Abstract
Leptin phenotype has been suggested to be a possible biomarker for the diagnosis and prognosis of different neoplasms. Nonetheless, there are conflicts among the outcomes found in several tumors, and little is proven concerning the correlation between the phenotype of leptin and its clinical significance in colorectal carcinomas. This study will describe the phenotype of leptin in colorectal adenocarcinomas, and investigate its correlation with clinicopathological factors.
Two hundred and twenty eight tissue samples include 155 colorectal carcinomas, 40 adenomas, and 33 noncancerous cases were utilized in constructing tissue microarrays which have been used in the revealing of leptin expression using leptin monoclonal antibody and immunohistochemistry staining protocol.
Immunoexpression of leptin was recognized in 145 (93.5%) of colorectal tumors and 56 (76.7%) cases of control group. Histotype was considerably associated with leptin phenotype (P = .000), there is up regulation in leptin expression in colorectal carcinoma cases. Significantly higher proportion of negative leptin immunostaining cases were observed in tumors which have size more than 5 cm (P = .045). Whereas, significant different survival patterns were observed in positive cases regarding tumor size, lymphovascular invasion, distant metastasis, local recurrence and relapse of disease (P-values .046, .011, .000, .013, and .001, respectively). On the other hand, positive leptin staining colorectal tumors with size <5 cm, and with no distant metastases, local recurrence, or disease relapse had significantly better survival estimates. However, leptin immunostaining did not show noteworthy associations with age, gender, differentiation, tumor location, stage, margins involvement, lymphovascular invasion, and lymph node metastasis.
The current study shows up regulation in leptin expression in colorectal adenocarcinoma compared with noncancerous control cases. Thus, immunohistochemical staining of leptin in colorectal cancer could be a helpful tool in the prediction of prognosis and survival pattern of colorectal cancer with certain clinicopathological factors (tumor size, lymphovascular invasion, distant metastasis, local recurrence, and relapse of disease).
Keywords: colorectal cancer, immunohistochemistry, leptin
1. Introduction
Clinicopathological parameters such as tumor, node, and metastases (TNM) stage, grade, tumor invasion, and lymph nodes involvement are important factors in deciding treatment modality for colorectal cancer.[1] Still these clinical factors are not satisfactory enough to predict disease outcomes and colorectal tumors remain till today a huge challenge to clinicians. This is because of considerable differences in the clinical outcomes of colorectal tumor cases of similar stages. Thus, the search for an effective molecular and histopathological prognosticating markers continues and is still main target to personalize treatment for cases of serious consequences.[1]
Leptin is an adipokine that has several functions and has an important role in the metabolism, lipogenesis, glucose homeostasis, inflammation, immune response, bone formation, tissue remodeling, and neoangiogenesis.[2–6] It is a peptide of 167 amino acids which is programmed by obese gene (Ob). Leptin is made by adipocytes and has been found to be expressed by some other tissues for instance stomach, liver, ovaries, placenta, and skeletal muscles.[7–9] Several reports have described leptin role in the proliferation, migration, and invasion of tumor cells and how it prevents apoptosis through different ways.[10–14] Histologically, many studies have attempted to test the relationship between leptin immunoexpression and clinical parameters in several tumors including colorectal cancer, but the findings are controversial,[15–25] and yet, not much is known about the clinical significance of leptin phenotype in colorectal carcinomas. Thus, the present study investigates the immunohistochemical staining of leptin in colorectal cancer, and assesses the correlation of its expression with the clinical characteristics and follow up information of these neoplasms.
2. Material and methods
All 155 colorectal adenocarcinoma tissue samples (83 males and 72 females) and 73 control group tissue samples (adjacent tissue, adenomas, and noncancerous condition including normal tissues) which have been used in this study were paraffin embedded specimens and were acquired from the pathology department of King Abdulaziz University. Paraffin blocks have been cut into 4 μm sections, hematoxylin and eosin stained and re-evaluated. Tumor characteristics and clinical data such as gender, age, cancer type, tumor volume, stage, grade, and metastasis) have been collected from King Abdulaziz University Hospital medical records (Table 1). All tissues of control group were selected from people who were sampled for noncancerous disorders. Recommendation of World Health Organization was applied in grading and staging of these colorectal cancer cases. All employed tissue samples of colorectal neoplasms and control group were employed to make tissue microarray. The present research was approved by King Abdulaziz University Biomedical Ethical Unit.
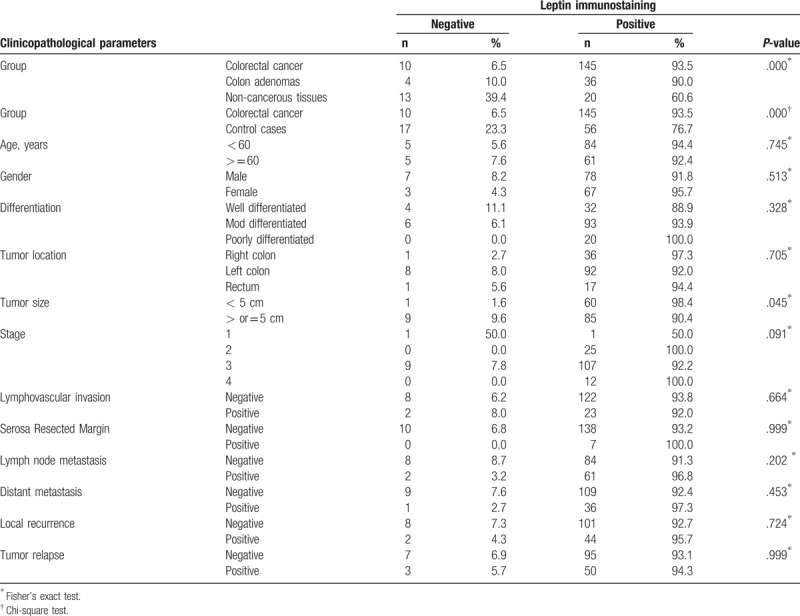
Table 1.
Distribution of various clinicopathological variables with leptin immunostaining in colorectal adenocarcinomas.
2.1. Tissue microarray (TMA) construction
Tissue microarrays were made from 155 colorectal cancer cases and 73 control tissue specimens as mentioned in our earlier papers.[26,27] TMA blocks have been sliced into 4 micron sections and sited on glass slides coated with aminosilane. Later, it was employed in leptin immunohistochemistry staining protocol.
2.2. Immunohistochemistry staining method
Immunohistochemical analysis was applied using immunohistochemistry autostainer (BenchMark ULTRA, Ventana, AZ) as previously reported.[26,27] Antileptin rabbit polyclonal antibody with dilution ratio of 1 to 100 (Santa Cruz Biotechnology), then ULTRAVIEW TM DAB visualizing protocol were used in immunohistochemistry staining. Every staining run contained a slide treated with tris buffer rather than antileptin antibody for using it as a negative control. A positive control slide contained JAR cell lysate from Santa Cruz Biotechnology. Cases with brown granular nuclear stain in more than 5% of transformed cells were counted positive.
Leptin immunoreactivity has been scored, by 2 pathologists, for staining intensity and positively stained cells percentage. The frequency of positive cells was evaluated applying semiquantitative method in 3 fields with lenses of 40 amplification power. Staining intensity has been given scores 0, 1, 2, and 3 demonstrating negative, faint, moderate, and strong staining, respectively. Scores of staining intensity has been presented as negative, low (1) and high (2 and 3). When a disparity between the 2 pathologists’ staining scores has happened, the lowest score value was reported.
2.3. Statistical analysis
All results were assessed by IBM-SPSS software (version 21). All data values were presented as percentages and incidences. The association between clinicopathological factors of colorectal cancer and Leptin expression was explored statistically by chi-square and Fisher's exact tests. Both unadjusted and adjusted comparison of survival distributions for various leptin immunohistochemistry staining intensity levels were assessed applying Beslow Generalized Wilcoxon test. STATA 14 is used to perform survival analysis. The point of importance was considered when P-value < .05.
3. Results
Clinicopathological factors of all colorectal tumor cases with leptin expression were presented in Table 1. Out of 155 colorectal adenocarcinoma cases, 145 (93.5%) cases showed positive leptin immunostaining, of which 33 (22.75%) cases showed moderate to strong stain. All positive cases showed leptin cytoplasmic immunostaining. The majority of positively stained colorectal cancer cases (89.6%) exhibited brown color in more than 50% of the neoplastic cells. On the other hand, 76.7% of control cases including adenomas and noncancerous tissues were positive for leptin immunostaining (Table 1 and Fig. 1A–D). Considerable heterogeneity in leptin immunohistochemical staining in colorectal cancer cases was identified regarding cells and glands, for example, some tumors showed positive staining in some cells or glands only and others revealed uniform immunostaining in all cellular or glandular components (Fig. 1F).
Figure 1.
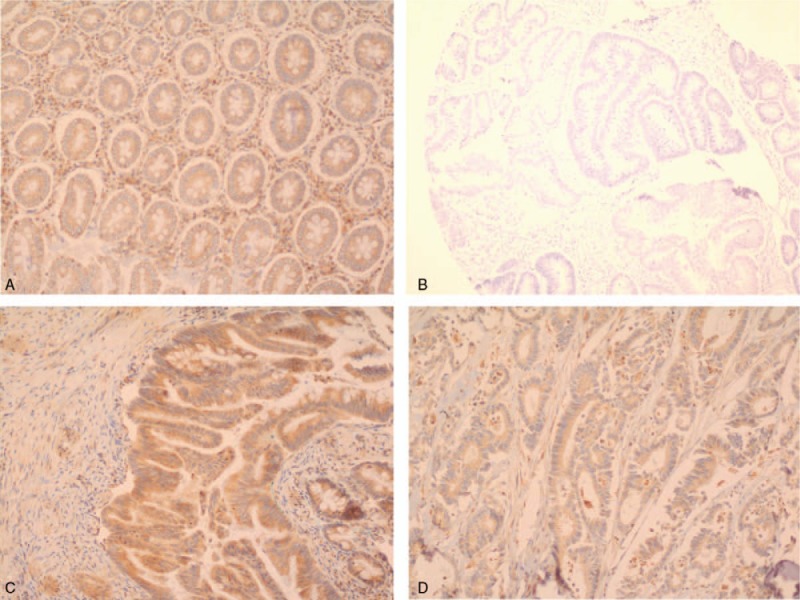
Leptin immunostaining pattern in colorectal cancer. (A) Strong positive staining in colorectal tissue (10 X); (B) negative staining in colorectal cancer (10×); (C) strong positive staining in colorectal cancer (10×); (D) moderate positive staining in colorectal cancer (10×).
Tissue type was considerably associated with leptin phenotype (P = .000), there is upregulation in leptin expression in colorectal carcinoma cases. Significantly higher proportion of negative leptin immunostaining cases were observed in tumors with size more than 5 cm (P = .045). Colorectal adenocarcinoma in both genders shared almost the same expression and distribution patterns of leptin with slight increase in staining frequency in female population. No significant associations, regarding immunostaining, were found with age, gender, differentiation, tumor size, tumor location, stage, margins involvement, vascular invasion, lymph node and remote metastases, local recurrence and disease relapse.
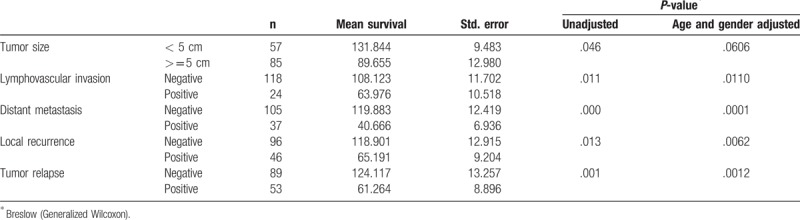
Beslow Generalized Wilcoxon test has been employed to liken the survival distribution in colorectal cancer cases of negative and positive leptin immunostaining. Table 2 describes the mean survival times of colorectal cancer patients with various clinicopathological factors adjusted for leptin immunostaining. Significant different survival patterns were observed with tumor size, lymphovascular invasion, distant metastasis, local recurrence, and disease relapse. Positive immunostaining cases with tumor size <5 cm shows better survival times than cases of large tumors (P = .046), similar pattern was observed when adjusted for age (P = .044); however, insignificant survival difference was observed when adjusted for gender (P = .057). Whereas positive cases with lymphovascular invasion, distant metastasis, local recurrence or disease relapse showed poor survival estimates (P = .011, .000, .013, and .001 respectively) and similar patterns were observed when adjusted for age and gender.
Table 2.
Comparison of survival distributions by various clinicopathological variables in positive leptin immunostaining colorectal adenocarcinomas.
Kaplan–Meier survival curves clearly shows that survival experiences are significantly better in cases of small tumors (<5 cm) and cases of no lymphovascular invasion, distant metastasis, local recurrence or disease relapse (Fig. 2).
Figure 2.
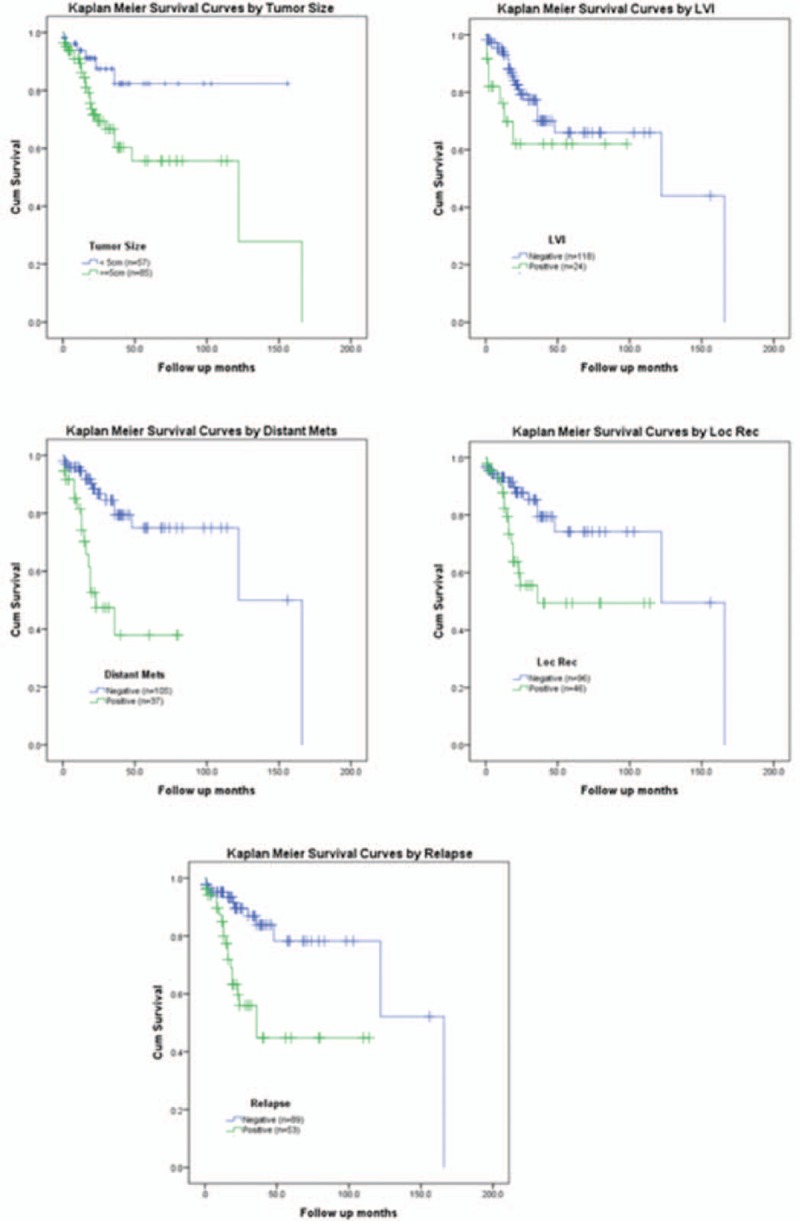
Kaplan–Meier survival curves by various clinicopathological variables with leptin immunostaining in colorectal adenocarcinoma.
4. Discussion
Colorectal cancer is one of the most important causes of death in Saudi Arabia. In 2013, there were 1387 cases of colorectal cancer, which totalled about 11.9% of all new cancer cases. Tumors of colon and rectum ranked first among males and third among females. It affected 736 (53.1%) males and 651 (46.9%) females with a ratio of 113:100. Sixty years was the average age at diagnosis amongst men and fifty 6 years among females. Morphologically, adenocarcinoma (NOS) was the most frequent type and far less common was mucinous carcinoma followed by signet ring cell carcinoma, and other histotypes. Youthful patients (below the age of 50) accounted for about 20% of all cases of colorectal cancers.[28]
Even the likely molecular mechanism of colorectal tumorigenesis is still ambiguous, some studies presented a considerable relationship between the serum leptin level and the risk of developing tumors in different sites including colon, telling that leptin could possibly be engaged in colorectal tumorigenesis. In a number of these published research papers, high concentrations of leptin have been allied with elevated hazard of colorectal tumor development.[29–31] However, there has been some disagreement about the correlation between serum level of leptin and colorectal tumor growth.[32,33] Furthermore, some in vitro studies indicated that leptin motivates DNA synthesis, stimulates proliferation, migration, invasion and survival of colorectal cancer cells. These analyses suggested the likely role of leptin in colorectal cancer evolution.[34–38] Although few studies has recommended that the immunohistochemical staining of leptin could be helpful as a marker to predict the characteristics and prognosis of colorectal carcinoma, but conflicting information has been reported.[20–25]
The present study found that high percentage of colorectal carcinomas (93.5%) were positive for leptin immunostaining, and in consistent with the findings of Jeong et al[25] study. But this frequency is higher than the results of Koda et al[20], Paik et al[21], Liu et al[22], and Wang et al[23] who reported (51.2%, 73.5%, 72.06, and 71.3%, respectively). The current study also showed significantly increased leptin cytoplasmic immunoexpression frequency from adjacent mucosa, normal tissue and noncancerous conditions to adenomas and carcinomas as transformation process; this finding is in harmony with Paik et al[21], and Liu et al[22] who reported a harmonious grown leptin staining frequency with neoplastic progression (adenoma-carcinoma sequence in colon).
However, Jeong et al[25] and the present study reported that leptin immunoexpression was not significantly associated with the clinicopathological features of colorectal carcinomas. On the other hand, Koda et al[20] significantly correlated leptin expression in colorectal cancer with tumor histological type and G2 grade but not with patients’ age and gender, tumor localization or lymph node involvement. Similarly Liu et al[22] stated that immunoexpression of leptin showed a significant relation with grades, Dukes’ stage, wall infiltration, lympho-vascular invasion, lymph node involvement, and distant metastasis, but did not display any link with patients’ age, gender, size of tumor, localization, and tumor histotypes. Furthermore, Paik et al[21] correlated leptin immunoexpression inversely with prognostic cancer characteristics such as stage, differentiation, infiltration, and lymph node involvement. Wang et al[23] significantly associated leptin expression with differentiation, stage and metastasis including lymph node, but not with the age and sex of patients. In Yoon et al[24] study, immunostaining of leptin was only considerably allied with stage of tumors.
In respect of survival distribution patterns, very few reports have researched the survival of colorectal cancer patients in proportion to leptin immunoexpression, and the information are insufficient to reach any conclusion. our findings opposed Paik et al[21] who reported “leptin-positive adenocarcinoma revealed better overall and disease-free survivals” while our positive staining cases with small tumor size, lymphovascular invasion, distant metastasis, local recurrence or disease relapse showed poorer survival estimates. In contrast, Jeong et al[25] showed no important changes in overall survival and disease-free survival rates among colorectal tumor cases.
However, the outcomes of our study showed that there is still some discrepancies between similar immunohistochemistry studies regarding the association of leptin immunostaining with clinicopathological features of colorectal carcinoma. These differences could be due to study sample size, diversity of populations, semiquantitative staining protocol, and procedures sensitivity. Therefore, larger comprehensive investigations are important for measuring the investigative and predictive competences of leptin staining in colorectal cancer.
5. Conclusion
Our findings propose a mounting rise in the expression of leptin during colorectal tumorigeneses. Immunoexpression of leptin could be a helpful biomarker in the prediction of prognosis and survival pattern of colorectal cancer with certain clinicopathological factors.
Acknowledgment
The authors acknowledge with thanks DSR for technical and financial support. The authors also acknowledge with thanks Dr Nadeem Shafique Butt for performing statistical analysis.
Author contributions
Conceptualization: Jaudah Ahmed Al-Maghrabi, Mohamad Nidal Khabaz.
Formal analysis: Mohamad Nidal Khabaz.
Funding acquisition: Jaudah Ahmed Al-Maghrabi.
Investigation: Jaudah Ahmed Al-Maghrabi, Imtiaz Ahmad Qureshi, Mohamad Nidal Khabaz.
Methodology: Jaudah Ahmed Al-Maghrabi, Imtiaz Ahmad Qureshi, Mohamad Nidal Khabaz.
Supervision: Mohamad Nidal Khabaz.
Visualization: Imtiaz Ahmad Qureshi.
Writing – original draft: Mohamad Nidal Khabaz.
Writing – review & editing: Imtiaz Ahmad Qureshi, Mohamad Nidal Khabaz.
Jaudah Ahmed Al-Maghrabi: 0000-0002-5298-7690
Footnotes
Abbreviations: IBM-SPSS = International Business Machines, Statistical Package for the Social Sciences, JAR cell lysate = choriocarcinoma cell lines, NOS = Not Otherwise Specified, Ob = obese gene, P-value = probability value, TM DAB = Multimer Technology, 3,3’-diaminobenzidine, TMA = tissue microarray, TNM = tumor, node, and metastases.
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, under grant number (G-124-140-38).
The authors have no conflicts of interest to disclose.
References
- [1].Puppa G, Sonzogni A, Colombari R, et al. TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues. Arch Pathol Lab Med 2010;134:837–52. [DOI] [PubMed] [Google Scholar]
- [2].Caro JF, Sinha MK, Kolaczynski JW, et al. Leptin: the tale of an obesity gene. Diabetes 1996;45:1455–62. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez RR, Simon C, Caballero-Campo P, et al. Leptin and reproduction. Hum Reprod Update 2000;6:290–300. [DOI] [PubMed] [Google Scholar]
- [4].Carino C, Olawaiye AB, Cherfils S, et al. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer 2008;123:2782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gao J, Tian J, Lv Y, et al. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci 2009;100:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science 1998;281:1683–6. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–32. [DOI] [PubMed] [Google Scholar]
- [8].Baratta M. Leptin--from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit 2002;8:RA282–92. [PubMed] [Google Scholar]
- [9].Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature 1998;394:790–3. [DOI] [PubMed] [Google Scholar]
- [10].Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res 2007;67:2497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bartucci M, Svensson S, Ricci-Vitiani L, et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer 2010;17:823–33. [DOI] [PubMed] [Google Scholar]
- [12].Dubois V, Jarde T, Delort L, et al. Leptin induces a proliferative response in breast cancer cells but not in normal breast cells. Nutr Cancer 2014;66:645–55. [DOI] [PubMed] [Google Scholar]
- [13].Lee KN, Choi HS, Yang SY, et al. The role of leptin in gastric cancer: clinicopathologic features and molecular mechanisms. Biochem Biophys Res Commun 2014;446:822–9. [DOI] [PubMed] [Google Scholar]
- [14].Zhou X, Li H, Chai Y, et al. Leptin inhibits the apoptosis of endometrial carcinoma cells through activation of the nuclear factor kappaB-inducing kinase/IkappaB kinase pathway. Int J Gynecol Cancer 2015;25:770–8. [DOI] [PubMed] [Google Scholar]
- [15].Khabaz MN, Abdelrahman A, Butt N, et al. Immunohistochemical staining of leptin is associated with grade, stage, lymph node involvement, recurrence, and hormone receptor phenotypes in breast cancer. BMC Womens Health 2017;1:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Khabaz MN, Butt NS, Al-Maghrabi B, et al. Leptin expression in stromal cells of endometrial carcinomas is associated with advanced stage and disease recurrence. Int J Clin Exp Pathol 2016;11:11774–80. [Google Scholar]
- [17].Gallina S, Sireci F, Lorusso F, et al. The immunohistochemical peptidergic expression of leptin is associated with recurrence of malignancy in laryngeal squamous cell carcinoma. Acta Otorhinolaryngol Ital 2015;1:15–22. [PMC free article] [PubMed] [Google Scholar]
- [18].Fan YL, Li XQ. Expression of leptin and its receptor in thyroid carcinoma: distinctive prognostic significance in different subtypes. Clin Endocrinol (Oxf) 2015;2:261–7. [DOI] [PubMed] [Google Scholar]
- [19].Lee KN, Choi HS, Yang SY, et al. The role of leptin in gastric cancer: clinicopathologic features and molecular mechanisms. Biochem Biophys Res Commun 2014;4:822–9. [DOI] [PubMed] [Google Scholar]
- [20].Koda M, Sulkowska M, Kanczuga-Koda L, et al. Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol 2007;8:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paik SS, Jang SM, Jang KS, et al. Leptin expression correlates with favorable clinicopathologic phenotype and better prognosis in colorectal adenocarcinoma. Ann Surg Oncol 2009;2:297–303. [DOI] [PubMed] [Google Scholar]
- [22].Liu H, Wan D, Pan Z, et al. Expression and biological significance of leptin, leptin receptor, VEGF, and CD34 in colorectal carcinoma. Cell Biochem Biophys 2011;3:241–4. [DOI] [PubMed] [Google Scholar]
- [23].Wang D, Chen J, Chen H, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci 2012;1:91–101. [DOI] [PubMed] [Google Scholar]
- [24].Yoon KW, Park SY, Kim JY, et al. Leptin-induced adhesion and invasion in colorectal cancer cell lines. Oncol Rep 2014;6:2493–8. [DOI] [PubMed] [Google Scholar]
- [25].Jeong WK, Baek SK, Kim MK, et al. Prognostic significance of tissue leptin expression in colorectal cancer patients. Ann Coloproctol 2015;6:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khabaz MN, Abdelrahman AS, Butt NS, et al. Cyclin D1 is significantly associated with stage of tumor and predicts poor survival in endometrial carcinoma patients. Ann Diagn Pathol 2017;30:47–51. [DOI] [PubMed] [Google Scholar]
- [27].Al-Maghrabi J, Al-Sakkaf K, Qureshi IA, et al. AMPK expression patterns are significantly associated with poor prognosis in breast cancer patients. Ann Diagn Pathol 2017;29:62–7. [DOI] [PubMed] [Google Scholar]
- [28].Al-Madouj A, Al-Shahrani Z, AI A, et al. Cancer Incidence Report Saudi Arabia 2012. Saudi Health Council web site, 2016. Available at: http://www.chs.gov.sa/Ar/HealthCenters/NCC/CancerRegistry/CancerRegistryReports/2013.pdf. Accessed January 30, 2018 [Google Scholar]
- [29].Stattin P, Palmqvist R, Söderberg S, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep 2003;6:2015–21. [PubMed] [Google Scholar]
- [30].Fenton JI, Lavigne JA, Perkins SN, et al. Microarray analysis reveals that leptin induces autocrine/paracrine cascades to promote survival and proliferation of colon epithelial cells in an Apc genotype-dependent fashion. Mol Carcinog 2008;1:9–21. [DOI] [PubMed] [Google Scholar]
- [31].Janeckova R. The role of leptin in human physiology and pathophysiology. Physiol Res Acad Sci Bohemoslov 2001;50:443–59. [PubMed] [Google Scholar]
- [32].Bolukbas FF, Kilic H, Bolukbas C, et al. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer 2004;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tessitore L, Vizio B, Jenkins O, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med 2000;4:421–6. [DOI] [PubMed] [Google Scholar]
- [34].Hardwick JC, Van Den Brink GR, Offerhaus GJ, et al. Leptin is a growth factor for colonic epithelial cells. Gastroenterology 2001;1:79–90. [DOI] [PubMed] [Google Scholar]
- [35].Mełeń-Mucha G, Lawnicka H. Leptin promotes the growth of Colon 38 cancer cells and interferes with the cytotoxic effect of fluorouracil in vitro. Endokrynol Pol 2007;1:2–6. [PubMed] [Google Scholar]
- [36].Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer 2008;11:2543–56. [DOI] [PubMed] [Google Scholar]
- [37].Ratke J, Entschladen F, Niggemann B, et al. Leptin stimulates the migration of colon carcinoma cells by multiple signaling pathways. Endocr Relat Cancer 2010;1:179–89. [DOI] [PubMed] [Google Scholar]
- [38].Hoda MR, Keely SJ, Bertelsen LS, et al. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg 2007;3:346–54. [DOI] [PubMed] [Google Scholar]


