Abstract
Purpose/Background:
Topiramate (TPM) and lorazepam (LZP) are two examples of frequently-prescribed medications that are associated with a high incidence of cognitive impairment; however, the factors that underlie inter-individual differences in side effect profiles have not been fully characterized. Our objective was to determine whether working memory capacity (WMC), the amount of information that can be stored and manipulated in memory over short time intervals, is one such factor.
Methods/Procedures:
Twenty-nine healthy volunteers completed a double-blind, randomized placebo-controlled crossover study during which they received placebo (PBO), TPM, and LZP in random order. Four hours after drug administration, a blood draw was taken to establish drug concentrations, and subjects performed a verbal working memory task while the accuracy and reaction time of their responses were recorded. WMC was calculated based on accuracy rates during the PBO session, and the role of WMC in moderating the severity of drug-related cognitive impairment was assessed by examining drug-related performance changes from PBO as a function of WMC.
Findings/Results:
Both TPM and LZP had a negative impact on task performance, though only TPM-related deficits were modulated by WMC: high WMC was associated with more severe impairments and heightened sensitivity to increasing TPM concentrations.
Implications/Conclusions:
We have identified a potential clinical risk factor, high WMC, which is associated with adverse cognitive events. These data provide objective evidence in support of clinical observations that high-functioning patients are more likely to experience severe cognitive impairments.
Keywords: working memory, anti-epileptic drugs, clinical neuropharmacology
1. INTRODUCTION
Many commonly prescribed medications can cause cognitive impairments severe enough to result in noticeable declines in quality of life and subsequent discontinuation of therapy. Despite a handful of studies that have identified clinically relevant risk factors such as age1–2, degree of drug exposure3, and titration rate4 that are associated with the incidence of adverse cognitive events, a full understanding of why particular individuals respond differently to the same dose of a drug has yet to be reached. As a consequence, it is currently impossible to predict which patients are most at risk of impairment, limiting our ability to personally tailor treatment regiments that enable clinicians to prospectively minimize the severity of these drug-related deficits and maximize treatment outcomes.
Topiramate (TPM), a second-generation broad spectrum anti-epileptic drug (AED) with formal indications for focal and generalized seizures, obesity (with phentermine), alcohol dependence, and migraine, is a prime example of a medication that can cause cognitive impairments severe enough to lead to discontinuation of an otherwise effective therapy5. TPM has repeatedly been shown to have a more pronounced negative impact than other AEDs on a wide range of cognitive functions6 including verbal fluency3,7, language comprehension8, attention9, psychomotor speed8, and short-term10 and working memory3,11.
Lorazepam (LZP) is a drug from the benzodiazepine class that is frequently prescribed for anxiety, sleep disorders, and as a rescue treatment for seizure clusters. Though LZP-related cognitive deficits have recently received less recent attention in the literature than those associated with TPM, LZP’s side effect profile has nonetheless been relatively well described. The drug primarily affects memory, both inducing long-term amnestic deficits in patients12 and impairing performance on a variety of memory tasks in laboratory settings13–14. LZP also has a pronounced effect on many different types of learning15–16, causes psychomotor slowing17, and also impacts attentional processes18. Unlike TPM, LZP does not cause impairments in verbal fluency3.
As is evident, both TPM and LZP have wide-ranging effects in a variety of cognitive domains. In this paper we focus specifically on each drug’s impact on the working memory system, the cognitive system responsible for the storage and manipulation of information over short time intervals19. Working memory capacity (WMC), the amount of information that can be held in working memory simultaneously, is limited, and this capacity limit differs across individuals20–21. This variability in WMC has been linked to differences in general intelligence22, reasoning ability23, controlling attention24, decision-making25, language comprehension26, and reading ability27. These findings clearly demonstrate the central role that working memory functions play in many essential complex behaviors and underline the importance of understanding drug-related working memory deficits: any negative effects that TPM and LZP have on working memory function will lead to impairments in a range of fundamental cognitive behaviors and potential consequent discontinuation of therapy.
Our primary objective in this study was to determine whether individual differences in WMC drive differences in patient responses by modulating the severity of the cognitive impairments associated with TPM and LZP.
2. MATERIALS AND METHODS
2.1. Participants
The experimental protocol was approved by the Institutional Review Board of the University of Minnesota prior to the commencement of the study. Exclusion criteria were: cardiovascular, endocrine, hematopoietic, hepatic, neurologic, psychiatric, or renal disease; a history of drug or alcohol abuse within the past five years; the use of concomitant medications known to affect cognitive function (including antidepressants, anxiolytics, psycho-stimulants, analgesics, and antipsychotics); prior history of hypersensitivity to TPM, LZP, or related compounds; a positive pregnancy test (administered to all females before the start of each study visit); use of any investigational drug within the previous thirty days; a native language other than English; diagnosis of a speech and/or language impairment; uncorrected poor vision or hearing; and a dominant left hand (to control for brain lateralization of language).
2.2. Protocol
We employed a double-blind, randomized, placebo-controlled crossover study design. Subjects signed informed consent, then completed a no-treatment baseline visit, during which they were familiarized with all study procedures and performed a modified Sternberg verbal working memory task28. Subjects were then assigned to one of six possible treatment sequences consisting of TPM, LZP, and placebo (PBO) administered once each. At the next three visits, separated by two-week intervals, subjects’ vital signs were checked before they randomly received either a single oral dose of TPM (subjects were randomized to receive either 100, 150, or 200 mg of TPM to induce a wide range of TPM concentrations across individuals), LZP (2 mg) or an inactive PBO, with drugs dispensed by the University of Minnesota Investigational Drug Services pharmacy. Four hours after drug administration, subjects completed a working memory task while their electroencephalogram (EEG) was recorded (EEG/ERP results reported elsewhere). Plasma samples were collected immediately prior to subjects completing the working memory task. The time of drug administration and the blood draw were recorded. Samples were immediately centrifuged and the plasma frozen until analysis. TPM and LZP plasma levels were quantified by liquid chromatograph-mass spectrometry (LCMS) assays29,Bathena et al., submitted).
Forty-six healthy right-handed volunteers gave written informed consent and completed all study visits. Seventeen subjects were excluded due to missing data resulting from technical issues with data acquisition or storage. Data from the remaining twenty-nine subjects (mean age=25.6 years (SD=8.04); 14 females) were included in these analyses. No adverse effects beyond those listed on the TPM and LZP package inserts were reported. All subjects were asked to refrain from consuming alcoholic beverages for at least 48 hours prior to testing.
2.3. Working Memory Task
A schematic of a single trial of the working memory task is presented in Figure 1. At the beginning of each trial, a cue string of one, three, or five pronounceable, nonsense syllables (low, moderate, and high memory loads, respectively) was displayed on a monitor for 1500ms (encoding phase). This was followed by a five second retention period (fixation cross displayed), during which subjects were instructed to retain the syllable string in memory. At the end of the retention period, a probe string was presented, and subjects were instructed to press a “yes” or “no” button to indicate whether or not the probe matched the cue (recall phase). The next trial was triggered by either a response, or the absence of one, within 5 seconds of probe onset. The entire task consisted of 10 blocks of 36 trials. There were an equal number of trials per memory load (1, 3, or 5 syllables), trials were randomized as a function of memory load, and response hands were counter-balanced across subjects. Subjects first completed a practice block in order to become familiarized with the task and there were brief breaks between blocks to reduce fatigue.
Figure 1:
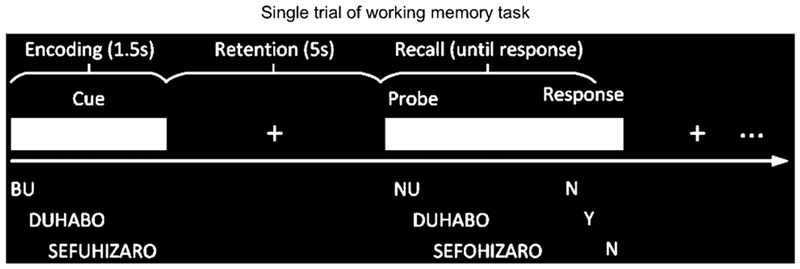
Single trial of working memory task. Time is represented in on the x-axis and encoding, retention, and recall phases of the task are shown separately.
2.3.1. Working Memory Task Performance Outcomes:
Accuracy (ACC) and Reaction Time (RT):
Accuracy on the EEG working memory task was defined as the number of correct responses divided by the number of trials with a response (computed overall and across all trials with the same memory load). Reaction Time was defined as the time (in msec.) between probe onset and a correct response and was averaged overall and across all trials with the same memory load.
Working Memory Capacity (WMC):
WMC was estimated for each subject using the Cowan’s K metric20. Accuracy data collected during the PBO session were entered into the formula [K = (hit rate + correct rejection rate – 1) x N], where N equals the size of the memory load (1, 3, or 5 syllables). This formula yielded a K value for each memory load for each subject; these load-specific values were then averaged to yield a global capacity measure for each individual that reflected performance on the task as a whole.
Relative Change Scores:
In order to assess the magnitude of drug-related working memory deficits, ‘relative change scores’ for ACC and RT were calculated using the formula ((treatment-PBO)/PBO). Relative change scores take the form of a percentage value that reflects the magnitude of drug-related changes in performance compared to PBO, enabling us to quantify the degree of impairment for each subject while normalizing across observed differences in ‘unimpaired’ performance during the PBO session. It is worth noting that all analyses reported here compared performance during the treatment sessions to PBO sessions, rather than to the no-treatment baseline (which subjects always completed first). In combination with a protocol in which subjects received treatment and PBO in random order, adopting this analytic approach allowed us to circumvent common sources of measurement error inherent to test-retest designs (i.e., practice effects and regression to the mean).
2.4. Data analysis
2.4.1. Accuracy (ACC) and Reaction Time (RT)
To examine whether there was an effect of treatment (PBO, LZP, TPM) or memory load (levels: 1, 3, or 5 syllables) on accuracy and reaction time, separate 3 × 3 repeated measures ANOVAs were used. ANOVAs were adjusted for treatment order, and a random factor was included to control for within-participant correlation across testing sessions. Post-hoc Bonferroni corrected t-tests were conducted to examine pairwise differences across treatments or memory loads.
2.4.2. Relationship between blood plasma levels and performance
To assess the extent to which variability in the degree of drug-related cognitive impairment was related to differences in drug concentration, we calculated Pearson’s correlations between blood plasma levels and each of the ACC and RT relative change scores. Correlations were calculated for the subject group as a whole, as well as separately for high and low WMC subject groups (see section 2.4.4).
2.4.3. Relationship between WMC and drug-related cognitive impairment
To determine the relationship between WMC at the PBO session and the severity of drug-related impairments associated with TPM and LZP administration, we first calculated Pearson’s correlations between WMC at PBO and each of the ACC and RT relative change scores. Correlations were calculated in two ways, using both overall and memory load specific measures. We then constructed linear mixed effects models for those performance measures that yielded significant correlations (p < .05). These models were used to assess the predictive power of WMC while accounting for differences in drug concentrations across individuals, and adjusted for treatment order, TPM dose group, gender, age, education, and estimated glomerular filtration rate.
2.4.4. Comparison between high and low WMC subjects
WMC scores at the PBO visit were rank-ordered and split into two groups based on the median value (≤ 1.65, > 1.65). The high WMC group included 14 participants with a mean WMC=2.02 and the low WMC group included 15 participants with a mean WMC=1.29 (p<.005). A Wilcoxon signed-rank test showed that drug concentrations did not differ across groups (LZP: p =.33, TPM: p =.39), and two-sided Fisher’s exact tests revealed that the two groups did not differ in terms of gender (p=.71), age (p =.88), years of education (p =.68), or treatment order (p=.39), i.e. there was no significant difference in the number of subjects who received treatment (LZP or TPM) before versus after PBO. We then calculated separate 2 × 3 repeated measures ANOVAs for RT and ACC data (one per drug per measure), with factors for group (high and low capacity) and memory load (1, 3, or 5 syllables). Post-hoc Bonferroni corrected t-tests were conducted to examine pairwise differences across treatments or memory loads. Correlations between performance and blood plasma levels were also assessed independently for each drug in each group.
3. RESULTS
3.1. Accuracy and reaction time
Accuracy
Accuracy percentages are shown in figure 2A. There were significant main effects of both memory load [F(2,28) = 12.7, p <.0001] and treatment [F(2,28) = 13.4, p <.0001] (see figure 2A), and the effect of treatment was also significant for each memory load independently (all p <.01). The treatment by memory load interaction did not reach significance.
Figure 2:
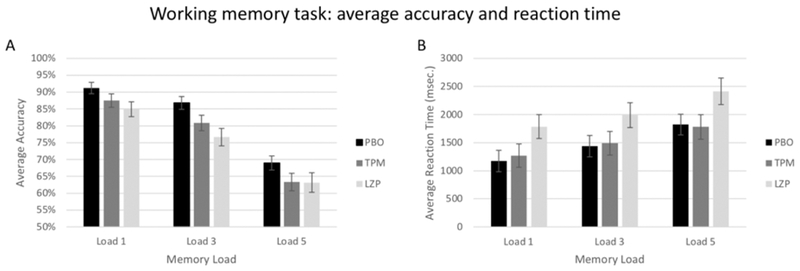
Working memory task average accuracy and reaction time (RT). Data are shown separately for PBO, LZP, and TPM sessions. Error bars represent standard error of the mean.
Reaction Time
Reaction times are shown in Figure 2B. There were significant main effects of both memory load [F(2,28) = 17.2, p <.0001] and treatment [F(2,28) = 17.1, p <.0001], and the effect of treatment was also significant for each memory load independently (all p <.01). The treatment by memory load interaction [F(4,28) = 4.3, p =.002] also reached significance due to the fact that RT during the LZP session was significantly higher than during both the PBO and TPM sessions, which did not differ.
3.2. Relationship between blood plasma levels and performance
We observed significant negative correlations between TPM concentration and the severity of TPM-related deficits; high concentrations were associated with larger declines in accuracy. This relationship held for average accuracy (Figure 3A: r = −.49, p=.006), and for each individual memory load (Figures 3B–3D: Load 1: r = −.45, p=.013; Load 3: r = −.42, p=.021; Load 5: r = −.38, p=.041). Correlations between TPM concentration and RT measures did not reach significance (all r <.32, all p >.08) and there were no significant correlations between LZP concentrations and relative change scores for any ACC or RT measure (all r <.22, all p >.26).
Figure 3:
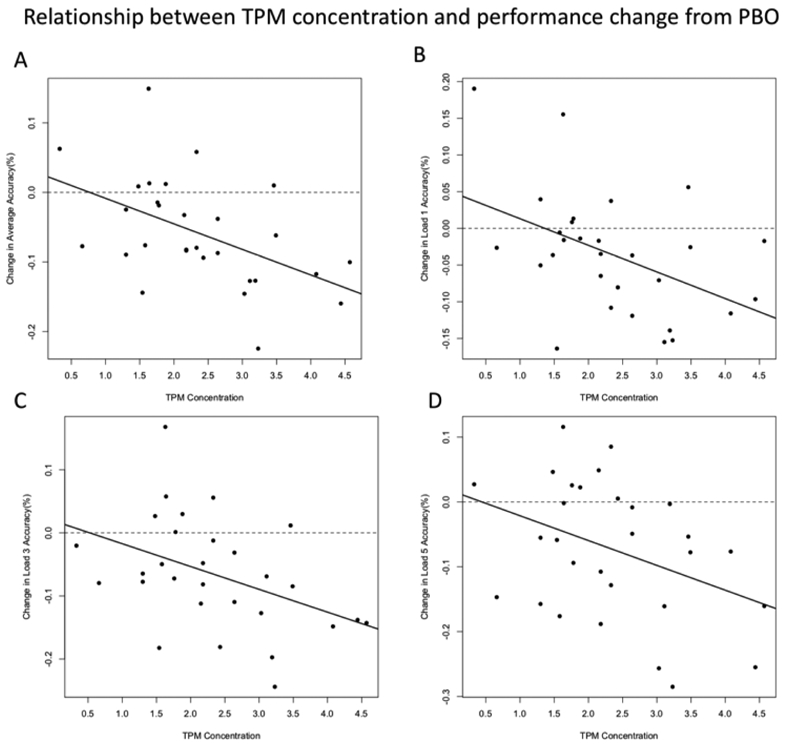
Relationship between concentration and performance change from PBO. Correlations between TPM concentrations and relative change scores ((TPM-PBO)/PBO) are shown separately for average accuracy and accuracy on Load 1, 3, and 5 trials.
3.3. Relationship between WMC and TPM-related cognitive impairment
There were robust relationships between WMC and the severity of TPM-related cognitive impairment. We observed a significant negative correlation between WMC and relative change in average accuracy (figure 4A: r = −.46, p =.011), and a significant positive correlation between WMC and relative change in average RT (figure 4B: r =.37, p =.046). When analyzing each memory load independently, this pattern of results also held for Load 1 (r=−0.37, p =.047) and Load 5 (r = −.644, p <.005) for ACC data, and for Load 1 (r =.42, p =.02) and Load 3 (r =.41, p =.02) for RT data. In the full mixed effect models we found that the effects of WMC on relative difference scores remained significant for both Load 1 (β = −.25, SE =.07, p =.0022) and Load 5 (β = −.04, SE =.02, p =.02) ACC data.
Figure 4:
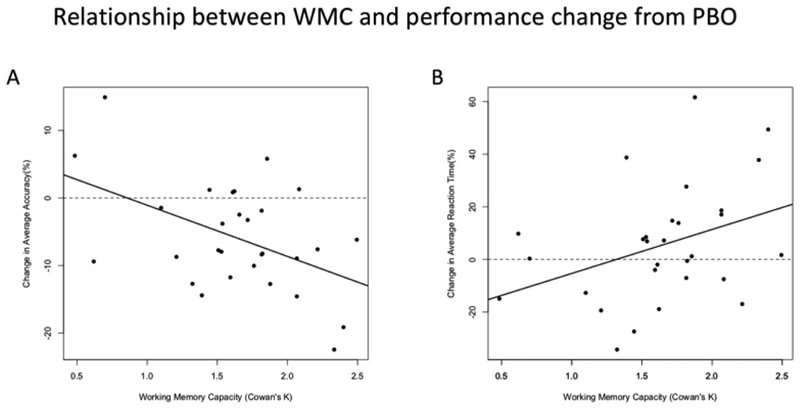
Relationship between WMC and performance change from PBO. Correlations between WMC and relative change scores ((TPM-PBO)/PBO) for average accuracy and reaction time (RT).
3.3.1. Differences in performance between high and low WMC subjects: TPM
Accuracy: Comparisons of the ACC relative change scores for high and low-capacity subjects are shown in Figure 5A. There was a significant main effect of group [F(1,28) = 5.44, p =.027, Cohen’s d’ =.29), due to the fact that the change in average ACC between PBO and TPM sessions was larger in the high capacity group than in their low capacity counterparts (8.1% compared to 3.7%). Neither the main effect of memory load nor the group x memory load interaction reached significance (both p >.1). Follow up pairwise comparisons revealed that the relative difference in ACC on Load 5 trials was highly significant (t(27) = 7.9, p =.009, Cohen’s d =.73).
Figure 5:
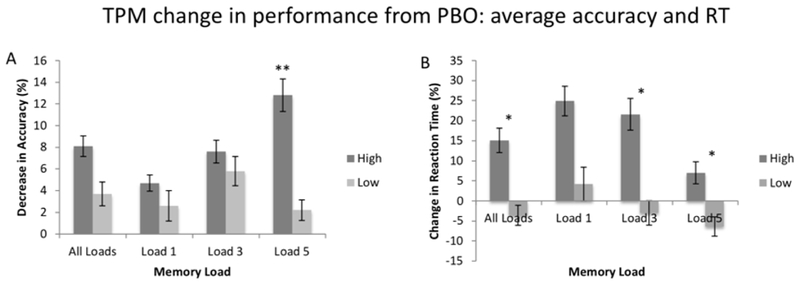
TPM change in performance from PBO: average accuracy and RT. Relative change scores ((TPM-PBO)/PBO) for accuracy and reaction time (RT) for both high and low WMC subjects. Error bars represent standard error of the mean. * p <.05; ** p <.01.
Reaction Time: Comparisons of the RT relative change scores for high and low-capacity subjects are shown in Figure 5B. There was a significant main effect of group [F(1,28) = 12.7, p = <.005, Cohen’s d =.77), due to the fact that the relative difference between PBO and TPM sessions was larger in the high capacity group compared to their low capacity counterparts (+15.1% compared to −3.6%). Neither the main effect of memory load nor the group x memory load interaction reached significance (both p > 0.1). Follow up pairwise comparisons revealed that the relative differences for each memory load were larger in the high capacity group, and these differences reached statistical significance for average RT (t(27) = 2.8, p =.02, Cohen’s d =.34), as well as for Load 3 (t(27) = 3.6, p = 0.01, Cohen’s d =.40) and Load 5 (t(27)=2.0, p = 0.03, Cohen’s d =.32) trials.
3.4. Relationship between WMC and LZP-related cognitive impairment
Compared to TPM, the relationship between WMC and the relative difference between LZP and PBO sessions was less robust. Only Load 5 ACC difference scores showed a significant correlation with WMC (r = −.42, p =.023). This effect remained significant in the full linear mixed effects model (β = −.054, SE =.02, p =.016).
3.4.1. Differences in performance between high and low WMC subjects: LZP
Comparisons of the relative change scores in ACC and RT between LZP and PBO sessions for high and low-capacity subjects are shown in figures 6A and 6B, respectively. There were no group effects for either ACC or RT data, due to the fact that LZP affected high and low capacity subjects approximately equally. The results of pairwise comparisons showed that the relative change scores for the two groups did not differ for any individual memory load, either for ACC or RT data (all p >.21).
Figure 6:
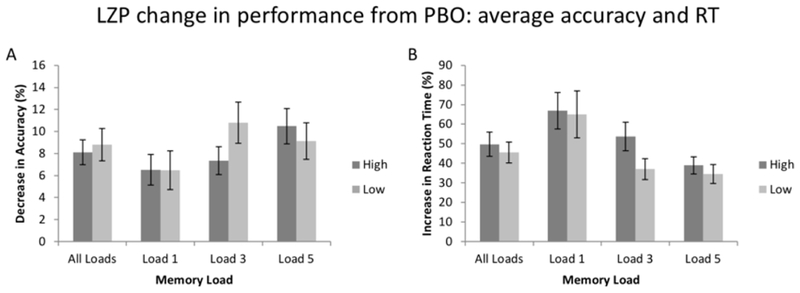
LZP change in performance from PBO: average accuracy and RT. Relative change scores ((LZP-PBO)/PBO) for accuracy and reaction time (RT) for both high and low WMC subjects. Error bars represent standard error of the mean.
3.5. Differential sensitivity to drug concentration across groups
In the high WMC group, analyses of the relationships between TPM concentrations and relative change scores revealed correlations with both average ACC (Figure 7A: r=−.52, p =.047) and RT (Figure 7C: r=.54, p =.044). These correlations did not reach significance in the low capacity group (both p >.14; Figures 7B and 7D). As these two groups had similar distributions for age, gender, education, and treatment order, and did not differ in terms of average drug concentration (see section 2.4.4), this differential sensitivity to increasing TPM concentrations can be plausibly attributed to differences in WMC. Analyses of the relationship between LZP concentrations and LZP-related performance declines revealed no significant correlations in either low- or high-capacity subjects (all p >.17).
Figure 7:
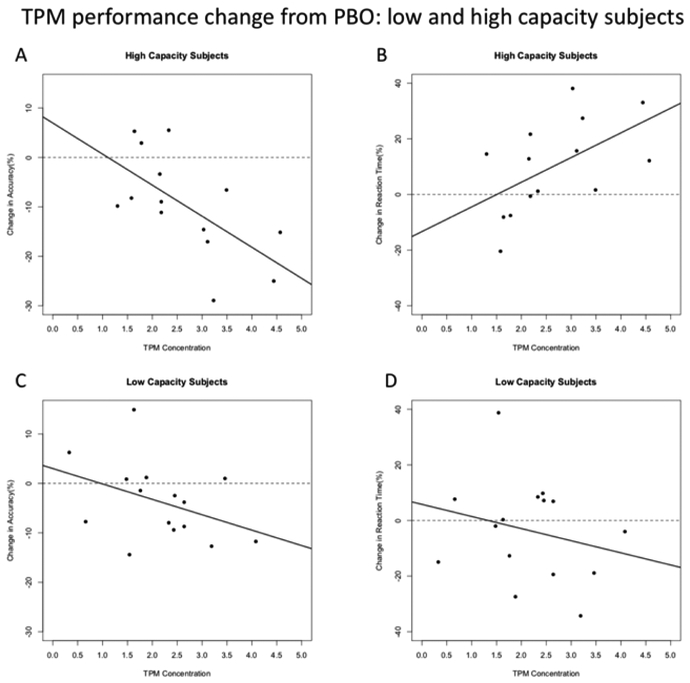
TPM performance change from PBO: high and low capacity subjects. Correlations between TPM concentration and relative change scores for accuracy and reaction time (RT) for high and low capacity subjects.
4. DISCUSSION
4.1. WMC and drug-related cognitive impairment
These findings demonstrate for the first time that WMC, measured after PBO administration, moderates the severity of drug-related cognitive impairment, and therefore that WMC is a factor that may be useful in predicting drug effects on cognition. For some of the behavioral measures that were analyzed, individual differences in WMC were significantly correlated with the magnitude of the deficits resulting from drug administration. This relationship appears to be of clinical value in the case of TPM, but not LZP, for the following reasons: first, the magnitude of TPM-related deficits was shown to be dependent on drug concentration, with higher concentrations associated with more severe declines in performance. Second, this relationship between concentration and TPM-related cognitive side effects was significantly moderated by WMC: concentration-performance relationships were significant in the high WMC group only. Finally, the results of a median split analysis revealed that high and low-capacity groups exhibited distinct patterns of behavioral changes after TPM administration. None of these patterns were observed in the LZP data, suggesting that LZP exerts its’ non-capacity-dependent effects on working memory via the type of generalized cognitive slowing associated with the benzodiazepine class. Taken together, these findings show that WMC is a clinically useful construct that should be considered when starting patients on TPM treatment regimens, but also that WMC is not a pertinent variable to consider for all drugs (e.g. LZP). In this study, we chose to initially focus on working memory deficits since they can cause debilitating impediments to normal function as a result of the essential cognitive functions that working memory plays a role in22–27. Future studies are needed to isolate additional cognitive factors that may also be useful in clinical settings when determining treatment approaches using other medications.
The most intriguing finding emerging from our data is that it was high capacity subjects that were most susceptible to experiencing severe TPM-related deficits, a somewhat counterintuitive finding that is inconsistent with the influential theory of cognitive reserve30–31. The basic assumption of this theory is that when the level of cognitive functioning is high prior to neurological insult, post-impairment outcomes will be more positive. Here we show the opposite pattern: high WMC subjects (i.e., those with high function after PBO administration) actually had worse outcomes after drug administration than their low WMC counterparts. This pattern provides support for the notion of functional adequacy32 that was initially forwarded to account for differences in post-surgical outcomes in patients with temporal lobe epilepsy. We feel that the most straightforward explanation of this pattern is also the best one: because high WMC subjects function so efficiently prior to drug administration, there is simply more potential for pronounced drug-related decline. In other words, negatively impacting a high functioning cognitive system will lead to more pronounced deficits than impacting a system that is already functioning at a low level. As an example, consider two subjects, one with high WMC and one with low, who both perform with 75% accuracy during their TPM sessions. During the PBO session, the elevated level of function conferred by higher WMC resulted in a 95% accuracy rate for the first subject, and the reduced functioning associated with low WMC resulted in an 85% accuracy rate for the second. Consequently, the magnitude of the performance decline is twice as large for the high capacity subject (20% vs. 10%). As such, high capacity individuals are at risk of experiencing a larger drug-related divergence from normal cognitive function and are therefore more likely to discontinue treatment due to pronounced declines in quality of life. These findings provide objective evidence confirming the intuitions of many clinicians who have long observed that high functioning patients are most likely to experience severe drug-related cognitive side effects.
In addition, we observed a robust relationship between TPM concentration and the severity of cognitive impairments in the high WMC group only. When combined with the findings on WMC, the clinical value of these data becomes evident; after using a simple assessment to classify a patient as low or high capacity, a clinician could potentially use these data to determine the risk of cognitive impairment and adjust dosing regimens and titration schedules accordingly.
4.2. Potential limitations
In order to successfully disentangle the independent cognitive deficits resulting from drug administration from those arising from underlying pathology, it is first necessary to study these drug-related impairments in healthy populations. Nonetheless, the single-dose protocol used here does limit our ability to generalize our findings to long-term drug effects on cognition resulting from repeated exposure. To address this limitation, we plan to test our hypotheses in patients on clinically relevant treatment regimens to further explore how titration, drug exposure, and underlying pathology interact with WMC to determine individual side effect profiles. In the present study, we adopted the admittedly simple Cowan’s K metric in order to quantify WMC, as it was not our aim to add to the vast literature on estimating WMC, but rather to assess the validity of WMC as a clinically useful cognitive feature. Determining the utility of multiple WMC measures simultaneously was not feasible within the scope of the current work, so it will be left for future research to determine the optimal approach to estimating WMC for use in clinical settings. In addition, the experimental task that was used in this study to estimate WMC may prove to be impractical to implement in clinical settings. However, now that the relationship between WMC and drug-related cognitive impairment has been demonstrated, it will be possible to move forward with the development of potentially more time and cost-effective methods of estimating WMC. These approaches may take the form of applying the results of traditional pencil-and-paper neuropsychological evaluations in novel ways or, more promisingly, developing an application that a could be used to present stimuli on a tablet or laptop, analyze responses, and determine whether a patient is low- or high-capacity.
4.3. Conclusions and future outlook
The ability to prospectively minimize the severity of drug-related cognitive deficits is directly tied to our ability to predict these impairments. We have shown that WMC is a variable that has predictive power with regards to the capacity-dependent cognitive deficits caused by drugs such as TPM, and as such may be an important factor for clinicians to consider when starting patients on TPM treatment regimens. At this point, we have successfully applied this approach to predicting drug effects on cognition to one drug using the results of one task that assesses one cognitive domain and shown, based on the LZP data, that WMC is not a factor that has universal clinical relevance for all drugs. In the future, we plan to extend this approach to other cognitive systems and drugs from other classes with the goals of using the data from multiple cognitive assessments to develop a full framework for predicting a wide range of drug effects on cognition.
Acknowledgments
This work was funded by NIH NINDS R01 NS076665; P.I. S.E Marino
Footnotes
Author disclosures: No authors report any disclosures.
REFERENCES
- [1].Mohamed K, Appleton R, Rosenbloom L Efficacy and tolerability of topiramate in childhood and adolescent epilepsy: a clinical experience. Seizure 2000; 9: 137–141. [DOI] [PubMed] [Google Scholar]
- [2].Ramsay R, Uthman B, Pryor F et al. Topiramate in older adults with partial-onset seizures: A pilot double-blind, dose-comparison study. Epilepsia 2008; 49: 1180–1185. [DOI] [PubMed] [Google Scholar]
- [3].Marino S, Pakhomov S, Han S et al. The effect of topiramate plasma concentration on linguistic behavior, verbal recall and working memory. Epilepsy Behav 2012; 24: 38–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mula M, Trimble M, Lhatoo S, et al. Topiramate and psychiatric adverse events in patients with epilepsy. Epilepsy Behav 2003; 44: 659–653. [DOI] [PubMed] [Google Scholar]
- [5].Meador K, Loring D, Vahle V et al. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology 2005; 64: 2108–2114. [DOI] [PubMed] [Google Scholar]
- [6].Javed A, Cohen B, Detyniecki K et al. Rates and predictors of patient-reported cognitive side effects of antepileptic drugs: An extended follow up. Seizure 2015; 29: 34–40. [DOI] [PubMed] [Google Scholar]
- [7].Thompson P, Baxendale S, Duncan J et al. Effects of topiramate on cognitive function. J. Neurol. Neurosurg. Psychiatry; 69: 2000: 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fritz N, Glogau S, Hoffman J et al. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav 2005; 6: 373–381. [DOI] [PubMed] [Google Scholar]
- [9].de Arajuo Filho G, Pasccalicchio T, Lin K et al. Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate. Epilepsy Behav 2006; 8: 606–609. [DOI] [PubMed] [Google Scholar]
- [10].Gomer B, Wagner K, Frings L et al. The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy Behav 2007; 10: 486–494. [DOI] [PubMed] [Google Scholar]
- [11].Jung K, Cho J, Joo E et al. Cognitive effects of topiramate revealed by standardised low-resolution brain electromagnetic tomography (sLORETA) of event-related potentials. Clinical Neurophysiology 2010; 121: 1494–1501. [DOI] [PubMed] [Google Scholar]
- [12].Lister R The amnesic action of benzodiazipenes in man. Neurosci Biobehav Rev 1985; 9: 87–94. [DOI] [PubMed] [Google Scholar]
- [13].Curran H, Gardiner J, Java R et al. Effects of lorazepam upon recollective experience in recognition memory. Psychopharmacology 1993; 110: 374–378. [DOI] [PubMed] [Google Scholar]
- [14].Mintzer M, Griffiths R Differential effects of scopolamine and lorazepam on working memory maintenance versus manipulation processes. Cognitive, Behavioral, and Affective Neuroscience 2007; 7: 120–129. [DOI] [PubMed] [Google Scholar]
- [15].File S, Bond A, Lister R Interactions between caffeine and lorazepam in performance tests and self-ratings. J Clin Psychopharmacol 1992; 2: 102–106. [PubMed] [Google Scholar]
- [16].File S, Lister R Do lorazepam-related effects on learning result from impaired rehearsal, reduced motivation, or increased sedation? Br J Clin Pharmacol 1982; 14: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Curran V, Allen D, Lader M The effects of single doses of alpidem and lorazepam on memory and psychomotor performance in normal humans. J Psychopharmacol 1987; 1: 81–89. [DOI] [PubMed] [Google Scholar]
- [18].Fluck E, Fernandes C, File S Are Lorazepam-related deficits in attention similar to those resulting from aging? J Clin Psychopharmacol 2001; 21: 126–130. [DOI] [PubMed] [Google Scholar]
- [19].Baddeley A Working Memory. Science 1992; 255: 556–559. [DOI] [PubMed] [Google Scholar]
- [20].Cowan N, Eliott E, Saults J et al. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology 2005; 51: 42–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vogel E, McCollough A, Machizawa M Neural measures reveal inidivudal differences in controlling access to working memory. Nature 2005; 438: 500–503. [DOI] [PubMed] [Google Scholar]
- [22].Ackerman P, Beier M, O’Boyle M Working Memory and Intelligence: The Same or Different Constructs? Psychol Bull 2005; 131: 30–60. [DOI] [PubMed] [Google Scholar]
- [23].Kyllonen C, Christal R Reasoning Ability is (little more than) Working Memory Capacity?! Intelligence; 14: 389–433. [Google Scholar]
- [24].Engle R Working Memory Capacity as Executive Attention. Current Directions in Psychological Science 2002; 11: 19–23. [Google Scholar]
- [25].Hinson J, Jameson T, Whitney P Impulsive Decision Making and Working Memory. Journal of Experimental Psychology: Learning, Memory, and Cognition 2003; 29: 298–306. [DOI] [PubMed] [Google Scholar]
- [26].Just M, Carpenter P A Capacity Theory of Comprehension: Individual Differences in Working Memory. Psychol Rev 1992; 99: 122–149. [DOI] [PubMed] [Google Scholar]
- [27].Daneman M, Carpenter P Individual Differences in Working Memory and Reading. Journal of Verbal Learning and Verbal Behavior 1980; 19: 450–456. [Google Scholar]
- [28].Sternberg S High Speed Scanning in Working Memory. Science 1969; 153: 652–654. [DOI] [PubMed] [Google Scholar]
- [29].Subramanian M, Birnbaum A, Remmel R High-Speed Simultaneous Determination of Nine Antiepileptic Drugs Using Liquid Chromatography-Mass Spectrometry. Ther Drug Monit 2008; 30: 347–356. [DOI] [PubMed] [Google Scholar]
- [30].Satz P Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology 1993; 7: 273–295. [Google Scholar]
- [31].Stern Y What is Cognitive Reserve? Theory and Research Application of the Reserve Concept. J Int Neuropsychol Soc 2002; 8: 448–460. [PubMed] [Google Scholar]
- [32].Chelune G Hippocampal adequacy versus functional reserve: Predicting memory functions following temporal lobectomy. Archives of Clinical Neuropsychology 1995; 5: 413–432. [PubMed] [Google Scholar]


