Abstract
Background:
In recent years, Trichuris suis ova (TSO) therapy in inflammatory bowel disease (IBD) has attracted much attention. However, efficacy and safety of TSO therapy are still not well described. The aim of the study was to perform a meta-analysis to assess the effectiveness of TSO therapy in IBD.
Methods:
PubMed, Embase, Web of Science, ClinicalTrials.gov, and Cochrane Library were searched from inception to August 2017. Only randomized, double-blind, placebo-controlled trials (RCTs) were included. The pooled estimate rates were performed by meta-analysis and reported according to the standard Cochrane guidelines and the PRISMA statement.
Results:
In ulcerative colitis study (3 RCTs, n = 74), the induced rates of clinical remission and clinical response were 10.8% (4/37) and 53.8% (21/39) in TSO group, while 6.7% (2/30) and 29.0% (9/31) in placebo group (all P > .26). Twenty-two (9/41) percent of patients in TSO group experienced at least 1 adverse event compared with 27.3% (9/33) of placebo [relative ratio (RR) 0.75, 95% confidence interval (95% CI) 0.17–3.27]. In Crohn disease study (3 RCTs, n = 538), 40.7% (74/182) of patients in TSO group achieved clinical remission compared with 42.9% (90/210) of placebo (RR 0.95, 95% CI 0.75–1.20); 45.9% (141/307) of patients in TSO group entered clinical response compared with 45.1% (151/335) of placebo (RR 1.02, 95% CI 0.86–1.21). There were sparse data of adverse events reporting both TSO and placebo group (RR 1.00, 95% CI 0.88–1.13).
Conclusion:
TSO therapy showed no statistical benefit for IBD patients, so it suggested clinicians consider its value carefully before putting into clinical practice. Perhaps continued investigations of larger sample size are necessary due to the previous results with lack of power.
Keywords: efficacy, inflammatory bowel disease, meta-analysis, safety, Trichuris suis ova
1. Introduction
Crohn disease (CD) and ulcerative colitis (UC) together consisted of inflammatory bowel diseases (IBD), is a group of chronic inflammatory disorders of the gastrointestinal tract, and the prevalence is increasing with industrialized development.[1] Although their underlying pathogenetic mechanism remains incompletely clear, it is acknowledged that the abnormality of immune regulation plays a pivotal role. Hence, IBD is referred to as one of immune-mediated diseases.[2] In theory, CD is a kind of Th1-mediated like disease and UC is a kind of Th2-mediated like disease, respectively.[3] For past decades, the treatment of IBD has been limited to sulphasalazine, 5-aminosalicylic acid (5-ASA), corticosteroids, immunomodulators, and biologicals, empirically employed to control the inflammatory disorder of IBD.[4–6]
It is known that environmental factors, including helminths exposure and smoking, show close association with the risk of developing IBD,[1,7] despite 18.8% and 50% of contribution from genes to UC and CD, respectively.[8] For instance, in sub-Saharan Africa where helminths of intestinal infestation were frequent, the prevalence of IBD was surprisingly low in these local black populations; however, the incidence of these diseases is approaching to white populations when African people immigrated in USA and UK, which cannot be explained only by genetic factors.[9] In other words, helminths are seemed to be inversely associated with the development of IBD.
About 10 years ago, the administration of Trichuris suis ova (T. suis, TSO), eggs from the porcine whipworm that is nonpathogenic in man, was promoted and used in 7 patients with IBD.[10] The encouraging results demonstrated that TSO treatment was effective for inducing clinical remission. More excitingly, TSO treatment could induce immune regulatory circuits that impedes Th1 and possibly even aberrant Th2 responses,[11,12] which are benefit for modifying the immune pathogenetic mechanism of IBD. To date, many observational or randomized controlled studies have investigated the efficacy of TSO treating IBD, which could provide a new insight into treatment of IBD.[10,13–15] In addition to this, the potential harmful effects of TSO might attract another attention, especially for gastroenterological physicians. In 2013, Sandborn et al[16] have ever concentrated on evaluating the safety and tolerability of TSO therapy in 36 patients with CD using a randomized placebo-controlled trial (RCT). Now, other larger randomized, double-blind, placebo-controlled trials are needed to confirm the efficacy and safety of TSO treatment in IBD.
Although a previous meta-analysis of TSO for the treatment of IBD has been conducted by Garg et al in 2014,[17] it only included 1 placebo-controlled RCT by Summers et al in 2005[15]; in addition, not enough information on the safety were provided because of limited data available. In recent 3 years, more double-blind, placebo-controlled RCTs of TSO, but no larger ones, for treating IBD have been reported. To assimilate these data, we conducted a systematic review and meta-analysis to assess the efficacy and safety of TSO therapy in IBD comparing to placebo.
2. Methods
A systematic review and meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements.[18]
2.1. Literature search and study selection
PubMed, Embase, Web of Science, ClinicalTrials.gov, and Cochrane Library were searched for relevant studies from inception to August 2017. The principal search key words and MeSH headings used were as follows: Trichuris suis ova, helminth, and IBD and its 2 subtypes. Articles in English were reviewed and other languages excluded. In addition, a manual search of references in the identified articles was performed to identify any other potentially eligible trials.
Firstly, duplicates among all searched articles were identified and removed by Endnote software. We included studies that met the following criteria: only randomized, double-blind, placebo-controlled RCTs were included, studies compared TSO therapy with placebo, and outcomes included efficacy or safety. Studies were excluded by the following exclusion criteria: studies without full texts, or reviews; other helminths except from T. suis ova; and the outcomes of studies without efficacy or safety.
2.2. Data extraction
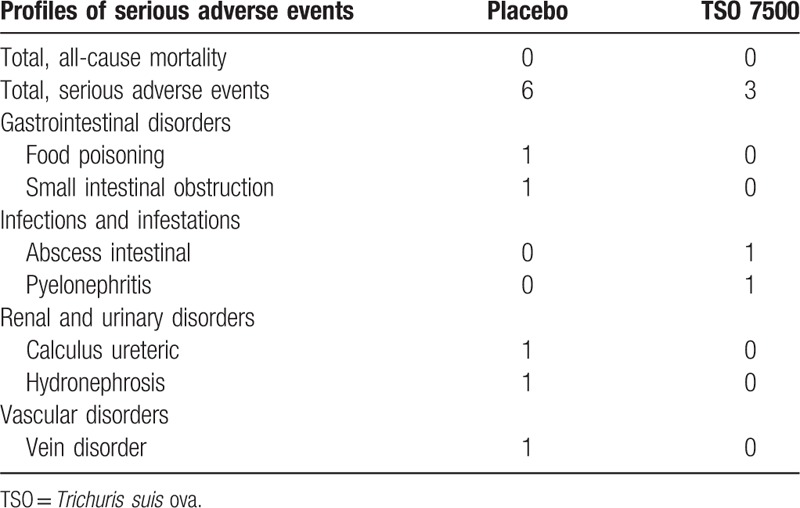
Full text articles that met inclusion criteria were reviewed and data extraction by 2 authors (Xing Huang and Li-Rong Zeng). All differences were resolved by discussions with other author. Data extracted were as follows: authors, year, region, study design, patient characteristics, number of patients, dose of TSO, follow-up (Table 1), induction of clinical response and clinical remission, the incidence and profiles of adverse events (including abdominal pain, nasopharyngitis, nausea or vomiting, diarrhea, fatigue, headache, etc) (Tables 2 and 3).
Table 1.
Characteristics of included trials in the meta-analysis.
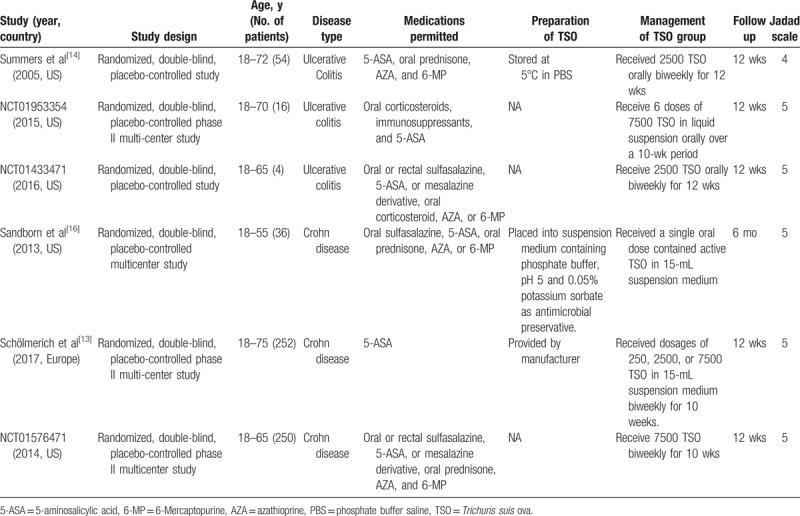
Table 2.
Profiles of adverse events (not including serious adverse events) developed when the treatment of Trichuris suis ova in inflammatory bowel disease.
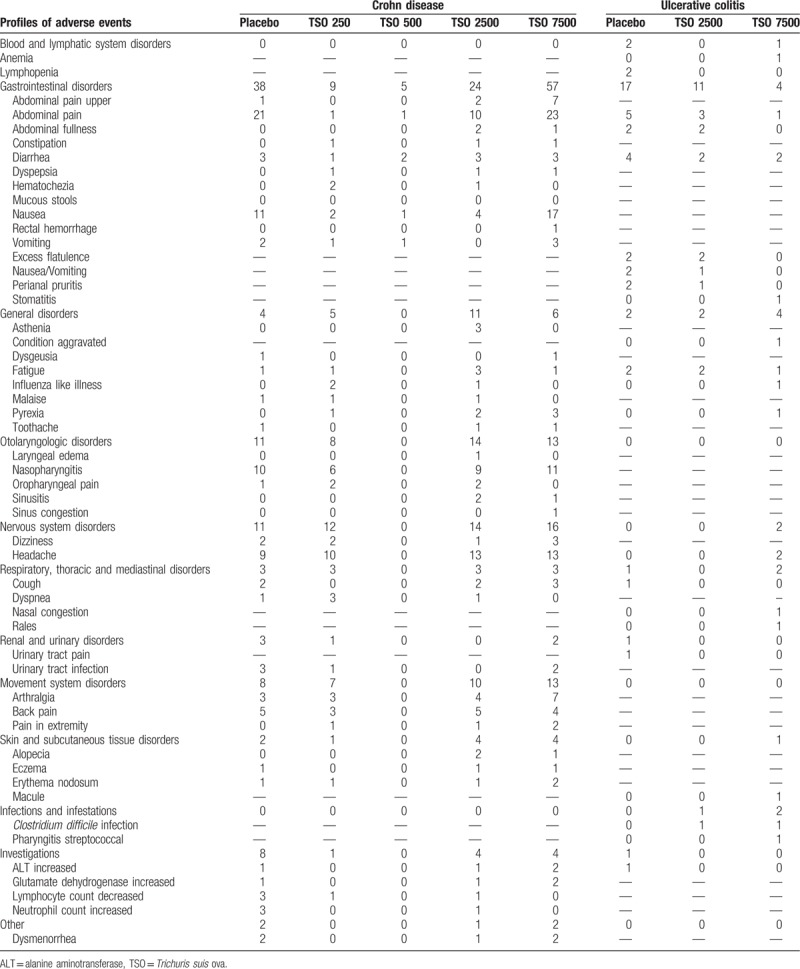
Table 3.
Profiles of serious adverse events developed when the treatment of Trichuris suis ova in Crohn disease.
2.3. Data synthesis and analysis
To assess TSO therapy, the outcomes of efficacy were pooled estimates of clinical remission and clinical response after TSO therapy for IBD patients. The outcome of safety was the incidence of total side effects.
We estimated aforementioned outcomes using the fixed or random effects model. Relative ratios (RRs) with 95% confidence intervals (95% CIs) as pooled estimates of efficacy and safety were calculated using an intention-to-treat analysis. Heterogeneity between studies was examined by Q test and I2 statistic with I2 > 50% representing substantial heterogeneity. All statistical analyses were performed using STATA 11.0 software (Stata Corporation, College Station, TX).
Meta-analysis is a systematic review based on previous studies and the ethical approval is not necessary.
3. Results
The literature search yielded a total of 384 records from 5 databases, of which 83 records were duplicates. These remaining 301 records were screened on the basis of selection criteria. Of the 21 potentially relevant clinical trials, 15 were removed due to specified reasons in Fig. 1. Among these removed trials, 1 trial (NCT03079700) reported no data on efficacy and safety; 2 published articles were also registered in ClinicalTrials.gov (NCT01434693 and NCT01279577).[13,16] Two observational studies by Summers et al[10,14] were excluded from this meta-analysis. Finally, 6 RCTs including 3 trials registered in ClinicalTrials.gov (NCT01953354, NCT01576471, and NCT01433471)[13,15,16] evaluating the efficacy and safety of TSO therapy in IBD patients were included in this meta-analysis.
Figure 1.
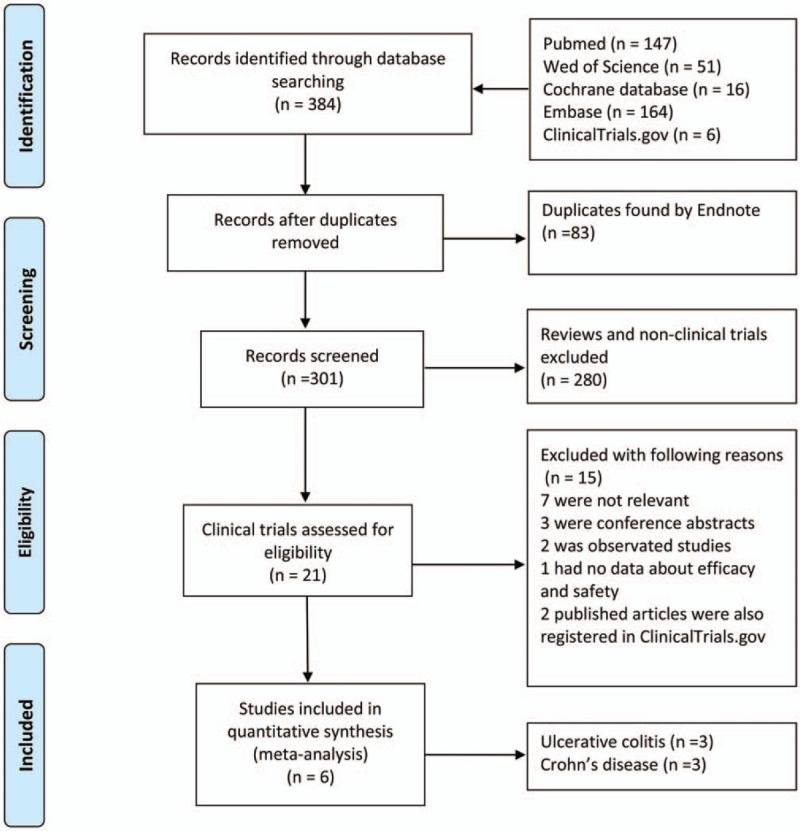
Identification process for eligible trials.
3.1. Study characteristics
These RCTs all involving adult patients were published between 2005 and 2017, all of which were conducted in Europe and United States. There were all randomized, double-blind, placebo-controlled trials, of which 4 multicenter trials were included (such as NCT01953354 and NCT01576471).[13,16] In TSO groups, IBD patients received one of four doses of TSO (such as 250 ova, 500 ova, 2500 ova, or 7500 ova) orally biweekly for more than 10 weeks. In addition, patients who were under TSO therapy were rechecked by colonoscopy or stool test for whether existing ova or parasites during the follow-up. The characteristics of 6 RCTs are summarized in detail in Table 1.
3.2. Efficacy and safety of TSO therapy in inflammatory bowel disease
Efficacy was regarded as whether achieved clinical remission or clinical response after TSO therapy. In addition, the sparse data of adverse events during follow-up period can be reported, such as abdominal pain, nasopharyngitis, nausea or vomiting, diarrhea, fatigue, headache, etc (Tables 2 and 3). If at least 1 adverse event occurred, it was regarded as total side effects. Of note, no worms or ova were identified in stools or endoscopy during the study period, even though being under TSO therapy.[13,15,16]
3.3. TSO therapy in ulcerative colitis
Three RCTs of UC (n = 74 patients) were eligible. In the UC study, patients took 2500 TSO or 7500 TSO orally biweekly for 12 weeks. The induced rates of clinical remission and clinical response were 10.8% (4/37) and 53.8% (21/39) in TSO group, while 6.7% (2/30) and 29.0% (9/31) in the placebo group (RR 1.64 and 1.66, 95% CI 0.32–8.34 and 0.69–4.03, respectively, Fig. 2A).
Figure 2.
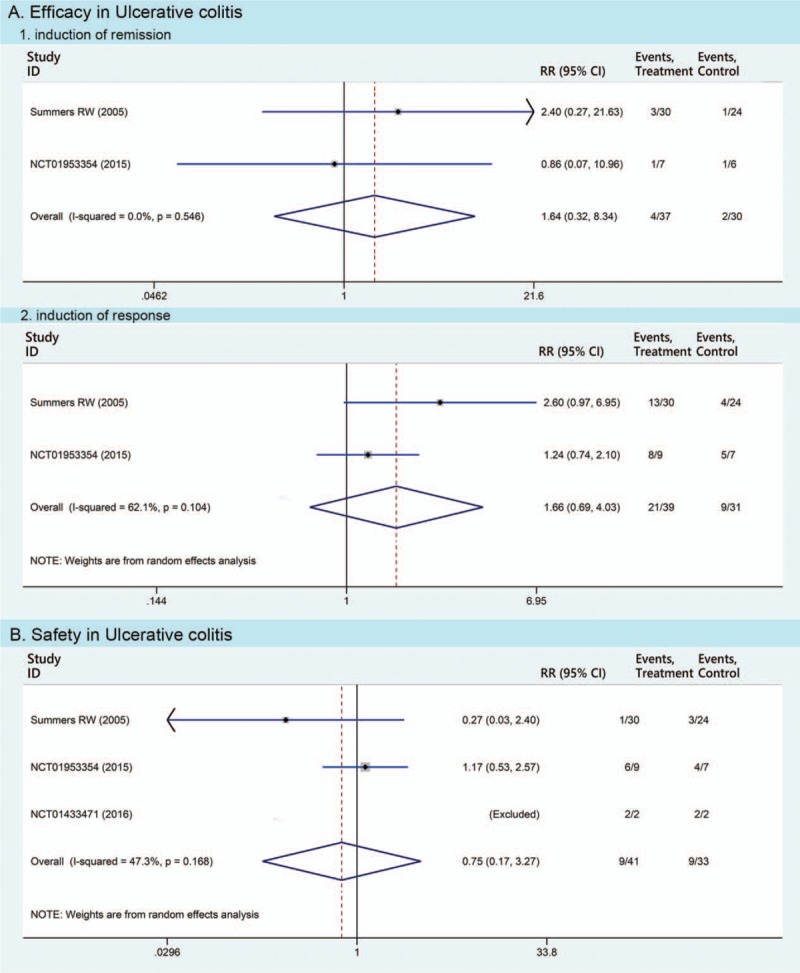
Pooled estimates of Trichuris suis ova therapy in ulcerative colitis compared with placebo in meta-analyses. Efficacy of Trichuris suis ova therapy includes the induction of remission and response, respectively.
In total, 22 different adverse events were monitored and reported in 3 RCTs of UC (Fig. 3A). The total side effects occurred in 22.0% (9/41) of patients in TSO group compared with 27.3% (9/33) of patients in placebo (RR 0.75, 95% CI 0.17–3.27, Fig. 2B). Moreover, none of the severe adverse events was reported in TSO therapy of this study.
Figure 3.
Profiles of adverse events when Trichuris suis ova therapy in inflammatory bowel disease.
3.4. TSO therapy in Crohn disease
In the CD study (3 RCTs, n = 538 patients), 40.7% (74/182) of patients in the TSO group achieved clinical remission compared with 42.9% (90/210) of placebo (RR 0.95, 95% CI 0.75–1.20); 45.9% (141/307) of patients in the TSO group entered clinical response compared with 45.1 (151/335) of placebo (RR 1.02, 95% CI 0.86–1.21) (Fig. 4A).
Figure 4.
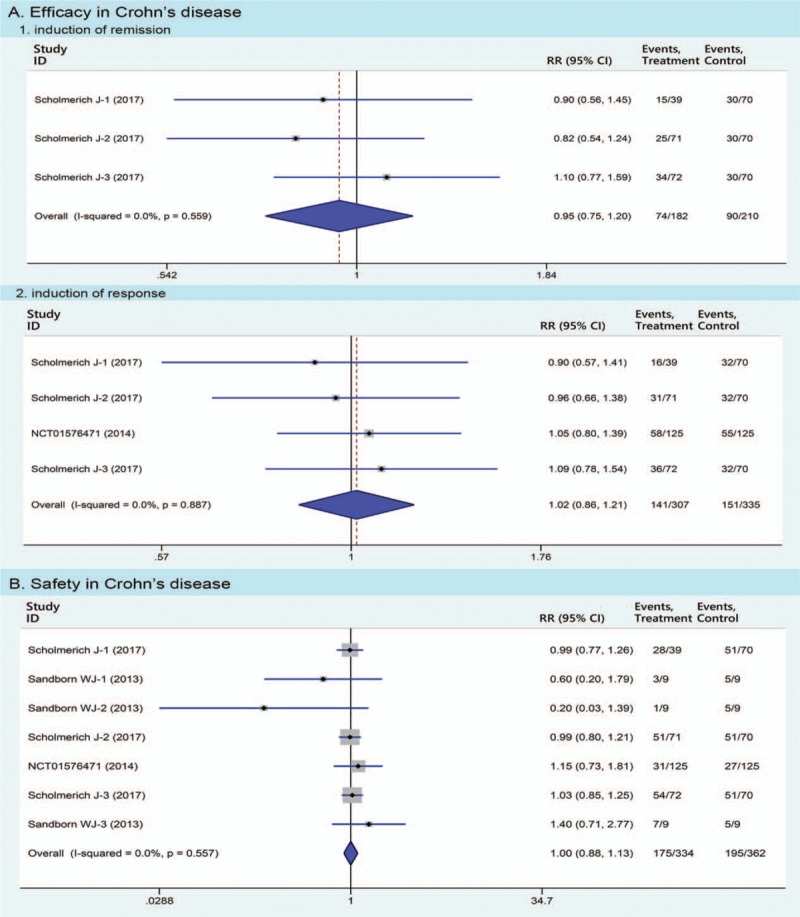
Pooled estimates of Trichuris suis ova therapy in Crohn disease compared with placebo in meta-analyses. Efficacy of Trichuris suis ova therapy includes the induction of remission and response, respectively.
In the CD study, 39 adverse events (including 7 serious adverse events) were reported (Fig. 3B). Fifty-two (175/334) of patients in TSO group experienced at least 1 adverse event compared with 53.9% (195/362) of placebo (RR 1.00, 95% CI 0.88–1.13, Fig. 4B).
3.5. Heterogeneity
There was no heterogeneity between trials of CD (I2 = 0%), while moderate heterogeneity (I2 = 62.1%) existed in data of clinical response of UC studies. Due to only 2 studies included, sensitivity analysis was not further conducted for clinical response.
4. Discussion
In the present study, we found that the absolute rate on efficacy in patients with UC treated by TSO is high, and the absolute rate of adverse events in patients exposed to TSO therapy is lower. However, these differences did not reach statistical significance on meta-analysis. Similarly, TSO therapy was not superior to placebo was also found in CD patients.
Given that these RCTs included a small number of patients with IBD, especially UC, so, a meta-analysis was needed to demonstrate the effectiveness of TSO therapy. This meta-analysis was performed using all available literature sources to date and in accordance with the recommendations of the Cochrane Collaboration. However, several limitations in our meta-analysis should be mentioned. First, few studies were included, and only published data were analyzed, despite large searches on databases. Some data may have been missed, which would result in conclusions lacking power. Second, biases in patient selection and differences in TSO dosage may influence the final results in terms of efficacy and safety. Besides, our meta-analysis could not draw a conclusion regarding TSO dosage due to the limited data. Third, most studies included in our meta-analysis had a short follow-up time of 12 weeks, which might be not enough to observe the effect of TSO therapy. Further research is therefore required to elucidate the long-term efficacy of TSO therapy in IBD. Finally, adverse events reporting was sparse or sporadic, which precluded further meta-analysis for the incidence of a specific adverse event.
To our knowledge, IBD probably results from an inappropriately vigorous immune response to contents of the intestinal lumen based on genetic susceptibility, which breaks the balance between Th1-like and Th2-like cytokines. However, not all intestinal microbes do harmfully. When helminths carried in CD patients whose disorder is mediated through Th1 and Th17 cells, the host evokes a strong Th2 immune response including release of Th2 cytokines such as interleukin (IL)-4, IL-5, and IL-13.[19] Helminths regulate their host's immune system and prevent excessive immune responses.[7] For example, products of T. suis induce immune response to protect the barrier function and suppress inflammatory cytokine production in intestinal epithelial cells.[20–23] Therefore, the administration of TSO has a potential efficacy for CD patients due to its counteraction to the aberrant Th1/Th17 response. Similarly, TSO therapy could also induce immune regulation for aberrant Th2 responses associated with UC.[11,12] Clinical trials have revealed encouraging evidences on efficacy when therapeutic use of TSO in IBD patients.[13,15,16] Epidemical data demonstrated that loss of exposure to helminths during childhood could lead to an increased risk of immune diseases, such as IBD.[6,20] In all, TSO therapy could activate immune regulations to be beneficial for IBD patients, because its therapeutic mechanism is partly converse to the pathogenesis of IBD. However, our study did not find the positive results. There are many possible explanations for no statistical difference on efficacy in IBD patients exposed to TSO therapy compared with placebo. First, the excreted/secreted products of T. suis, may not its eggs, could regulate their host's immune system.[23,24] Furthermore, T. suis, a porcine whipworm, has the differences in morphology, exogenous, and endogenous development from T. trichiura of man.[25] However, using human helminths may have disease potential or raise public health concerns. Second, the quality and dosage of TSO have an important impact on effectiveness in IBD.[26] Only the fully embryonated eggs that contain distinct larvae have the potential to hatch and invade the intestinal mucosa, which is believed to be the key factor activating immune regulations.[26] Eggs without visible larvae do not have this effect, and therefore, the concentration of embryonated eggs in suspension medium is essential for the dosage of the pharmaceutical effect.[27] Finally, positive results may be observed if the sample size is larger and follow-up time is longer.
As for its safety, it may be of more concern for us, but less is known. Therefore, this meta-analysis was conducted for better understanding of it. The results demonstrated that TSO therapy in IBD did not improve the risk of developing adverse events compared with placebo. In the profiles of adverse events, gastrointestinal signs and symptoms were the most common in both CD and UC patients. In addition, CD patients were more susceptible to these adverse events by TSO therapy than UC patients. Fortunately, less serious adverse events or no all-cause mortality of TSO therapy occurred. Of note, no worms or ova were identified in stools or endoscopy during the study period. Overall, TSO therapy was well tolerated in IBD patients.
In addition, the TSO are eggs of the porcine whipworm, a parasitic animal. TSO treatment to IBD cannot help to raise other safety concerns, such as parasitism.[28] The natural host of T. suis is pig, not humans, although it could be capable of colonizing a human host for several weeks, and no diseases developed when human volunteers were exposed to the living TSO.[25] Clinical studies verified that worms or ova were not found in stools or endoscopy during the study period.[13,15,16] Also raised is the question of whether administration of helminth ova contaminated by other pathogens promote the coinfection of these pathogens, such as secondary bacterial infection. It is not possible because these ova are usually collected, washed extensively, and incubated as well as stored in specific pathogen-free environment. Furthermore, the development of TSO is requested under good manufacturing practice (GMP) and appropriate safety testing by the United States Food and Drug Administration.[29] Thus, the risk of contaminating the TSO with other infections is removed.
In theory, TSO therapy provides a new immunopathology for treating IBD patients. However, TSO therapy in IBD patients had no statistically or clinically significant effect in this meta-analysis. The dosage of TSO or treatment duration may have an influence on efficacy. In addition, these results based on 3 studies only had to be interpreted carefully. Furthermore, the limited sample size of the available studies mean that future studies are needed with much larger samples to ensure more reliable findings.
Acknowledgment
We acknowledge all clinical researchers of the selected studies and patients related to these studies.
Author contributions
Data curation: Li-Rong Zeng.
Formal analysis: Meng-Hua Zhu.
Methodology: Xing Huang, Meng-Hua Zhu.
Resources: Li-Rong Zeng.
Software: Li-Rong Zeng, Feng-Song Chen, Jing-Ping Zhu.
Supervision: Feng-Song Chen, Jing-Ping Zhu.
Validation: Feng-Song Chen.
Visualization: Jing-Ping Zhu.
Writing – original draft: Xing Huang, Li-Rong Zeng.
Writing – review & editing: Xing Huang, Meng-Hua Zhu.
Footnotes
Abbreviations: 5-ASA = 5-aminosalicylic acid, CD = Crohn disease, CI = confidence interval, GMP = good manufacturing practice, IBD = inflammatory bowel disease, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized, double-blind, placebo-controlled trials, RR = relative ratio, TSO = Trichuris suis ova, UC = ulcerative colitis.
XH and L-RZ contributed equally to this work.
The authors declare that they have no competing interests.
The authors of this work have nothing to disclose.
References
- [1].Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- [2].Choy MC, Visvanathan K, De Cruz P. An overview of the innate and adaptive immune system in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:2–13. [DOI] [PubMed] [Google Scholar]
- [3].Plevy SE, Landers CJ, Prehn J, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol 1997;159:6276–82. [PubMed] [Google Scholar]
- [4].Morrison G, Headon B, Gibson P. Update in inflammatory bowel disease. Aust Fam Physician 2009;38:956–61. [PubMed] [Google Scholar]
- [5].De Vroey B, Colombel J-F. IBD in 2010: optimizing treatment and minimizing adverse events. Nat Rev Gastroenterol Hepatol 2011;8:74–6. [DOI] [PubMed] [Google Scholar]
- [6].Ardizzone S, Porro GB. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med 2002;252:475–96. [DOI] [PubMed] [Google Scholar]
- [7].Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis 2009;15:128–33. [DOI] [PubMed] [Google Scholar]
- [8].Halfvarson J, Bodin L, Tysk C, et al. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 2003;124:1767–73. [DOI] [PubMed] [Google Scholar]
- [9].Fiasse R, Latinne D. Intestinal helminths: a clue explaining the low incidence of inflammatory bowel diseases in Subsaharan Africa? Potential benefits and hazards of helminth therapy. Acta Gastroenterol Belg 2006;69:418–22. [PubMed] [Google Scholar]
- [10].Summers RW, Elliott DE, Qadir K, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 2003;98:2034–41. [DOI] [PubMed] [Google Scholar]
- [11].Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 2002;296:490–4. [DOI] [PubMed] [Google Scholar]
- [12].Elliott DE, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 2003;284:G385–391. [DOI] [PubMed] [Google Scholar]
- [13].Schölmerich J, Fellermann K, Seibold FW, et al. A randomised, double-blind, placebo-controlled trial of Trichuris suis ova in active Crohn's disease. J Crohns Colitis 2017;11:390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy in Crohn's disease. Gut 2005;54:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005;128:825–32. [DOI] [PubMed] [Google Scholar]
- [16].Sandborn WJ, Elliott DE, Weinstock J, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment Pharmacol Ther 2013;38:255–63. [DOI] [PubMed] [Google Scholar]
- [17].Garg SK, Croft AM, Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev 2014;CD009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maizels RM, Balic A, Gomez-Escobar N, et al. Helminth parasites: masters of regulation. Immunol Rev 2004;201:89–116. [DOI] [PubMed] [Google Scholar]
- [20].Laan LC, Williams AR, Stavenhagen K, et al. The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells. FASEB J 2017;31:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Parthasarathy G, Mansfield LS. Trichuris suis excretory secretory products (ESP) elicit interleukin-6 (IL-6) and IL-10 secretion from intestinal epithelial cells (IPEC-1). Vet Parasitol 2005;131:317–24. [DOI] [PubMed] [Google Scholar]
- [22].Elliott DE, Summers RW, Weinstock JV. Helminths and the modulation of mucosal inflammation. Curr Opin Gastroenterol 2005;21:51–8. [PubMed] [Google Scholar]
- [23].Hiemstra IH, Klaver EJ, Vrijland K, et al. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol Immunol 2014;60:1–7. [DOI] [PubMed] [Google Scholar]
- [24].Klaver EJ, Kuijk LM, Laan LC, et al. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol 2013;43:191–200. [DOI] [PubMed] [Google Scholar]
- [25].Beer RJ. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Res Vet Sci 1976;20:47–54. [PubMed] [Google Scholar]
- [26].Reddy A, Fried B. The use of Trichuris suis and other helminth therapies to treat Crohn's disease. Parasitol Res 2007;100:921–7. [DOI] [PubMed] [Google Scholar]
- [27].Bruun JM, Carstensen JM, Vejzagic N, et al. OvaSpec: a vision-based instrument for assessing concentration and developmental stage of Trichuris suis parasite egg suspensions. Comput Biol Med 2014;53:94–104. [DOI] [PubMed] [Google Scholar]
- [28].Van Kruiningen HJ, West AB. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflamm Bowel Dis 2005;11:515. [DOI] [PubMed] [Google Scholar]
- [29].Heylen M, Ruyssers NE, Gielis EM, et al. Of worms, mice and man: an overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol Ther 2014;143:153–67. [DOI] [PubMed] [Google Scholar]


