with the PI patients, the area with increased gray matter volume was more than that with the decreased volume, thereby indicating that the structural abnormality might be related to the pathogenesis in PID patients. To control the depression and strengthen the cognitive competence, the reaction of PI patients was enhanced, and the brain regional volumes of the related ACC, temporal lobe, parietal lobe, occipital lobe, and cerebellum increased.
4.2. Investigation of rs-fcMRI
ACC is a core component in cerebral limbic system, playing integrative roles in the regulation of behavior, cognition, and emotion. The lesion in ACC may generate a series of symptoms, including attention-deficit disorder, vegetative nervous function disturbances, and abepithymia. A few studies are available describing the ACC network in PI. Thomas et al[25] reported that the whole brain metabolism decreased in healthy subjects with sleep deprivation for >24 hours. The study used 18F-deoxyglucose (18FDG) PET scanning, especially in the frontal lobe, parietal lobe cortex, and thalamus, indicating that sleep is the restock of brain function of cognition-related thalamus cortex network, wherein the basal forebrain and ACC play crucial roles in regulating the awakening. Furthermore, we speculated that depression greatly influenced on the functional connectivity of ACC network in PI patients. Anand et al[26,27] found that the regulatory function of ACC on the emotion circuit was weakened. After antidepression therapy, the regulatory effect of ACC was improved, and the remission of the depressive disorder symptoms was related to the enhanced regulatory effect. Our results also indicated that the volumes of ACC in PI and PID patients decreased, especially in PI patients. Thus, ACC was selected as the seed point to investigate the functional change in the brain region in ACC cognitive network in PI and PID patients and the neuromechanism underlying depressive disorder in PI patients.
Rs-fcMRI does not require the comparison between experimental conditions and basal level; the time series correlation of BOLD signal fluctuation is the primary factor in different brain regions distal to the observation space. Spontaneous, organized, and continuous functional activities exist under the no-task waking and resting states, which comprises the default mode network (DMN). DMN mainly includes the prefrontal cortex, parietal lobe cortex, and ACC, useful in maintaining the waking state, and is associated with the extraction of episodic memory, monitoring the surrounding environment and introspectiveness, as well as the interaction between continuous cognition and emotion.[28,29] In this study, we proved that the brain function under resting-state had a DMN in normal individuals. DMN is still active under normal light sleep; however, an abnormality has been found after sleep deprivation.[30] The extensive changes in DMN under resting-state occurs in the depressive disorder-related insomnia patients.[31] The results showed that the brain functional connectivity of the frontal lobe, parietal lobe, occipital lobe, temporal cortex, and cerebellum area with ACC were weakened, and the enhanced area was mainly the limbic system. Under resting-state, DMN primarily remains as the waking-state of the brain; in the present study, the decreased activity of DMN function might be related to PI neuropathological changes. Recently, PI patients were reported to have abnormal consistency, mostly in the limbic system, which might be attributed to the long-term accumulation of negative emotion caused by insomnia. To adapt the changes of the inner emotional environment, the baseline activity level of the limbic system (especially anterior cingulate cortex) increased corresponding,[32] which was in agreement with our result. This phenomenon suggested that the enhanced functional activity of the limbic system might be correlated with the emotional fluctuations in PI patients. The weakened area of ACC brain functional connectivity in PID patients primarily included the parietal lobe cortex, and the enhanced area was mainly in the frontal and limbic lobes. Under sleep deprivation, the functional activities of the parietal lobe cortex and ACC decreased, indicating a decrease in the cognitive functions (such as attention and execution). Our results also indicated that the cognitive function was damaged in PID patients. Hamilton et al[33] reported that the functional connectivity of the prefrontal lobe and ACC in depression patients increased, and the results showed that the enhancement of functional connectivity of ACC with frontal lobe and limbic lobe was the underlying pathological mechanism for the emotional disorder in PI patients. The altered DMN in PI and PID patients, decrease in functional connectivity of the brain region, and imbalance of the DMN functional regulation indicated that both PI and PID patients exhibited damage in the regional function of the brain, and functional consistency disorder among neurons. The abnormality of ACC neural network might be the underlying pathological machinery for the changes in cognitive and emotional function in PI patients.
The bilateral ACC was considered as a seed point to compare the brain functional connectivity among the groups. Compared with the NC group, the right inferior temporal gyrus, right superior parietal gyrus/superior parietal lobule/inferior parietal lobule, and the left limbic lobe/cingulate gyrus had a negative correlation with ACC, and left cerebellum anterior lobe/culmen/lingual gyrus had a positive correlation with the ACC functional connectivity. The brain region showing a weak negative correlation with the ACC functional connectivity showed that the results of VBM indicated a decreased volume in the related brain region. Frings et al[34] found that the damage to superior parietal lobule caused the disorders of topesthesia, direction, and extremity spatial position. Therefore, the weakened functional connectivity of the right superior parietal gyrus/superior parietal lobule/inferior parietal lobule with ACC was closely related to the abnormality of attention control and lack of executive capacity in spatial memory of PI patients. The right inferior temporal gyrus and left limbic lobe/cingulate gyrus is one of the main regions of DMN, and the ACC functional connectivity was weakened; thus, the cognitive function was damaged in PI patients. The enhanced ACC functional connectivity with the left cerebellum anterior lobe/culmen/lingual gyrus might be related to the functional abnormality of visual cortex in occipital lobe lingual gyrus and disorder of the cognitive function. The high RCFT score in the PI group corresponded to the relevant brain region with abnormal functional connectivity. Therefore, the abnormal regions of functional connectivity in PI patients and NC group did not show a significant correlation with the PSQI score. Thus, the abnormal region of functional connectivity in ACC and PI patients might be the underlying pathological mechanism for the cognitive function damage in PI patients.
Compared with the NC group, the brain region was negatively correlated with the ACC functional connectivity in PID patients, including brainstem/midbrain, left middle temporal gyrus, and the left posterior cerebellar lobe/cerebellum Crus1 area. The region positively correlated with the ACC functional connectivity included left parietal lobe/subgyrus/Rolandic operculum, left parietal lobe/subgyrus/pars triangularis inferior frontal gyrus, right parietal lobe/middle occipital gyrus, and right superior parietal gyrus. The region with weakened ACC functional connectivity was consistent with the decreased volume in the related brain region. The weakened degree of functional connectivity in PID patients was negatively correlated with the PSQI score, among which the decrease in the degree of midbrain brain functional connectivity was strongly correlated with PSQI score. The network structure was closely related to the cerebellum, the loop formed between them affected the brain excitatory state and retention of the awake state. Thus, it was deduced that the weakened functional connectivity of these regions with ACC was closely related to the insomnia status and pathogenesis in PID patients. The left middle temporal gyrus and cerebellum Crus 1 area involved in the cognitive function process and the functional connectivity with ACC was weakened. The functional connectivity degree of the left inferior frontal gyrus was positively correlated with HAMD, suggesting that the vicious circle formed by PID patients’ sleep and emotional disorder influence the cognitive function. The brain regions positively correlated with the ACC functional connectivity mainly lie in the frontal parietal lobe. The adaptive compensatory effect of the enhanced functional connectivity indirectly proved the significance of the missing cognitive function in PI patients. The PSQI, HAMD, and RCFT scores in PID group were high, which also further improved the function in the relevant brain regions associated with sleep, emotion, and spatial memory.
Compared with PI patients, PID patients showed a negative correlation of the left corpus callosum/posterior cingulate with ACC functional connectivity, whereas the midbrain was positively correlated with the ACC functional connectivity. The occurrence of depression in PI patients might be related to the decrease in functional activity in brain region regulating the emotions. Reportedly, the abnormality of posterior cingulate in the depression patients is associated with persistent emotional burn.[35] In the present study, the decrease in the degree of functional connectivity of posterior cingulate was positively correlated with HAMD and the ACC functional connectivity with posterior cingulate was weakened, suggesting the onset of the neurological function in depressive disorder. The decrease in the corpus callosum and ACC functional connectivity indicated the damage in the cognitive function of PID patients. PID hyperarousal leads to dysfunction in a network system, changes in circadian rhythms, and no correlation between midbrain and the HAMD score. Thus, the functional connectivity between midbrain and ACC is enhanced, which is an adaptive response during chronic insomnia in PID patients.
Nonetheless, the brain structural connection and functional connectivity are interdependent. PI exhibits abnormality in intracephalic multisystem structure and neural network connection. The interaction and influence between depression and insomnia aggravate the damage to cognitive function. This study provides a theoretical basis for exploring the neuropathology of PID and cognitive function. It also indicates that VBM and rs-fcMRIcan effectively evaluate the changes in PI-associated brain structure and functional connectivity, which provides clinical value for the prospective investigation on neurocognition.
Author contributions
Conceptualization: Gang Li, Xiaoqi Zhang, Yongli Li.
Data curation: Gang Li, Jiewen Zhang, Hongju Zhang.
Formal analysis: Gang Li, Xiaoqi Zhang, Enfeng Wang, Yongli Li.
Investigation: Gang Li, Xiaoqi Zhang, Jiewen Zhang, Yongli Li.
Methodology: Xiaoqi Zhang, Hongju Zhang.
Project administration: Jiewen Zhang.
Resources: Enfeng Wang, Hongju Zhang.
Software: Enfeng Wang.
Supervision: Enfeng Wang.
Validation: Jiewen Zhang, Yongli Li.
Visualization: Hongju Zhang.
Writing – original draft: Gang Li, Xiaoqi Zhang.
Writing – review and editing: Jiewen Zhang, Enfeng Wang, Hongju Zhang, Yongli Li.
Footnotes
Abbreviations: ACC = anterior cingulate cortex, BA = Brodmann area, BOLD = blood oxygenation level-dependent signal, DMN = default mode network, DSM-IV = diagnostic and statistical manual, DWI = diffusion-weighted imaging, EPI = echo planar imaging, Fc = functional connectivity, FLAIR = fluid-attenuated inversion recovery, fMRI = functional magnetic resonance imaging, fourth edition, FOV = field of view, FWHM = full width at half maximum, HAMA = Hamilton Anxiety Rating Scale, HAMD = Hamilton Depression Rating Scale, MMPI = Minnesota Multiphasic Personality Inventory, MNI = Montreal Neurological Institute, PI = primary insomnia, PID = primary insomnia with depression, PSQI = Pittsburgh Sleep Quality Index, RCFT = Rey Complex Figure Test, ROI = region of interest, rs-fMRI = resting state functional magnetic resonance imaging, SPM = statistical parametric mapping, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging, TI = inversion time, TR/TE = repetition time/echo time, VBM = Voxel-based morphometry.
GL and XZ equally contributed to this work.
This work was supported by the Natural Science Foundation of Henan Province, China (grant number 162300410285) and Scientific and Technological Research Program of Henan Province, China (grant number 1721023100115).
The authors have no conflicts of interest to disclose.
References
- [1].Waine J, Broomfield NM, Banham S, et al. Metacognitive beliefs in primary insomnia: developing and validating the Metacognitions Questionnaire–Insomnia (MCQ-I). J Behav Ther Exp Psychiatry 2009;40:15–23. [DOI] [PubMed] [Google Scholar]
- [2].Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 2010;14:19–31. [DOI] [PubMed] [Google Scholar]
- [3].Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone 2003;5:5–15. [DOI] [PubMed] [Google Scholar]
- [4].Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev 2010;14:9–15. [DOI] [PubMed] [Google Scholar]
- [5].Buysse DJ, Thompson W, Scott J, et al. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med 2007;8:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med Rev 2010;14:47–60. [DOI] [PubMed] [Google Scholar]
- [7].Harper DG, Plante DT, Jensen JE, et al. Energetic and cell membrane metabolic products in patients with primary insomnia: a 31-phosphorus magnetic resonance spectroscopy study at 4 tesla. Sleep 2013;36:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salgado-Pineda P, Fakra E, Delaveau P, et al. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res 2011;125:101–9. [DOI] [PubMed] [Google Scholar]
- [9].Agosta F, Pievani M, Geroldi C, et al. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging 2012;33:1564–78. [DOI] [PubMed] [Google Scholar]
- [10].Li J, Zhang Z, Shang H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy Res 2012;98:97–103. [DOI] [PubMed] [Google Scholar]
- [11].Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci 2007;7:367–79. [DOI] [PubMed] [Google Scholar]
- [12].Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 1995;2:244–52. [DOI] [PubMed] [Google Scholar]
- [13].Di Paola M, Macaluso E, Carlesimo GA, et al. Episodic memory impairment in patients with Alzheimer's disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. J Neurol 2007;254:774–81. [DOI] [PubMed] [Google Scholar]
- [14].Labate A, Cerasa A, Gambardella A, et al. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study. Neurology 2008;71:1094–101. [DOI] [PubMed] [Google Scholar]
- [15].Mane A, Falcon C, Mateos JJ, et al. Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res 2009;114:136–43. [DOI] [PubMed] [Google Scholar]
- [16].Colloby SJ, Firbank MJ, Vasudev A, et al. Cortical thickness and VBM-DARTEL in late-life depression. J Affect Disord 2011;133:158–64. [DOI] [PubMed] [Google Scholar]
- [17].Joo EY, Noh HJ, Kim JS, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep 2013;36:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Winkelman JW, Plante DT, Schoerning L, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep 2013;36:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Persson J, Lustig C, Nelson JK, et al. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci 2007;19:1021–32. [DOI] [PubMed] [Google Scholar]
- [20].Pillay SS, Renshaw PF, Bonello CM, et al. A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Psychiatry Res 1998;84:61–74. [DOI] [PubMed] [Google Scholar]
- [21].Li XL, Sun XJ. Research development of integrated application of VBM, DTI and resting-state BOLD-fMRI on major depressive disorder. Radiol Pract 2013;28:276–8. [Google Scholar]
- [22].Peng J, Liu J, Nie B, et al. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol 2011;80:395–9. [DOI] [PubMed] [Google Scholar]
- [23].Taki Y, Kinomura S, Awata S, et al. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord 2005;88:313–20. [DOI] [PubMed] [Google Scholar]
- [24].Chen CH, Ridler K, Suckling J, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 2007;62:407–14. [DOI] [PubMed] [Google Scholar]
- [25].Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000;9:335–52. [DOI] [PubMed] [Google Scholar]
- [26].Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 2005;57:1079–88. [DOI] [PubMed] [Google Scholar]
- [27].Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology 2005;30:1334–44. [DOI] [PubMed] [Google Scholar]
- [28].Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA 2001;98:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp 2008;29:839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baglioni C, Spiegelhalder K, Lombardo C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev 2010;14:227–38. [DOI] [PubMed] [Google Scholar]
- [31].Liang MJ, Li J, Zhou Q, et al. The fMRI of depression insomnia related patients of default mode network in resting state. J Clin Radiol 2012;31:1528–33. [Google Scholar]
- [32].Liang MJ, Zhou Q, Yang XL, et al. The study of resting state fMRI changes in primary insomnia patients based on regional homogeneity. J Clin Radiol 2014;33:10–4. [Google Scholar]
- [33].Hamilton JP, Chen G, Thomason ME, et al. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry 2011;16:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Frings L, Wagner K, Quiske A, et al. Precuneus is involved in allocentric spatial location encoding and recognition. Exp Brain Res 2006;173:661–72. [DOI] [PubMed] [Google Scholar]
- [35].Yao ZJ, Wang L, Lu Q, et al. Altered default mode network functional connectivity in patients with depressive disorders:resting-state fMRI study. Chin J Nervous Mental Dis 2008;34:278–82. [Google Scholar]


