Abstract
Objective
Numerous studies have investigated different operative procedures for treating chronic subdural hematoma (CSDH); however, the results are controversial. This meta-analysis was performed to evaluate the efficacy of burr hole drainage without irrigation (BHD) and burr hole drainage with irrigation (BHDI) for CSDH.
Methods
We searched the following electronic databases to identify all studies from their inception to September 2017: Cochrane Library, Science Direct, MEDLINE, EMBASE, Scopus, Google Scholar, the China Biomedical Database (CBM), and the Chinese National Knowledge Infrastructure (CNKI). Randomized clinical trials (RCTs), prospective cohort studies, retrospective observational cohort studies, and case-control studies investigating BHD and BHDI for the treatment of CSDH were included. The Cochrane Collaboration's RevMan 5.3 software was used for meta-analysis.
Results:
In total, 7 retrospective cohort studies and 2 RCTs involving 993 participants were included. Comprehensive analysis results of 9 studies indicated that the recurrence of the BHDI was similar to that in BHD (odds ratio [OR] = 1.27, 95% confidence interval [CI] = .61–2.63, P = .53). Moreover, analysis for comparing recurrence in the 2 RCTs was not significantly different (OR = 1.14, 95% CI = .16–8.24, P = .95).In addition, meta-analysis of pneumocephalus (OR = 5.91, 95% CI = .61–56.86, P = .12) and mortality (OR = 0.94, 95% CI 0.14–6.16, P = .95) was not significantly different.
Conclusions:
The results of this meta-analysis demonstrated that procedures with or without irrigation in the treatment of CSDH might have similar effect regarding recurrence and complications; therefore, irrigation might not be necessary. However, well-conducted RCTs and high-quality observational studies are still required to corroborate this issue.
Keywords: burr hole drainage, irrigation, meta-analysis, recurrence, systematic review
1. Introduction
Chronic subdural hematoma (CSDH) is one of the most commonly encountered condition in neurosurgery. The prevalence of CSDH in elderly individuals is predicted to increase as the average age of the population in most countries continues to rise.[1] The treatment strategy for CSDH depends on the presence of symptoms and clinical signs of cerebral parenchymal compression.[2] In addition to conservative treatment, multiple surgical techniques include twist drill craniotomy (TDC), burr hole drainage with irrigation (BHDI) or without irrigation (BHD), and large craniotomy.[3] However, there is no universal consensus regarding the optimal surgical procedure for treating CSDH.
Burr hole drainage is a common procedure for the treatment of CSDH. Briefly, burr hole was performed by drilling a hole approximately 1.5 cm in diameter. Next, the dura mater and the hematoma membrane are dissected. Subsequently, the catheter is inserted into the cavity. In BHDI, body temperature saline is irrigated until the fluid ran clear; however, irrigation is not performed in BHD group. Finally, the wound is sutured, and closed drainage systems are used in all patients in both groups.[4] Numerous studies have investigated the recurrence rate of these 2 techniques. Ishibashi et al[4] reported the recurrence rate that was 3-fold higher for drainage without irrigation (10.3%) versus drainage with irrigation (2.9%, P = .191). In Kuroki et al’ trail, recurrence rates were 3.6% in the BHD group and 13.3% in the BHDI group. However, some studies have reported that the recurrence rate was not significantly associated with surgical procedures. Thus, determining the type of surgical technique that is more effective and safer is still under debate. There are no systematic reviews comparing the recurrence rates of BDHI and BHD. Therefore, we conducted the present review to determine which technique is more effective in terms of prevention recurrence, and achieving better postoperative clinical outcomes.
2. Methods
2.1. Search strategies
We searched the Cochrane Library, Science Direct, MEDLINE, EMBASE, Scopus, Google Scholar, the China National Knowledge Infrastructure (CNKI), and the Chinese Biomedical Database (CBM) from their inception to September 2017 for available studies using appropriate combinations of MeSH terms. The following terms were searched: “chronic subdural hematoma" [All Fields] OR “hematoma, subdural, chronic" [MeSH Terms] OR (“hematoma" [All Fields] AND “subdural" [All Fields] AND “chronic" [All Fields]) OR “chronic subdural hematoma" [All Fields] OR (“chronic"[All Fields] AND “subdural"[All Fields] AND “hematoma" [All Fields]), and key words, including: Surgery, Burr hole drainage; Irrigation; Recurrence; and mortality. Corresponding Chinese terms were also searched. We analyzed studies published in English and Chinese. The following types of studies were excluded: those not evaluating recurrence and complications of the procedure, those analyzing BHDI and BHD, those not able to provide sufficient primitive data, those published in other languages, those including patients with CSDH secondary to cranial procedures, and those referring to irrigation with artificial cerebrospinal fluid or urokinase or other liquid besides normal saline.
2.2. Study selection
Two authors (Y.Y. and Q.P.W.) independently assessed titles, abstracts, and full texts of the primary selected studies. All RCTs, prospective cohort studies, retrospective observational cohort studies, and case-control studies comparing the recurrence rates of CSDH after BHD and BHDI were considered in this analysis. Included studies had to conform to standardized criteria: patients diagnosed with CSDH; patients treated with BHD and BHDI; and data were available. Exclusion criteria were: studies with a diagnosis of other diseases such as chronic subdural effusion, acute subdural hematoma, etc; those analyzing patients undergoing other surgical procedures other than BHD or BHDI; those wherein data were integrated by another study or were missing data.
2.3. Data extraction
According to the predefined eligibility criteria, 2 reviewers (Y.Y. and Q.P.W.) independently reviewed the eligible studies using a standardized data abstraction table. Disagreements were resolved by discussion and finally reached a consensus. Two authors independently extracted data and cross-checked the outcome .We extracted data for: study characteristics (author, year of publication, country, study design and sample size); patient baselines; 3) treatment details (different interventions for BHDI vs. BHD); and study outcomes (incidence of recurrence, mortality, and pneumocephalus).
2.4. Methodological quality of included studies
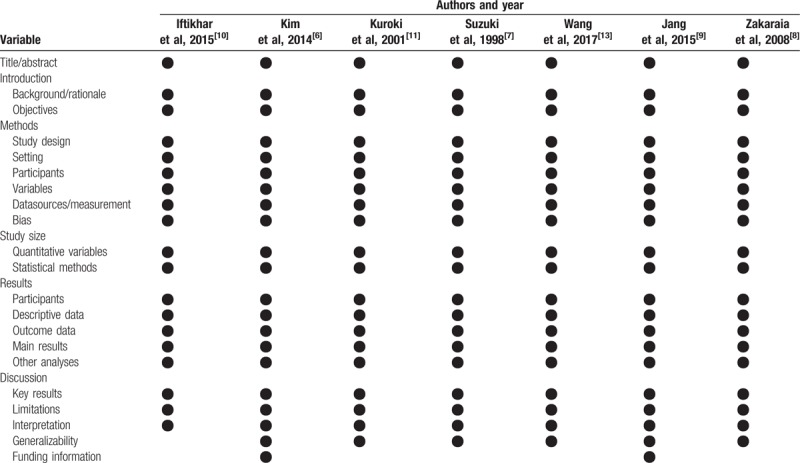
Studies in our systematic review were RCTs and retrospective observational studies. The Jade Quality Assessment Scale was used to reach a methodological evaluation of the included RCTs (Table 1). For observational studies, we used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Table 2).
Table 1.
Methodology quality assessment—Modified Jaded Score (7-points).
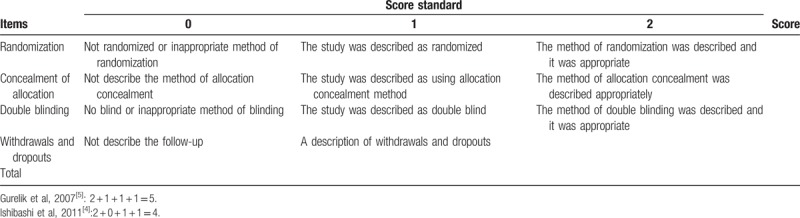
Table 2.
Methodological quality of included 6 retrospective observational studies (STROBE criteria).
2.5. Statistical analysis
We used Review Manager Software (version 5.3, Cochrane Collaboration) to conduct statistical analyses and P < .05 was considered a statistically significant difference. Data from all trials were combined to estimate the pooled odds ratio (OR) with 95% confidence intervals (CI) for recurrence. ORs were used for dichotomous variables. We contacted the corresponding authors to acquire sufficient information and verify sample details when necessary. We used the χ2 test and the Higgins I2 test to assess heterogeneity. If statistical heterogeneity was not obvious as assessed, data were pooled across studies using fixed-effects models. The analysis was conducted using random-effects models when the I2 value exceeded 50% and heterogeneity testing was statistically significant. Funnel plots were constructed to assess publication bias.
2.6. Quality of evidence
Studies were ranked from level 1 (strongest evidence) to level 5 (weakest evidence) and assigned to a quality rating according to the Centre for Evidence-Based Medicine criteria.
3. Results
3.1. Study selection and methodological quality
Overall, we reviewed 94 potentially relevant and related articles addressing CSDH. In total, 66 articles were excluded by screening the title and abstract. After reading the full texts, 9 studies involving 993 participants were analyzed in our study (Table 3).[4–12] All of the studies included in our analysis were published articles. General information on the 2 RCTs and 7 observational studies is listed in Table 4. Three studies were conducted in Japan,[4,7,11] 1 in Turkey,[5] 1 in China,[13] 1 in Pakistan,[10] 1 in Malaysia,[8] and 2 in Korea.[6,9] All patients were adults rather than children with CSDH. We defined recurrence as reappearance of symptoms and signs owing to an ipsilateral hematoma identified on computed tomography scan from the initial surgical drainage. All trials reported the incidence of recurrence in both the treatment and control groups. Additionally 2 studies[8,11] evaluated the pneumocephalus rate of each group, 2 studies[13,6] described the volume of pneumocrania (on the postoperative day), and 4 studies reported postoperative mortality within 3 months. Furthermore, 6 studies had >3-month follow-up periods,[13,5,6,8,9,11] 1 had a 1- to 12-month follow-up,[7] and 2[10,13]did not report the precise follow-up times (Table 4).
Table 3.
Flowchart of study selection.
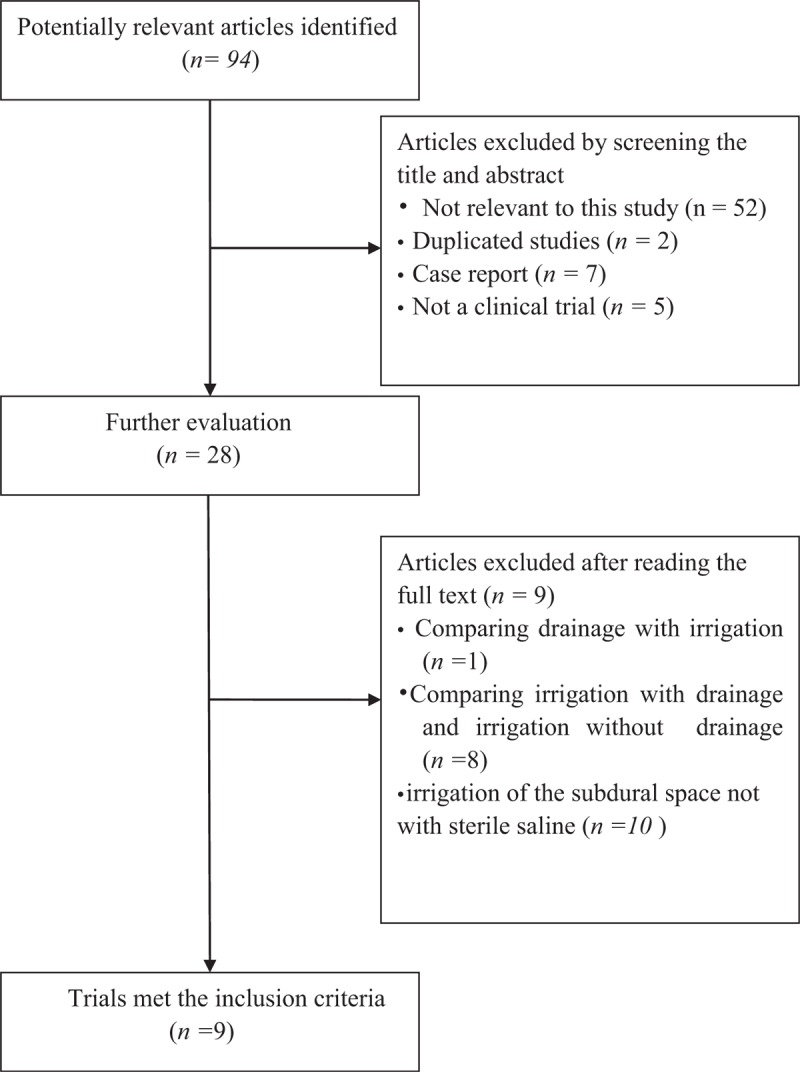
Table 4.
Characteristics and outcomes of the studies included in the meta-analysis.
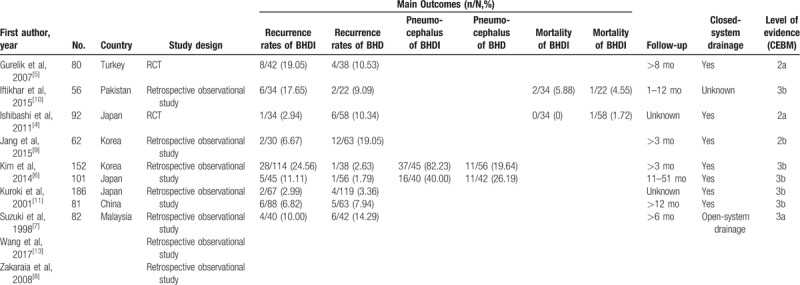
The 2 RCTs[5,7] were relativity high-quality studies, but one[5] did not report the details of random sequence generation and the other[4] had a high risk of random sequence generation bias, and allocation concealment was unclear in both groups, resulting in attrition bias. The 7 observational cohort studies[6,7,6,10,12] clearly described their study population (including missing data and lost follow-up). Furthermore, 6 studies[4,5,7,8,10,11] did not disclose their funding sources, 2[6,9] showed no support fund, and 1[13] was supported by hospital funds.
3.2. Outcome measures
All studies compared the incidence of recurrence in the BHD group with that in the BHDI group. The Q test was used to assess statistical evidence for heterogeneity between trials and the I2index was used as an estimate of the extent between trial variability. Moderate heterogeneity between trials was observed (χ2 = 14.32, P = .07, I2 = 44%, Fig. 1) according to Q test, and heterogeneity test was statistically significant; thus, we conducted the meta-analysis using random-effects models. The meta-analysis showed no significant difference between 2 groups when it came to the risk of recurrence (OR = 1.27, 95% CI .59–2.71, P = .54, Fig. 1).
Figure 1.
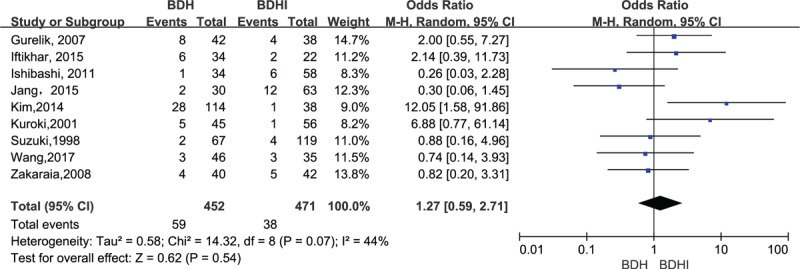
Forest plot comparing of burr hole drainage with or without irrigation in relation to recurrence for the nine included trails. The pooled estimates were obtained using a fixed-effects model.
In subgroup of the 2 RCTs, heterogeneity was (χ2 = 2.55, P = .11, I2 = 61%, Fig. 2) not significantly different for recurrence between the 2 groups (OR = 1.14, 95% CI .16–8.24, P = .89, Fig. 2)
Figure 2.
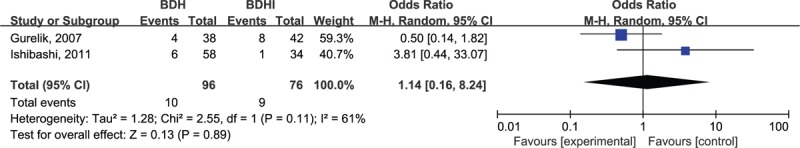
Forest plot summary of the 2 randomized control trials.
In the analysis of pneumocephalus, heterogeneity was (χ2 = 10.83, P = .001, I2 = 91%, Fig. 3) not significantly different between the 2 groups (OR = 5.91, 95% CI .61–56.86, P = .12, Fig. 3).Analysis of the subgroup of 4 studies comparing mortality showed no significant difference among the studies (OR = 0.94, 95% CI = .14–6.16, P = .95, Fig. 4).
Figure 3.
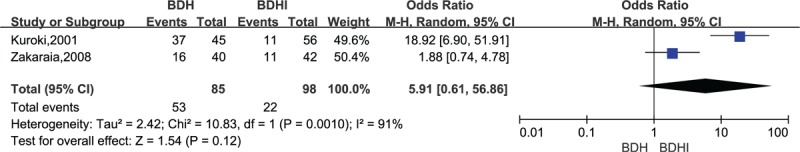
Forest plot for pneumocephalus comparison.
Figure 4.
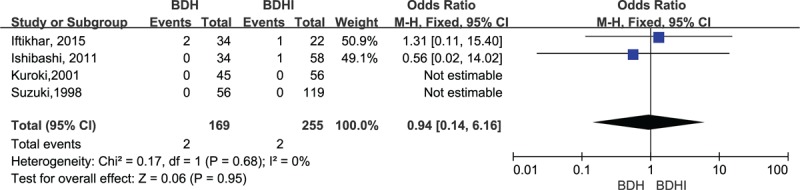
Forest plot for mortality comparison.
3.3. Bias
The risk of bias summary for the RCTs is shown in Figure 5. Funnel plot was performed to screen the publication bias of the nine included studies (Fig. 6). Visual inspection of the corresponding symmetrical funnel plots indicated no publication bias.
Figure 5.
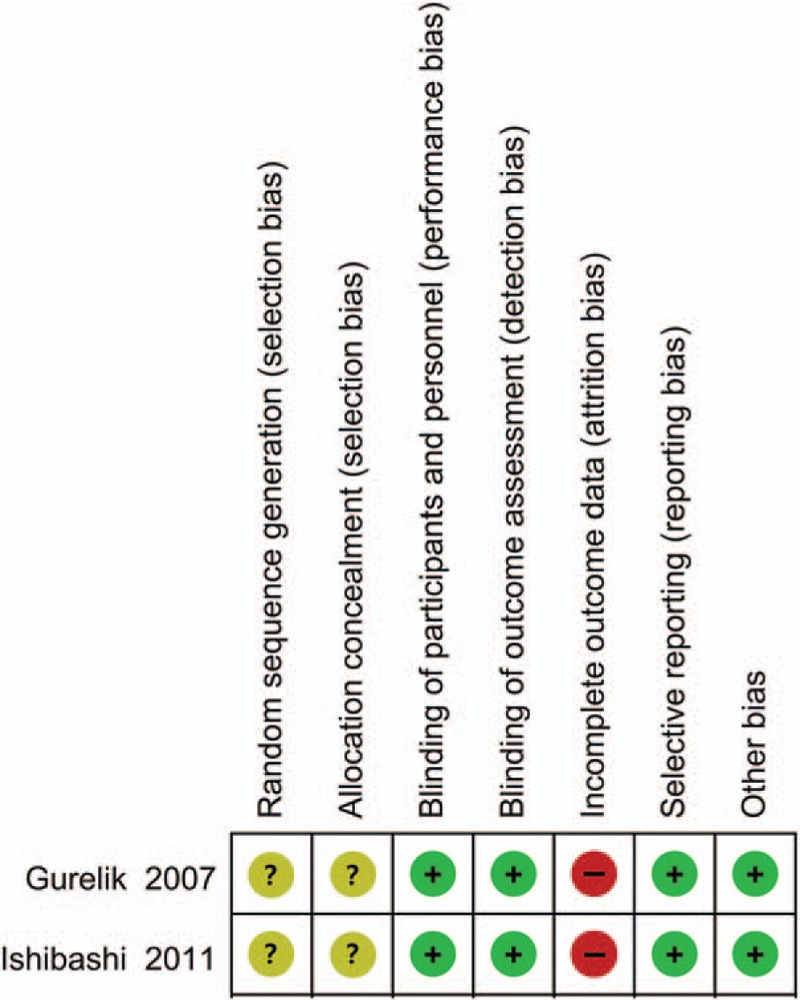
Risk of bias summary for the 2 randomized control trials.
Figure 6.
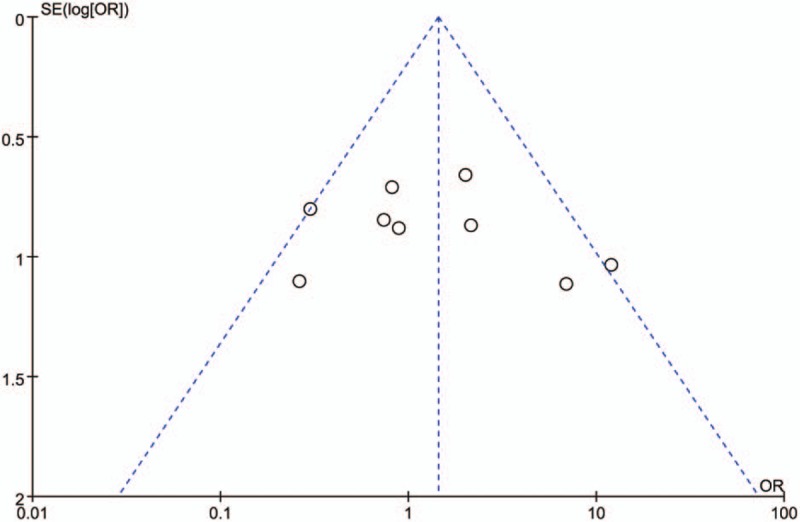
Funnel plot evaluating publication bias in comparing chronic subdural hematoma treated using burr hole drainage with or without irrigation. The plot showed that the 9 included trials in this meta-analysis appear approximately symmetric. The pooled estimates were obtained using a fixed-effects model, and no publication bias was found.
4. Discussion
CSDH occurs in approximately 5 of 1000 individuals per year and is much more common in the elderly.[14] The clinical manifestations of CSDH vary from no symptoms to headache, language difficulties, seizures, and confusion. Computed tomography (CT) scans reveal typically hypodense, isodense, or mixed-density hematomas.[15]
Surgery is the best option for moderate to large hematoma with neurodeficit or mental status changes, and hematoma on serial CT scans progressively increases in size or subdural hematoma with maximum thickness >1 cm.[16] CSDH may be associated with new intracranial hematoma, infection, seizure, and tension pneumocephalus. Prognosis is superior in patients with higher preoperative Glascow coma scale and in younger patients.[17] Although CSDH is a treatable disease, overall surgical mortality ranges from 0% to 32%, whereas recurrence rates range from 0.36% to 33.3%.[18] The data demonstrate that despite the modifications to improve the surgical technique, a certain recurrence rate with this procedure persists. Of the many available treatment options, it is generally accepted that immediate operative evacuation be performed in individuals with focal symptoms or significant deterioration in neurological status. For instance, craniotomy is the most common treatment for acute subdural hematoma, or CSDH with significantly thick membranes, but is less common because of its high rates of morbidity and mortality rates.[19] Ivamoto et al[20] conducted a systematic review indicating that the use of closed-system drainage following burr hole is related to a lower incidence of recurrence compared to burr hole without drainage. Furthermore, neurosurgeons never stop exploring whether BDHI or BDH should be the best choice all the time.
Thus far, the pathogenesis of CSDH needs clarification. Proposed theories include the osmotic gradient theory,[21] the inflammatory process theory,[22,23] and hematoma capsule rebleeding connected with hyperfibrinolysis.[24] Researchers have never explained how it works and multiple treatment concepts and options are available. In addition, recurrence of CSDH following burr-hole drainage is inevitable, and may require reoperation. Major mechanisms explaining CSDH expansion include recurrent microhemorrhages, osmotic gradient formation, and the role of anticoagulation factors in avoiding clot formation.[25] Vasoactive cytokines, infammatory mediators, fibrinolytic factors[26] angiogenic growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), inflammatory mediators, and fibrinolysis factors are reported to be present and active in the hematoma cavity.[24] Saito et al[27] proposed that because substances in high concentrations may result in an increased risk of rebleeding, irrigating and completely washing out the residual hematoma is a very important surgical goal. From this perspective, BHDI should be the most effective technique because of cleaning the pivotal nidus.
Although some studies recommended the use of BDHI, several studies have discouraged to use it, because of baffling studies reporting recurrence or based on personal experience. First, BHDI for CSDH sharply reduces intracranial pressure. The abrupt decrease in intracranial pressure may cause further damage to the traversing vein and lead to reappearance of CSDH in the group with irrigation. Moreover, the sudden decline in intracranial pressure may induce tension pneumocephalus[7,28] or intracapsular hemorrhage.[29]
Second, rebleeding may also be because of the rapid parenchymal shift, fragile cortical vessel injury, and an abrupt rise in cerebral blood flowing hematoma evacuation.[30] Third, poor brain reexpansion is shown to be responsible for recurrence. Despite the caution, the irrigation may still bring air into the hematoma cavity, hindering brain expansion. The presence of a postoperative massive subdural air collection contributes to a bad re-expansion of parenchymal and tends to be associated with the hematoma recurrence. Reducing the air in the hematoma cavity is crucial for a good outcome in surgery of CSDH. Some studies recommend that the patient's head should be fixed to ensure that the burr hole is always situated at the highest point, so that the cavity can be filled with saline instead of air before closure.[31] Although there were no reports of higher infection rates in the BDHI group, repeated irrigation could increase the opportunity of contamination of the cavity.
From the 2 sides, pursuing incompatible objectives does not appear to be easy. Without irrigation, catheter occlusion owing to the clot can lead to incomplete drainage. An irrigation procedure may be preferable in patients with clots in the hematoma. During surgery, care should be taken to irrigate with a small amount of fluid at a uniform and slow speed, ensuring that input/output volumes are equal.
This meta-analysis indicated that existing studies were unable to provide adequate evidence regarding which procedure is the better choice for any condition. When comparing BDH and BDHI, recurrence rates were similar and mortality rates relatively low in both groups. The analysis also shows no significant differences in the outcome between BHD and BHDI, indicating that the 2 procedures have equivalent efficacy. The incidence of new neurological deficits (NNDs) in these study groups has only been compared once by Iftikhar et al.[11] Of the 34 patients in the irrigation group, 6 developed NNDs, 3 of whom had new-onset seizures. None of the patients who had surgery without irrigation developed any NND, and the difference was not statistically significant (P = 0.12). Additionally, 2 studies compared infection rates, and Kuroki et al did not report any postoperative complications, such as infection or intracapsular haemorrhage.[11] Wang et al observed no statistically significant difference for each group has 1 case (1.1% in BHDI and 1.6% in BHD).[13] Thus, this study aimed to present the existing results, and provide references for further study.
There are several limitations of this study. First, the included studies are limited, only 2 RCTs were found, for which allocation for surgery occurred by medical record number or the order of operation. We included 6 observational cohort studies, which was prone to selection, performance, and attrition bias. Thus, confounding variables such as CSDH thickness were not controlled, possibly affecting the results of this meta-analysis. Second, 3 studies had relatively small sample sizes of <40 cases in each group, which may have resulted in an over- or underestimation of the outcome. Third, follow-up varied among the investigated trials, making it difficult to extract definitive conclusive recommendations on outcome. Jang reported that follow-up CT was performed immediately after surgery, 1 week and 3 months after surgery. Kim et al reported that follow-up CT was checked on the third and tenth postoperative days. Kuroki et al reported that both groups underwent daily follow-up CTs during drainage and weekly or monthly thereafter until the haematoma disappeared. We recommended that it is better to perform repeat imaging immediately after surgery, and at 1 week and 3 months after surgery for follow- up monitoring. Fourth, 8 studies reported they used the close-system drainage.[4–8,11,12] One reported that they use the open-system drainage, it can also have influence on the outcome.[9]
5. Conclusion
The results of this systematic review demonstrate that the procedure with or without irrigation may have similar effects in the treatment of CSDH for the outcomes of recurrence, pneumocephalus and mortality. Furthermore, high-quality randomized controlled trails are needed to explore this issue in the futher.
Author contributions
Authorship: Y.Y. and Q-P.W. contribute equally to the article; Y.Y. and N-X.X. were involved in study concept and design. Q-P.W., Y-L.C. and H-R.Z. were involved in analysis and interpretation of data. Y.Y. drafted the manuscript. Q-P.W. and Y-L.C. were involved in critical revision of the manuscript; N.X.X. was involved in final approval of the manuscript.
Conceptualization: Nanxiang Xiong.
Data curation: Ye Yuan, Qiangping Wang, Yu-lin Cao, Hong-ri Zhang, Nanxiang Xiong.
Formal analysis: Ye Yuan, Qiangping Wang, Yu-lin Cao.
Methodology: Ye Yuan.
Supervision: Nanxiang Xiong.
Validation: Nanxiang Xiong.
Visualization: Nanxiang Xiong.
Writing – original draft: Ye Yuan, Qiangping Wang.
Writing – review & editing: Mohammad Shah Nayaz Burkutally, Kamile Budryte, Nanxiang Xiong.
Footnotes
Abbreviations: BHD = burr hole drainage without irrigation, BHDI = burr hole drainage with irrigation, CI = confidence interval, CSDH = chronic subdural hematoma, NNDs = new neurological deficits, OR = odds ratio, TDC = twist drill craniotomy.
YY and Qi-P. W contributed equally to this work.
Funding: The study was funded by The Funds for Creative Research of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (02.03.2017–65).No external funding was received for this study.
The authors report no conflicts of interest.
References
- [1].Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci 2005;32:501–6. [DOI] [PubMed] [Google Scholar]
- [2].Gelabert-Gonzalez M, Rico-Cotelo M, Aran-Echabe E. [Chronic subdural hematoma]. Med Clin (Barc) 2015;144:514–9. [DOI] [PubMed] [Google Scholar]
- [3].Ducruet AF, Grobelny BT, Zacharia BE, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev 2012;35:155–69. [DOI] [PubMed] [Google Scholar]
- [4].Ishibashi A, Yokokura Y, Adachi H. A comparative study of treatments for chronic subdural hematoma: burr hole drainage versus burr hole drainage with irrigation. Kurume Med J 2011;58:35–9. [DOI] [PubMed] [Google Scholar]
- [5].Gurelik M, Aslan A, Gurelik B, et al. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci 2007;34:84–7. [DOI] [PubMed] [Google Scholar]
- [6].Kim DH, Kim HS, Choi HJ, et al. Recurrence of the chronic subdural hematoma after burr-hole drainage with or without intraoperative saline irrigation. Korean J Neurotrauma 2014;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Suzuki K, Sugita K, Akai T, et al. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol 1998;50:231–4. [DOI] [PubMed] [Google Scholar]
- [8].Zakaraia AM, Adnan JS, Haspani MSM, et al. Outcome of 2 different types of operative techniques practiced for chronic subdural hematoma in Malaysia: an analysis. Surg Neurol 2008;69:608–15. [DOI] [PubMed] [Google Scholar]
- [9].Jang KM, Kwon JT, Hwang SN, et al. Comparison of the outcomes and recurrence with three surgical techniques for chronic subdural hematoma: single, double burr hole, and double burr hole drainage with irrigation. Korean J Neurotrauma 2015;11:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iftikhar M, Siddiqui U, Rauf M, et al. Comparison of irrigation versus no irrigation during burr hole evacuation of chronic subdural hematoma. J Neurol Surg Cent Eur Neurosurg 2016;77:416–21. [DOI] [PubMed] [Google Scholar]
- [11].Kuroki T, Katsume M, Harada N, et al. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien) 2001;143:1041–4. [DOI] [PubMed] [Google Scholar]
- [12].Okada Y, Akai T, Okamoto K, et al. A comparative study of the treatment of chronic subdural hematoma—burr hole drainage versus burr hole irrigation. Surg Neurol 2002;57:405–9. 410. [DOI] [PubMed] [Google Scholar]
- [13].Wang QP, Yuan Y, Guan JW, et al. A comparative study of irrigation versus no irrigation during burr hole craniostomy to treat chronic subdural hematoma. BMC Surg 2017;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miranda LB, Braxton E, Hobbs J, et al. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 2011;114:72–6. [DOI] [PubMed] [Google Scholar]
- [15].Miranda LB, Braxton E, Hobbs J, et al. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 2011;114:72. [DOI] [PubMed] [Google Scholar]
- [16].Yadav Y, Parihar V, Namdev H, et al. Chronic subdural hematoma. Asian J Neurosurg 2016;11:330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Adachi A, Higuchi Y, Fujikawa A, et al. Risk factors in chronic subdural hematoma: comparison of irrigation with artificial cerebrospinal fluid and normal saline in a cohort analysis. PLoS One 2014;9:e103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Almenawer SA, Farrokhyar F, Hong C, et al. Chronic subdural hematoma management. Ann Surg 2014;259:449–57. [DOI] [PubMed] [Google Scholar]
- [19].2015;Regan JM, Worley E, Shelburne C, et al. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysisJT PLoS One. 10:e115085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ivamoto HS, Lemos HP, Atallah AN. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg 2016;86:399–418. [DOI] [PubMed] [Google Scholar]
- [21].Labadie EL, Glover D. Chronic subdural hematoma: concepts of physiopathogenesis. A review. Can J Neurol Sci 1974;1:222–5. [DOI] [PubMed] [Google Scholar]
- [22].Heula AL, Ohlmeier S, Sajanti J, et al. Characterization of chronic subdural hematoma fluid proteome. Neurosurgery 2013;73:317–31. [DOI] [PubMed] [Google Scholar]
- [23].Frati A, Salvati M, Mainiero F, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg 2004;100:24–32. [DOI] [PubMed] [Google Scholar]
- [24].Tokmak M, Iplikcioglu AC, Bek S, et al. The role of exudation in chronic subdural hematomas. J Neurosurg 2007;107:290–5. [DOI] [PubMed] [Google Scholar]
- [25].Javadi A, Amirjamshidi A, Aran S, et al. A randomized controlled trial comparing the outcome of burr-hole irrigation with and without drainage in the treatment of chronic subdural hematoma: a preliminary report. World Neurosurg 2011;75:731–6. [DOI] [PubMed] [Google Scholar]
- [26].2009;Hong H, Kim Y, Yi H, et al. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematomaJT Surg Neurol. 71:161–5. [DOI] [PubMed] [Google Scholar]
- [27].Saito A, Narisawa A, Takasawa H, et al. Expression of the TGF-beta-ALK-1 pathway in dura and the outer membrane of chronic subdural hematomas. Neurol Med Chir (Tokyo) 2014;54:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ihab Z. Pneumocephalus after surgical evacuation of chronic subdural hematoma: Is it a serious complication? Asian J Neurosurg 2012;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Honda M, Tanaka K, Tanaka S, et al. [A case of infected subdural hematoma following chronic subdural hematoma irrigation]. No To Shinkei 2002;54:703–6. [PubMed] [Google Scholar]
- [30].Chang SH, Yang S, Son BC, et al. Cerebellar hemorrhage after burr hole drainage of supratentorial chronic subdural hematoma. J Korean Neurosurg Soc 2009;46:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–81. [DOI] [PubMed] [Google Scholar]


