Abstract
To study the relationship between pigment epithelium-derived factor (PEDF) rs1136287, rs1894286 polymorphisms and the risk of age-related macular degeneration (AMD) in northern Chinese populations.
The study was carried out on case–control methods. Ninety-six patients with AMD and 98 health controls were recruited who were matched with the former by age and gender, rs1136287 and rs1894286 were genotyped by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP). Hardy–Weinberg equilibrium (HWE) was also checked by χ2 test. The distribution frequencies of genotype, allele, and haplotype were calculated by direct counting method. The genotype, allele, and haplotype distribution differences between the case and control groups were analyzed by chi-square test, and odds ratio (OR) and 95% confidence interval (CI) was used to express the relative risk of AMD in northern Chinese populations. The linkage disequilibrium (LD) and haplotype analyses were conducted with Haploview software.
The genotype and allele distribution frequencies in rs1136287 were obviously between in cases and controls (P < .05). TT genotype might lead to 3.24 times risk of AMD occurrence compared with CC genotype (OR = 3.24, 95% CI = 1.26–8.32), and C allele also played an increased risk role in the attack of AMD (OR = 1.58, 95% CI = 1.06–2.38). The T–C haplotype frequency of rs1136287-rs1894286 in PEDF were significantly correlated to the increased susceptibility to AMD (OR = 1.57, 95% CI = 1.02–2.40).
The rs1136287 polymorphism in PEDF may be related to the occurrence risk of AMD. Additionally, a haplotype is also a non-ignorable risk factor.
Keywords: age-related macular degeneration, PEDF, polymorphism
1. Introduction
Age-related macular degeneration (AMD) also known as senile macular degeneration (SMD) is a complex genetic disease of the photoreceptor-RPE-Bruch film-Choroidal capillary,[1,2] which affects the central area of the retina and choroid, and can result in the loss of central vision. Is the main cause of blindness disease over 50 years old population in western developed countries.[3,4] With the development of the economy and the aging of the population intensified, the incidence of AMD indicates a trend of the rising year by year, being the third major cause of blindness in our country. Etiology studies indicated that AMD is a complex disease caused by multi-gene genetic factors and environmental risk factors and their interaction.[5] Among them, genetic factors take an important role in the occurrence of AMD, which has been confirmed in a large number of family studies.[6] Therefore, we should further explore the causes of the disease, it has important clinical significance for the early prevention and treatment of disease development.
Pigment epithelium-derived factor (PEDF) is a kind of neurotrophic factor, which is also a kind of secreted glycoprotein was extracted from the conditioned medium of cultured human fetal retinal pigment epithelial cells (RPE-conditioned medium) by Tombram-Tink et al in 1989.[7]PEDF is encoded by a single gene encoding, an encoding gene located on chromosome 17p13.1, the human PEDF gene length is about 16 kb, containing 8 exons and 7 introns.[8] The production and distribution of PEDF is very extensive. The cerebrospinal fluid, liver, heart, skeletal muscle, placenta, and ovary and other tissues and organs are PEDF synthesis. It can be seen that the distribution of PEDF is a wide and functional diversity, which suggests that it plays a certain role in the physiological or pathological process of many organs of the human body.[9–11]
PEDF not only has the function of nerve nutrition and nerve protection but also is a natural and powerful new blood vessel inhibitor in the intraocular. Research findings of immunohistochemistry, compared with normal eyes, which the activity of PEDF in patients with AMD was significantly decreased.[12] Ogata et al determined the PEDF content in underwent vitrectomy of patients with diabetic retinopathy, ruptural detachment of retina or idiopathic macular hole, and further proved that PEDF has the ability to inhibit the formation of new blood vessels.[13] It is reported that PEDF plays an important role in the pathogenesis of AMD. Therefore, PEDF polymorphisms are speculated to be a correlation to the susceptibility of AMD, but rare articles introduce the relationship between the 2, especially in northern Chinese populations. So in this study, the correlation between PEDF rs1136287, rs1894286, and AMD susceptibility were analyzed in order to provide bases for the pathological explanation and clinical diagnosis of AMD.
2. Materials and methods
2.1. The case and control groups
This study is a case–control study, divided into AMD case group and healthy control group. All of the study subjects were randomly selected from the north of China. This clinical trial program was approved by the Ethics Committee of Aerospace Central Hospital and complied with Helsinki declaration. All participants fully understood the purpose, procedure, and method of this study, and signed the informed consent.
The case group included 96 patients with AMD selected from the clinical inpatients in Aerospace Central Hospital during June 2012 to January, 2014, including 59 males and 37 females. The inclusion criteria of AMD patients were as follows: ≥50 years old; AMD was diagnosed by fluorescein fundus angiography (FFA) or optical coherence tomography (OCT); the cases did not suffer from other retinal diseases. Exclusion criteria: high myopia ≥6.00D; polypoidal choroidal vasculopathy (PCV) patients; retinopathy caused by other reasons, such as diabetes; other diseases influencing the results of this study. Their age range was 56 to 87, with the average age of 70.6 ± 9.8. The controls were all healthy people from the medical examination center of Aerospace Central Hospital in the same period with the cases, and they were frequency-matched in age and sex with the case group, including 60 males and 38 females. Their age was a group of 59 to 92 with the average age of 73.9 ± 10.4.
All patients were conducted visual acuity, slit lamp, ophthalmoscopy, the lens slit lamp examination, OCT, fundus photography system and FFA by 2 professional doctors of fundus disease using standard test procedure, and in line with the international age related maculopathy (ARM) epidemic research group developed ARM and AMD international classification standards.[14] The healthy control group has been excluded from the family hereditary disease, renal insufficiency, blood disease, benign or malignant tumor, and other retinal choroidal diseases, such as high myopia, central serous choroidal retinopathy, etc.
3. Methods
3.1. DNA extraction
Two milliliter peripheral venous blood were extracted from all subjects who fasted for 12 hours, and placed in the centrifugal tube containing dipotassium ethylenediaminetetraacetic acid (EDTA-2K) anticoagulation. The peripheral blood leucocyte genome DNA was extracted using Beijing TIANGEN biochemical blood genome DNA extraction kit according to the manufacturer's instructions, and then stored in −20°C refrigerator for standby application.
3.2. PEDF genotyping
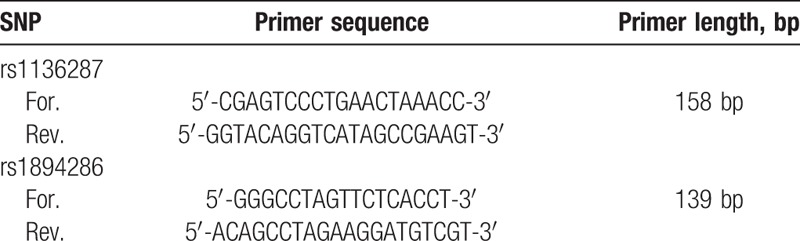
Polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) was applied for the genotyping of PEDF rs1136287, rs1294385 polymorphisms. Then we search Genebank database to find the complete sequence of PEDF in human chromosome 17. We designed the primers using Primer Premier 5.0 software (Canada). The primer sequences were listed in Table 1.
Table 1.
Primer sequences of PEDF gene in rs1136287, rs1894286.
PCR reaction system was a volume of 20 μL mixture, including 2.0 μL DNA template, each 1.0 μL of forward and reverse primers, 2.0 μL 10 × PCR Buffer, 0.4 μL dNTP, 0.2 μL Taq DNA polymerases and 13.4 μL ddH2O. The reaction is carried out on the thermal cycling apparatus. Reaction conditions were 94°C pre-denaturation for 8 minutes; followed by 30 cycles of 94°C degeneration for 30 seconds, 60°C annealing for 30 seconds, 72°C extension for 60 seconds, and finally 72°C extension for 10 minutes. Enzyme reaction system was a volume of 20 μL, including 10 μL PCR products, 2.0 μL 10 × Buffer solution, 2.0 μL restriction enzyme (BsstSI for rs1136287 and HincII for rs1294385) and double distilled water. And then the mixture was digested for 16 hours in water bath of 37°C. The enzyme-digested products were separated by 2% advanced glycation end products (AGE) and the Vilber Lourmat were used to analyze the genetyping of the 2 SNPs.
3.3. Statistical analysis
The statistical analysis was accomplished by PASW Statistics 18.0 software (Chicago). Hardy–Weinberg was used to test all groups whether from the same Mendelian population. Allele and genotype frequencies of 2 loci were calculated by direct counting method. Linkage disequilibriumn (LD), its correlation coefficient (D′ value) and haplotypes between rs1136287, rs1894286 polymorphisms were calculated by Haploview software. Measurement data were expressed by 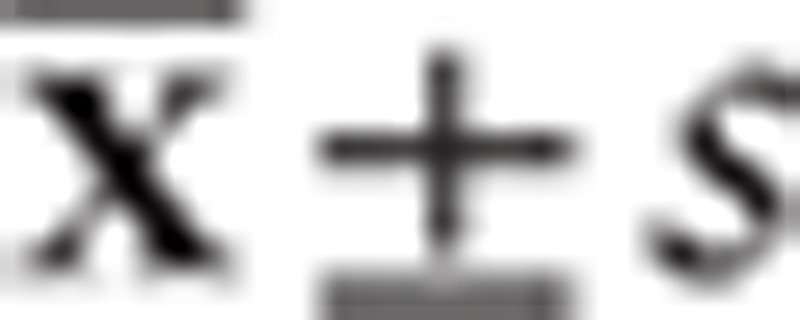 and %. The alleles, genotypes and haplotypes comparisons of PEDF polymorphisms between the cases and controls were also analyzed by χ2 test. The effect of PEDF polymorphisms on AMD was evaluated with odd ratio (OR) and 95% confidence interval (95% confidence interval [CI]). And P < .05 was considered as the statistical significance.
and %. The alleles, genotypes and haplotypes comparisons of PEDF polymorphisms between the cases and controls were also analyzed by χ2 test. The effect of PEDF polymorphisms on AMD was evaluated with odd ratio (OR) and 95% confidence interval (95% confidence interval [CI]). And P < .05 was considered as the statistical significance.
4. Results
4.1. General conditions of research objects
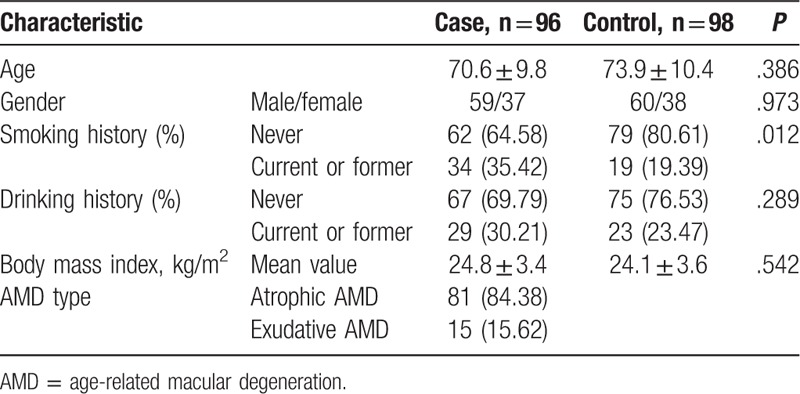
In this study, 96 AMD patients and 98 healthy controls were collected. In the case group, the sex ratio was 1.59:1.00 in males and females with the average age of 70.6 ± 9.8. In the control group the sex ratio was 1.58:1.00 and the average age was 73.9 ± 10.4. The differences of age and gender between the 2 groups had no statistical significance via Chi-square test (P > .05), which indicated a good study population. We found over one-third of AMD patients had the smoking history (current or former), and the ratio was less than a fifth in controls. It was significantly different between the 2 groups and was the risk factor of AMD (P = .012). Drinking history and body mass index (BMI) were not found the significant difference between the case and control groups (P > 0.05). Additionally, in AMD patients, atrophic AMD accounted for nearly 85% of all cases in this study and the rest 15% were exudative AMD. The detailed data were showed in Table 2.
Table 2.
The basic characteristics of subjects in the case and control groups.
4.2. The genotypes distribution of PEDF polymorphisms in case and control groups and the relationship with AMD risk
The genotypes distribution of PEDF rs1136287, rs1894286 polymorphisms both conformed to Hardy–Weinberg equilibrium (HWE) between the 2 groups, which indicated our subjects had a group representative. The genotype and allele distributions were shown in Table 3.
Table 3.
Frequency comparisons of genotypes and alleles in PEDF gene polymorphisms.
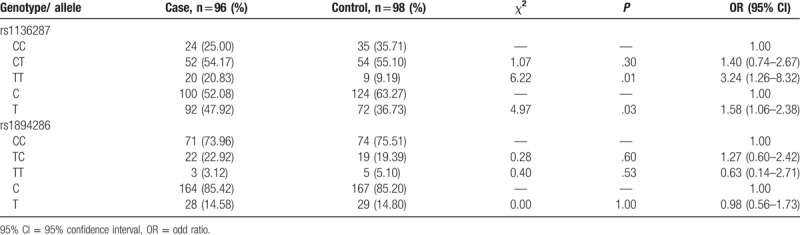
The frequencies of rs1136287 CC, CT, TT genotypes and C, T alleles were 25.20%, 54.17%, 20.83%, and 52.08%, 47.92% in cases, and 35.71%, 55.10%, 9.19%, and 63.27%, 36.73% in controls respectively. Therefore, the TT genotype and T allele frequencies had a significant difference between 2 groups (P < .05). Moreover, TT genotype increased 3.24 times risk to suffer from AMD, compared with CC genotype (OR = 3.24, 95% CI = 1.26–8.32), and C allele carriers had 1.58 times risk of AMD than T allele carriers (OR = 1.58, 95% CI = 1.06–2.38).
CC, TC, TT genotype and C, T alleles frequencies of rs1894286 were 73.96%, 22.92%, 3.12%, and 85.42%, 14.58% in the case group, and 75.51%, 19.39%, 5.10%, and 85.20%, 14.80% in the control group respectively, which showed that the genotype and allele distributions of rs1894286 between 2 groups had no statistical significance (P > .05).
4.3. Haplotype analysis of PEDF rs1136287, rs1894286 polymorphisms
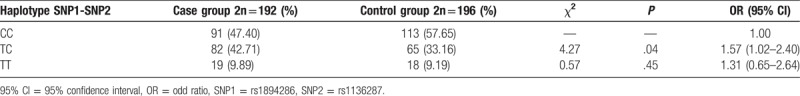
Haploview software was used to analyze LD between rs1136287 and rs1894286 of PEDF. A strong linkage disequilibrium was found between the 2 SNPs (D′ = 1.0), and a total of 3 haplotypes were constructed including A–G, G–G, and G–A, and the relative information was displayed in Table 4. The data showed that the distributions of T–C haplotype had an obvious difference in 2 groups (P = .04), which indicated that it could increase the risk of AMD occurrence (OR = 1.57, 95% CI = 1.02–2.40).
Table 4.
Analyses of LD and haplotypes in PEDF rs1136287, rs1894286 polymorphisms.
5. Discussion
AMD is a severe macular disease of which the incidence increases with the patients’ age growing. This diseases characterized by progressive deterioration of the retinal pigment epithelium (RPE) and macula, leading to irreversible decrease or loss of central vision. AMD in clinically divided into atrophic (dry) and effusion (wet). Wet AMD is the main factor of visual impairment in AMD, the main performance is the formation of choroidal neovascularization (CNV), which can cause repeated bleeding and exudation, and eventually led to the scar formation and vision loss. Despite a large number of basic and clinical studies on the pathogenesis of AMD, the pathogenesis is still not clear. The World Health Organization reports that at least 8,000,000 people in the world were caused serious loss of vision and seriously affect the quality of life due to the late AMD. At present, with the aging of the population, the prevalence of AMD increased year by year. Epidemiological studies have shown that the risk factors associated with AMD include age, family history, smoking, white people, light iris, UV exposure, diet, and nutritional status, and so on.[15–18]
PEDF is a 50-kDa protein secreted by the RPE. PEDF gene is highly conserved, it belongs to the serine protease inhibitor gene family. PEDF has 2 functional areas: the N side of the neurotrophic region and the C terminal of serine protease inhibitor superfamily reaction ring.[19]PEDF has the function of nerve nutrition, nerve protection and nerve differentiation on many parts of the central and peripheral nervous system. The latest research results show that PEDF can be used as a mitotic factor or survival factor to maintain the internal environment stability of retinal micro vessel. PEDF in Adult intraocular is mainly produced by RPE cells, to reach retinal photoreceptors matrix through paracrine modes; in aqueous humor and vitreous chamber also contain high concentration of PEDF, Which is secreted by the retinal ganglion cells, kernel cell layer, photoreceptor cells, ciliary body epithelium, corneal epithelial cells and endothelial cells, and so on.[20] A large number of studies have also confirmed that PEDF can protect retinal cells from light damage,[21] oxidative stress injury,[22] ischemia-reperfusion injury[23] and glutamate-induced toxicity,[24] and so on, which plays an important role in the maintenance of retinal tissue differentiation and function of normal form.
PEDF was strongly expressed in human fetal and adult RPE cells, and the expression decreased in the senescence of RPE cells.[20] RPE cells cultured in vitro can also synthesize and secrete PEDF, but with the increase of the number of cell passages, PEDF secretion may also be decreased, which may be related to the senescence of RPE cells, which may be involved in the pathological process of some age related diseases, such as AMD, and so on.[25] Holekamp et al[26] found that patients with the formation of CNV in AMD, whose PEDF concentration in the vitreous body was significantly lower than that in the control group, while there was no significant difference in VEGF, which indicated that the decrease of PEDF could promote the formation of CNV. Yamagishi et al[27] found that Met72Thr (rs1136287) SNP was associated with age related macular degeneration, but had nothing to do with diabetic microvascular complications. Most of these studies are only for foreigners; to Chinese people are almost no research. In this study, we studied the relationship between PEDF gene polymorphism and the risk of AMD in the northern Chinese populations. The results showed that the genotype and allele frequencies of rs1894286 polymorphism had no statistically significant difference in the AMD group and healthy control group. However, this study only aimed at the northern Chinese populations therefore we need to get further verification with larger sample size and more races to see whether rs1894286 polymorphism is related to AMD in other regions. In contrast, we found that the genotype and allele distributions of rs1136287 polymorphism had obvious differences between 2 groups in this study. This is consistent with the results of Yamagishi et al In addition, the result was checked by the correlation between the haplotypes in PEDF rs1136287, rs1894286 polymorphisms and AMD, A–A haplotype was discovered to obviously increase the risk suffering from AMD in old adults.
In a word, the outcome of this article proved that PEDF gene polymorphisms had a connection with the occurrence of AMD in the northern Chinese populations, which can provide a theoretical basis for the development and prognosis of the disease from gene level, to achieve the purpose of early detection, early diagnosis and early treatment. Meanwhile, there are many limitations in our study, such as small sample size, avoiding the environmental factors. In addition, due to the relatively small sample size and low mutation rate of the studied polymorphisms, the association of PEDF gene polymorphisms with type of AMD had not been explored in our study. Therefore, further studies should be performed to improve our conclusions.
Author contributions
Conceptualization: Jie Cheng.
Data curation: Jie Cheng.
Formal analysis: Jie Cheng.
Funding acquisition: Jie Cheng.
Investigation: Jie Cheng.
Methodology: Jie Cheng.
Project administration: Zhongchen Zhang.
Resources: Zhongchen Zhang.
Software: Zhongchen Zhang.
Supervision: Xiaolin Hao, Zhongchen Zhang.
Validation: Xiaolin Hao, Zhongchen Zhang.
Visualization: Xiaolin Hao.
Writing – original draft: Xiaolin Hao.
Writing – review & editing: Xiaolin Hao.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, AMD = age-related macular degeneration, ARM = age-related maculopathy, BMI = body mass index, CNV = choroidal neovascularization, EDTA = ethylenediaminetetraacetic acid, FFA = fluorescein fundus angiography, HWE = Hardy–Weinberg equilibrium, LD = linkage disequilibrium, OCT = optical coherence tomography, OR = odd ratio, PCR–RFLP = polymerase chain reaction–restriction fragment length polymorphism, PCV = polypoidal choroidal vasculopathy, PEDF = pigment epithelium-derived factor, RPE = retinal pigment epithelium, SMD = senile macular degeneration.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Holz FG, Pauleikhoff D, Klein R, et al. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol 2004;137:504–10. [DOI] [PubMed] [Google Scholar]
- [2].Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48:257–93. [DOI] [PubMed] [Google Scholar]
- [3].Swaroop A, Branham KE, Chen W, et al. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Hum Mol Genet 2007;16 Spec No. 2:R174–82. [DOI] [PubMed] [Google Scholar]
- [4].Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology 2007;114:253–62. [DOI] [PubMed] [Google Scholar]
- [5].Haddad S, Chen CA, Santangelo SL, et al. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol 2006;51:316–63. [DOI] [PubMed] [Google Scholar]
- [6].Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Nat Acad Sci U S A 2010;107:7401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res 1991;53:411–4. [DOI] [PubMed] [Google Scholar]
- [8].Abramson LP, Stellmach V, Doll JA, et al. Wilms’ tumor growth is suppressed by antiangiogenic pigment epithelium-derived factor in a xenograft model. J Pediatr Surg 2003;38:336–42. [DOI] [PubMed] [Google Scholar]
- [9].Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: new insights into phylogeny, function, and signaling. Front Biosci: J Virtual Libr 2005;10:2131–49. [DOI] [PubMed] [Google Scholar]
- [10].Sawant S, Aparicio S, Tink AR, et al. Regulation of factors controlling angiogenesis in liver development: a role for PEDF in the formation and maintenance of normal vasculature. Biochem Biophys Res Commun 2004;325:408–13. [DOI] [PubMed] [Google Scholar]
- [11].Tombran-Tink J, Barnstable CJ. Osteoblasts and osteoclasts express PEDF, VEGF-A isoforms, and VEGF receptors: possible mediators of angiogenesis and matrix remodeling in the bone. Biochem Biophys Res Commun 2004;316:573–9. [DOI] [PubMed] [Google Scholar]
- [12].Witmer AN, Vrensen GF, Van Noorden CJ, et al. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retinal Eye Res 2003;22:1–29. [DOI] [PubMed] [Google Scholar]
- [13].Ogata N, Tombran-Tink J, Nishikawa M, et al. Pigment epithelium-derived factor in the vitreous is low in diabetic retinopathy and high in rhegmatogenous retinal detachment. Am J Ophthalmol 2001;132:378–82. [DOI] [PubMed] [Google Scholar]
- [14].Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39:367–74. [DOI] [PubMed] [Google Scholar]
- [15].Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108:697–704. [DOI] [PubMed] [Google Scholar]
- [16].Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol 2001;119:1191–9. [DOI] [PubMed] [Google Scholar]
- [17].Hirvela H, Luukinen H, Laara E, et al. Risk factors of age-related maculopathy in a population 70 years of age or older. Ophthalmology 1996;103:871–7. [DOI] [PubMed] [Google Scholar]
- [18].Klein R, Klein BE, Moss SE. Relation of smoking to the incidence of age-related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol 1998;147:103–10. [DOI] [PubMed] [Google Scholar]
- [19].Bilak MM, Corse AM, Bilak SR, et al. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J Neuropathol Exp Neurol 1999;58:719–28. [DOI] [PubMed] [Google Scholar]
- [20].Karakousis PC, John SK, Behling KC, et al. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis 2001;30:154–63. [PubMed] [Google Scholar]
- [21].Cao W, Tombran-Tink J, Elias R, et al. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Investig Ophthalmol Vis Sci 2001;42:1646–52. [PubMed] [Google Scholar]
- [22].Cao W, Tombran-Tink J, Chen W, et al. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J Neurosci Res 1999;57:789–800. [PubMed] [Google Scholar]
- [23].Stellmach V, Crawford SE, Zhou W, et al. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Nat Acad Sci USA 2001;98:2593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pang IH, Zeng H, Fleenor DL, et al. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci 2007;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hjelmeland LM, Cristofolo VJ, Funk W, et al. Senescence of the retinal pigment epithelium. Mol Vis 1999;5:33. [PubMed] [Google Scholar]
- [26].Holekamp NM, Bouck N, Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol 2002;134:220–7. [DOI] [PubMed] [Google Scholar]
- [27].Yamagishi S, Nakamura K, Inoue H, et al. Met72Thr polymorphism of pigment epithelium-derived factor gene and susceptibility to age-related macular degeneration. Med Hypoth 2005;64:1202–4. [DOI] [PubMed] [Google Scholar]


