Abstract
Neutrophils fight infections by generating ROS and extracellular traps (NETs). However, how neutrophils modulate ROS/NET generation is mechanistically unclear. Kindlin-3, an essential integrin activator expressed in hematopoietic cells, is required to support integrin-mediated neutrophil recruitment during inflammation. Here we characterized a novel role of kindlin-3 in regulating ROS/NET generation in neutrophils. When overexpressing kindlin-3 in neutrophil-like differentiated HL-60 cells (HL-60N), ROS/NET generation from these cells were significantly suppressed. Interestingly, overexpression of a kindlin-3 mutant that is defective for interacting with integrins in HL-60N cells still inhibited ROS/NET generation, suggesting that the role of kindlin-3 in inhibiting ROS/NET signaling may be independent of its binding to integrins. Consistently, we observed that knockdown of kindlin-3 in HL-60N cells led to enhanced ROS/NET generation. In addition, bone marrow neutrophils isolated from kindlin-3-deficient mice showed elevated ROS/NET generation when compared with WT counterparts. As expected, overexpression of exogenous kindlin-3 in mouse neutrophils could suppress NET release ex vivo and in vivo. Collectively, these results demonstrate that kindlin-3 in neutrophils is involved in modulating the ROS/NET signaling, providing a novel mechanism for fine-tuning neutrophil behaviors during inflammation.
Keywords: Inflammation, Innate immunity, ROS
INTRODUCTION
Neutrophils employ multiple mechanisms in fighting against infections, one of which is generation of neutrophil extracellular traps (NETs) upon activation. NETs are web-like structures of decondensed chromatin released from activated neutrophils in complex with granular proteins, which can prevent further spreading of invaded pathogens and meanwhile perform potent antimicrobial activity as well.[1] NETs are also pathologically associated with many difficult-to-treat diseases, such as autoimmunity, venous thrombosis and cancer.[2–4] Therefore, the function of releasing NETs needs to be tightly controlled to ensure proper neutrophil behaviors. Mechanistically, NET release can be facilitated by reactive oxygen species (ROS) produced by NADPH oxidase.[5] Neutrophils from patients with chronic granulomatous disease caused by deficiency of gp91phox, a key component of NADPH oxidase, are incapable of forming NETs; and such a functional defect can be rescued by restoring the expression of gp91phox in neutrophils in these patients.[6] Although the functional significance of ROS/NET signaling has been well recognized, the detailed mechanisms by which neutrophils control the generation of ROS/NETs still remain poorly understood.[7]
Kindlin-3 is an important binding partner of the integrin cytoplasmic tails in hematopoietic cells and plays a key role in supporting integrin activation.[8–10] Kindlin-3’s deficiency in humans leads to type-III leukocyte adhesion deficiency (LAD-III), and these patients are featured with recurrent infections and severe bleeding disorders due to dysfunction of integrins in leukocytes and platelets.[11, 12] Using a kindlin-3 gene knockin (K3KI) mouse model that carries QW/AA mutations in kindlin-3 that leads to disconnection of kindlin-3 with integrins, we previously demonstrated that the interaction between kindlin-3 and integrins is required to support integrin-mediated platelet aggregation and neutrophil recruitment.[13, 14] Interestingly, we also observed that neutrophils isolated from K3KI mice show a moderately reduced ability to release NETs,[14] indicating that kindlin-3 may be involved in the ROS/NET signaling. To further delineate the role of kindlin-3 in regulating NET release in current study, we manipulate the expression levels of kindlin-3 in both in vitro and in vivo systems, and demonstrate that kindlin-3 in neutrophils actually can act as a negative regulator for fine-tuning the generation of ROS/NETs, providing a novel pathway for developing anti-inflammatory strategies.
MATERIAL AND METHODS
HL-60 and HL-60N cells
To manipulate the expression of kindlin-3 in HL-60 cells, lentiviral particles expressing EGFP/EGFP-kindlin-3 or non-specific/kindlin-3-specific shRNA (co-expressing with Cerulean) were prepared. After transducing HL-60 cells with these lentiviral particles, cells positive for EGFP or Cerulean were sorted, expanded and frozen in multiple aliquots for further uses. To differentiate HL-60 cells into neutrophil-like cells (HL-60N), cells were treated with 1.25% DMSO for 7 days in tissue culture.
Mice
Mice carrying the floxed kindlin-3 alleles were bred with Mx1-Cre mice to generate kindlin-3fl/flMx1-Cre mice. Deletion of kindlin-3 gene in kindlin-3fl/flMx1-Cre mice was induced by intraperitoneal injection of poly(I:C). Meanwhile, inbred Kindlin-3fl/fl littermates undergone the same injections were used as controls for comparative studies. For exogenously overexpressing kindlin-3 in neutrophils in mice, bone marrow cells isolated from WT mice were transduced with lentiviral particles expressing either EGFP or EGFP-kindlin-3 and further used to transplant lethally irradiated recipient mice. All these mouse experiments were performed with approval of the IACUC.
Isolation of mouse bone marrow neutrophils
Bone marrow neutrophils were isolated from mice as previously described.[14, 15] Briefly, the mouse bone marrow cells were collected from the tibia and the femur using HBSS buffer supplemented with 1% BSA and 5mM EDTA and passed through a 70-µM sterile cell strainer for collecting the single cells. Total bone marrow cells were loaded on a discontinuous Percoll gradient consisting of 52%, 69% and 78% Percoll in PBS. After centrifugation for 30 min at 1500 g, mature neutrophils were collected from the interphase between 69% and 78% Percoll and washed 3 times with PBS. Viability of the purified neutrophils was measured by trypan blue staining and the purity was evaluated by the expression of Gr1 using flow cytometry.
Quantification of NET-DNA release
HL-60N cells or mouse bone marrow neutrophils were allowed to reside on chamber slides coated with poly-L-lysine and stimulated with PMA (100 nM) for 2 hours. The formed NET-DNA structures were visualized by staining the cells with Hoechst. Alternatively, HL-60N cells or mouse neutrophils were stimulated with PMA in non-stick tubes followed by incubation with micrococcal nuclease to liberate the NET-DNA fibers from cells. After centrifugation, the solubilized DNA fragments in supernatants were quantified using SYTOX green, as we previously described.[14]
Quantification of ROS production
Superoxide radical anion (O2•–) generated from the PMA-stimulated HL-60N and mouse neutrophils was quantified by a HPLC-based assay using hydroethidine (HE) probe, as previously described.[16, 17] In brief, cells were stimulated with PMA (100 nM) for 1 hours at a cell density of 1×105 cells/ml in the presence of hydroethidine (HE, 20 µM). HE reacts with superoxide radical anion to produce red fluorescent 2-OH-E+, which was separated and quantified by HPLC equipped with absorption and fluorescence detectors.
RESULTS AND DISCUSSION
Kindlin-3 negatively regulates ROS/NET generation in HL-60N cells
HL-60 cell can be differentiated into neutrophil-like cells (termed as HL-60N) after treatment with DMSO.[16, 18] Compared to undifferentiated HL-60 cells, HL-60N cells expressed higher levels of neutrophil marker CD11b (Figure 1A). Upon stimulation, HL-60N cells but not HL-60 cells were found to release NET-DNA fibers (Figure 1B), which could be abolished by DNase treatment (not shown). The release of NET-DNA from stimulated HL-60N cells could be mostly inhibited by DPI, an NADPH oxidase inhibitor (Figure 1C), suggesting that the release of NET-DNA from HL-60N cells is ROS-dependent. NET release from HL-60N cells could also be induced by LPS and TNF-α; however, the responses were much weaker than PMA stimulation and less sensitive to DPI treatment.
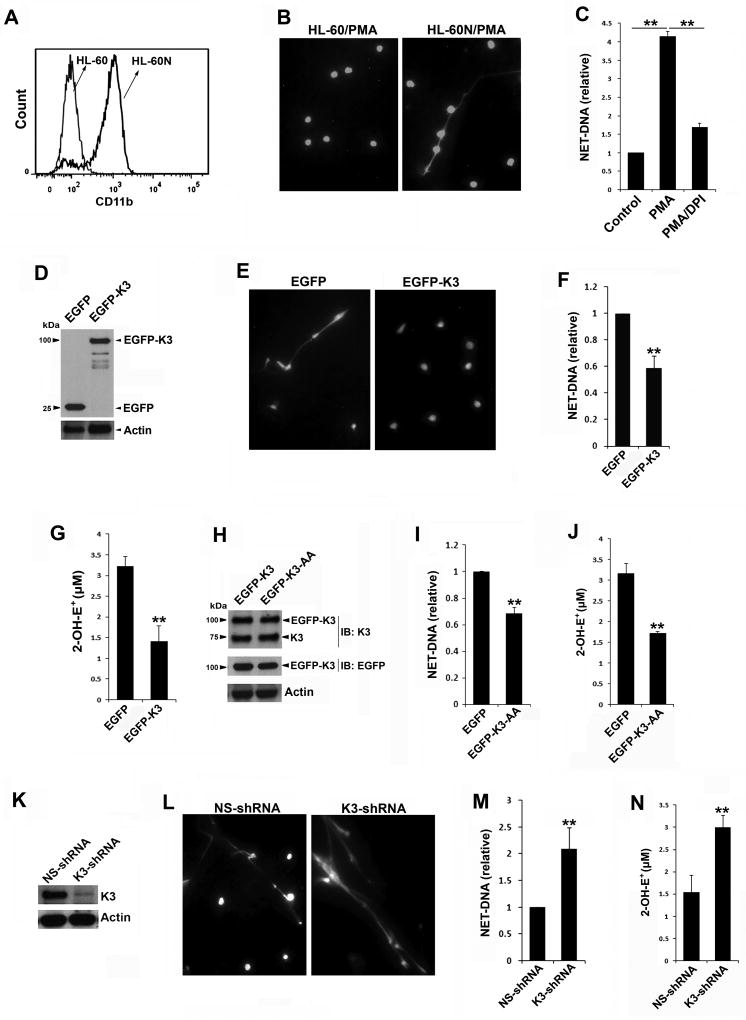
Figure 1.
Kindlin-3 inhibits the generation of ROS/NETs in neutrophil-like HL-60 (HL-60N) cells. (A) FACS histograms to show the expression levels of CD11b in undifferentiated HL-60 and differentiated HL-60N cells. (B) HL-60 and HL-60N cells were resided on chamber slides and stimulated with PMA (100 nM) for 2 hours, and fixed, permeabilized and stained with Hoechst to display the NET-DNA fibers under a fluorescence microscope (10× objective). (C) The HL-60N cells were stimulated with PMA (100 nM) for 2 hours in the presence or absence of DPI (10 µM). HL-60N cells without PMA stimulation were used as a control. NET-DNA released from these cells was quantified by SYTOX green. (D) Western blots to show the expression of EGFP and EGFP-kindlin-3 (EGFP-K3) in HL-60N cells. (E) As described in (B), HL-60N cells expressing either EGFP or EGFP-K3 were stained with Hoechst after PMA stimulation. (10× objective) (F) HL-60N cells expressing either EGFP or EGFP-K3 were stimulated with PMA and NET-DNA released from these cells was quantified by SYTOX green. (G) HL-60N cells expressing either EGFP or EGFP-K3 were stimulated with PMA (100 nM) in the presence of hydroethidine (HE, 20 µM). The oxidation of HE were evaluated by the production of 2-OH-E+ using a HPLC-based assay. (H) Western blots to show the expression levels of endogenous kindlin-3, and EGFP-K3 with or without the QW/AA mutations (K3-AA) in HL-60N cells. (I) HL-60N cells expressing either EGFP or EGFP-K3-AA were stimulated with PMA (100 nM) and NET-DNA release was quantified by SYTOX green. (J) HL-60N cells expressing either EGFP or EGFP-K3-AA were stimulated with PMA (100 nM) in the presence of HE for 60 minutes. The production of 2-OH-E+ was measured. (K) Western blots to show the expression of endogenous kindlin-3 in HL-60N cells expressing either a non-specific shRNA (NS-shRNA) or a kindlin-3-specific shRNA (K3-shRNA). (L & M) NETs released from HL-60N cells expressing either NS-shRNA or K3-shRNA under PMA stimulation were evaluated by Hoechst staining (L) or SYTOX green (M). (N) Production of 2-OH-E+ in HL-60N cells expressing either NS-shRNA or K3-shRNA under PMA stimulation (100 nM, 60 minutes) was quantified. Data represent means ± SD of 3 or more independent experiments; **P<0.01 (paired Student t test).
To test if kindlin-3 is involved in regulating NET release, HL-60N cells expressing either EGFP-kindlin-3 or EGFP were used. The expression levels of EGFP-kindlin-3 and EGFP in HL-60N cells were confirmed by Western blots (Figure 1D). After stimulation with PMA, we found that NET-DNA fibers released from HL-60N cells expressing EGFP-kindlin-3 were less than those released from HL-60N cells expressing EGFP (Figure 1E). Further quantification of the release DNA using the SYTOX green assay confirmed that overexpression of EGFP-kindlin-3 could compromise NET-DNA formation in HL-60N cells (Figure 1F). Since ROS is required to promote NET release (Figure 1C), we next quantified the production of ROS by HL-60N cells, and found that upon PMA stimulation the production of superoxide radical anion in EGFP-kindlin-3 expressed HL-60N cells was significantly suppressed (Figure 1G). In addition, the production of hydrogen peroxide was also inhibited in these cells (not shown). These results suggest that kindlin-3 can act as a negative factor in regulating ROS/NET generation in HL-60N cells.
To examine if such a role of kindlin-3 in inhibiting ROS/NET generation depends on its binding to integrins, we employed a kindlin-3 mutant (K3KI) carrying the QW/AA mutations in the F3 subdomain that can disconnect kindlin-3 with integrins.[13, 14, 19] The expression level of EGFP-fused K3KI mutant in HL-60N cells was comparable to EGFP-kindlin-3; and endogenous kindlin-3 was normally expressed in these cells (Figure 1H). Substantially, overexpressing the K3KI mutant in HL-60N cells also suppressed NET-DNA release and superoxide radical anion production (Figure1, I & J), indicating that the inhibition of kindlin-3 on ROS/NET generation may not (or may not merely) rely on its interaction with integrins. In a previous study, we actually found that disconnection of kindlin-3 with integrins in K3KI mouse neutrophils led to a moderate reduction of NET-DNA release,[14] which may be attributed to the enhanced availability of the mutated kindlin-3 in activated neutrophils for performing the integrin-independent functions.
Further, we employed an shRNA-based approach to knockdown endogenous kindlin-3 in HL-60N cells (Figure 1K). The formation of NET-DNA structures significantly enhanced in HL-60N cells when endogenous kindlin-3 was silenced (Figure 1, L & M). Consistently, production of superoxide radical anion from these kindlin-3-deficient HL-60N cells also significantly increased (Figure 2N). As a note, overexpression or down-regulation of kindlin-3 has no significant effect on HL-60 differentiation, as evidenced by the expression levels of neutrophil marker CD11b. Together, these results suggest that kindlin-3 is a negative regulator for ROS/NET generation in HL-60N cells.
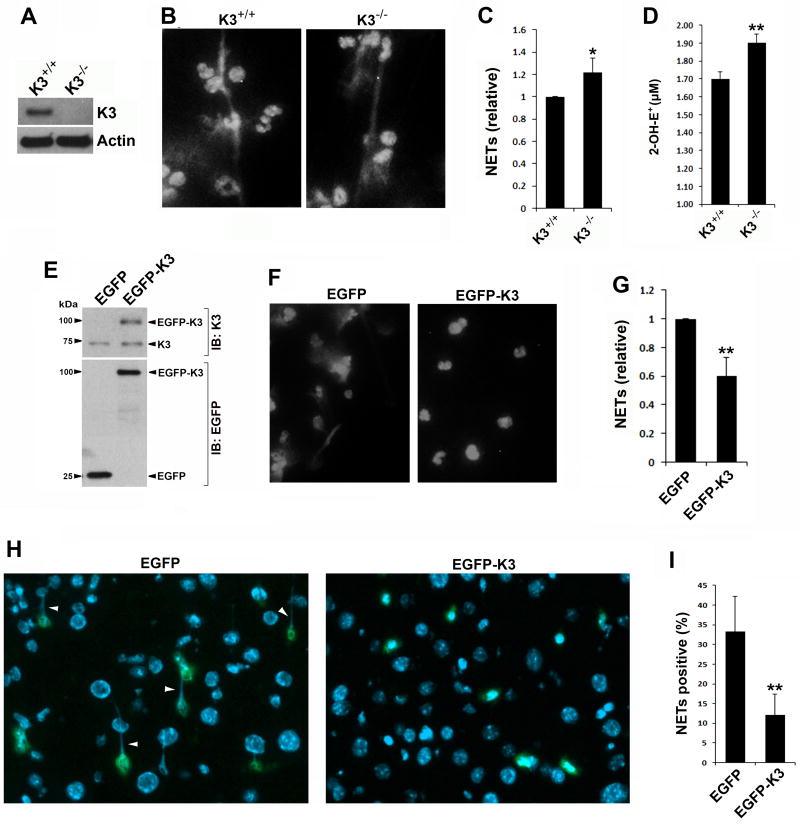
Figure 2.
Kindlin-3 suppresses the generation of ROS/NETs in mouse neutrophils. (A) Western blots to show the expression of endogenous kindlin-3 in bone marrow neutrophils isolated from kindlin-3-deficient mice (K3−/−) and WT control mice (K3+/+). (B) Bone marrow neutrophils isolated from K3+/+ and K3−/− mice were resided on immobilized ploy-l-lysine and stimulated with PMA (100 nM) for 2 hours. After stimulation, neutrophils were fixed, permeabilized and stained with Hoechst for visualizing the released NET-DNA fibers under a fluorescence microscope (20× objective). (C) NET-DNA released from K3+/+ and K3−/− neutrophils after stimulation with PMA (100 nM, 2 hours) was quantified by SYTOX green. (D) Oxidation of hydroethidine (HE) were quantified for K3+/+ and K3−/− neutrophils by measuring the production of 2-OH-E+ using the HPLC-based assay. (E) Western blots to show the expression levels of endogenous kindlin-3, EGFP and EGFP-K3 in mouse bone marrow cells after bone marrow transplant. (F) Mouse bone marrow neutrophils expressing EGFP or EGFP-K3 were selected and used for Hoechst staining after stimulation with PMA (100 nM, 2 hours), followed by observation under a fluorescence microscope (20× objective). (G) NET-DNA released from PMA-stimulated mouse bone marrow neutrophils expressing either EGFP or EGFP-K3 was quantified by SYTOX green. (H) Representative fluorescence images (20× objective) of liver tissues isolated from LPS-treated mice expressing either EGFP or EGFP-K3 in bone marrow cells. The EGFP signal was enhanced by immunostaining with an anti-EGFP antibody. Cell nuclei were stained with DAPI. The formed NET-DNA fibers were pointed out (arrow heads). (I) Quantification of EGFP-positive cells attached with NET-DNA fibers. Data represent means ± SD of 3 or more independent experiments; *P<0.05; **P<0.01 (paired Student t test).
Kindlin-3 negatively regulates ROS/NET generation in mouse neutrophils
Next, we sought to evaluate the role of kindlin-3 in regulating ROS/NET generation in mouse neutrophils. Bone marrow neutrophils were isolated from kindlin-3-deficient kindlin-3fl/flMx1-Cre mice (kindlin-3−/−) and inbred WT control mice (kindlin-3+/+). Deficiency of kindlin-3 in kindlin-3−/− neutrophils was confirmed by Western blotting (Figure 2A). After PMA treatment, NET-DNA fibers could be detectable on both kindlin-3+/+ and kindlin-3−/− neutrophils (Figure 2B). Further quantification of the released NET-DNA showed that kindlin-3−/− neutrophils had an enhanced ability to release NET-DNA when compared to kindlin-3+/+ neutrophils (Figure 2C). In addition, production of superoxide radical anion also increased in kindlin-3−/− neutrophils (Figure 1D). These results suggest that kindlin-3 can negatively regulate ROS/NET generation in mouse neutrophils.
To further verify the role of kindlin-3 in regulating NETs, a strategy of overexpressing exogenous kindlin-3 in mouse neutrophils was utilized. Bone marrow cells isolated from WT mice were transduced with lentiviral particles to express either EGFP or EGFP-kindlin-3 and then transplanted into lethally irradiated WT recipient mice. The respective expression levels of EGFP, EGFP-kindlin-3 and endogenous kindlin-3 in bone marrow cells of transplanted mice were confirmed by Western blotting (Figure 2E). Bone marrow neutrophils with positive expression of EGFP or EGFP-kindlin-3 were collected for functional analysis. As expected, in response to PMA treatment, neutrophils expressing EGFP-kindlin-3 showed a significantly reduced ability to release NET-DNA when compared to neutrophils only expressing EGFP (Figure 2, F and G). Although purified mouse bone marrow neutrophils are poorly responsive to LPS treatment for releasing NETs ex vivo, significant in vivo NET release can be detected in LPS-stimulated endotoxemic mice.[14] Therefore, we evaluated in vivo NET formation in the transplanted mice. Mice were treated with LPS to induce endotoxemia and their liver tissues were harvested for histological analysis. As shown in Figure 2 (H and I), NET-DNA fibers in liver tissues could be clearly observed on locally recruited neutrophils expressing EGFP control; however, neutrophils expressing EGFP-kindlin-3 showed a significantly reduced formation of NET-DNA fibers. These results verify that kindlin-3 acts as an effective suppressor for NET formation in vivo.
Taken all together, our findings disclose a novel role of kindlin-3 in regulating neutrophil functions. During inflammatory responses, the killing function of neutrophils needs to be very carefully controlled to minimize collateral damage to normal tissues. Theoretically, the newly identified role of kindlin-3 in limiting ROS/NET generation in neutrophils may serve as an important mechanism to restrict the aggressiveness of activated neutrophils. Given the fact that kindlin-3 is also required to support integrin-mediated neutrophil recruitment during inflammation, kindlin-3 may have multiple roles in balancing neutrophil functions in both integrin-dependent (for initial neutrophil recruitment) and integrin-independent (for later ROS/NET generation) manners. Presumably, while the presence of kindlin-3 in patrolling neutrophils is required for supporting integrin activation under stimulatory conditions, it may also play an important role in preventing unnecessary ROS/NET generation under resting conditions. When neutrophils encounter inflammatory challenges, kindlin-3 needs to interact with integrins to mediate neutrophil recruitment,[14] which, together with a possibly degradation mechanism for kindlin-3,[20] may alleviate the inhibition of kindlin-3 on ROS/NET signaling, thus facilitating ROS/NET generation. Certainly, more future studies are required for understanding the mechanistic details of how kindlin-3 temporally fine-tunes neutrophil behaviors under different stimulations. Especially, to specifically delineate the integrin-independent functions of kindlin-3, the subdomain/key residues in kindlin-3 that are responsible for inhibiting the ROS/NET signaling need to be identified for further functional evaluation, and these future studies will be useful for developing more specific anti-inflammatory strategies.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL131654) and the NNSFC (31770967, 31571177 and 31370748).
ABBREVIATION
- NETs
Neutrophil extracellular traps
- ROS
Reactive oxygen species
- NADPH
Nicotinatmide adenine dinucleotide phosphate
- K3KI
Kindlin-3 gene knock-in
- LAD-III
Type III leukocyte adhesion deficiency
- HL-60N
Neutrophil-like differentiated HL-60 cells
Footnotes
AUTHORSHIP
Z.X., B.N., Z.C., J.Z. and J.G. contributed to acquisition of the data and reviewed the manuscript; F.C., B.K. and G.C.W. contributed to interpretation of the data and reviewed the manuscript; Y.Q.M. contributed to design of the experiments, analysis of the data and wrote the manuscript.
CONFLICTS OF INTEREST DISCLOSURE
The authors declare no conflict of interest.
References
- 1.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. Journal of Experimental Medicine. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, Wagner DD. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016;5:e1134073. doi: 10.1080/2162402X.2015.1134073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. Journal of Cell Biology. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127:1243–1252. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin.Immunopathol. 2013;35:513–530. doi: 10.1007/s00281-013-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fassler R. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat.Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 9.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, Moser M, Metin A, Fried M, Tomlinson I, Hogg N. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat.Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat.Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 11.Kuijpers TW, van dV, Weterman MA, de BM, Tool AT, van den Berg TK, Moser M, Jakobs ME, Seeger K, Sanal O, Unal S, Cetin M, Roos D, Verhoeven AJ, Baas F. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009;113:4740–4746. doi: 10.1182/blood-2008-10-182154. [DOI] [PubMed] [Google Scholar]
- 12.Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma YQ, Podrez EA, Tosi M, Lennon DP, Caplin AI, Shurin SB, Plow EF, Byzova TV. A point mutation in kindlin-3 ablates activation of three integrin subfamilies in humans. Nature Medicine. 2009;15:313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Chen X, Zhi H, Gao J, Bialkowska K, Byzova TV, Pluskota E, White GC, Liu J, Plow EF, Ma YQ. Direct interaction of kindlin-3 with integrin alphaIIbbeta3 in platelets is required for supporting arterial thrombosis in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:1961–1967. doi: 10.1161/ATVBAHA.114.303851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z, Cai J, Gao J, White GC, Chen F, Ma YQ. Interaction of kindlin-3 and beta2-integrins differentially regulates neutrophil recruitment and NET release in mice. Blood. 2015;126:373–377. doi: 10.1182/blood-2015-03-636720. [DOI] [PubMed] [Google Scholar]
- 15.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J.Innate.Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielonka J, Cheng G, Zielonka M, Ganesh T, Sun A, Joseph J, Michalski R, O'Brien WJ, Lambeth JD, Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J Biol Chem. 2014;289:16176–89. doi: 10.1074/jbc.M114.548693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zielonka J, Zielonka M, VerPlank L, Cheng G, Hardy M, Ouari O, Ayhan MM, Podsiadly R, Sikora A, Lambeth JD, Kalyanaraman B. Mitigation of NADPH Oxidase 2 Activity as a Strategy to Inhibit Peroxynitrite Formation. J Biol Chem. 2016;291:7029–44. doi: 10.1074/jbc.M115.702787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. Journal of Cell Biology. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. Journal of Biological Chemistry. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Wang X, Zhan J, Chen Y, Fang W, Zhang L, Zhang H. Smurf1 inhibits integrin activation by controlling Kindlin-2 ubiquitination and degradation. J Cell Biol. 2017;216:1455–1471. doi: 10.1083/jcb.201609073. [DOI] [PMC free article] [PubMed] [Google Scholar]