Abstract
The neurotrophin Brain-Derived Neurotrophic Factor (BDNF) has been implicated in a number of neuropsychiatric disorders, including alcohol use disorder. Studies have shown that BDNF activity in cortical regions, such as the medial prefrontal cortex (mPFC) mediates various ethanol-related behaviors. We previously reported a significant down-regulation in Bdnf mRNA in mPFC following chronic ethanol exposure compared to control mice. The present study was conducted to extend these findings by examining whether chronic ethanol treatment reduces BDNF protein expression in mPFC and whether reversing this deficit via direct injection of BDNF or viral-mediated overexpression of BDNF in mPFC alters voluntary ethanol consumption in dependent and nondependent mice. Repeated cycles of chronic intermittent ethanol (CIE) exposure was employed to model ethanol dependence, which produces robust escalation of ethanol intake. Results indicated that CIE treatment significantly increased ethanol intake and this was accompanied by a significant decrease in BDNF protein in mPFC that lasted at least 72 hr after CIE exposure. In a separate study, once dependence-related increased drinking was established, bilateral infusion of BDNF (0, 0.25, 0.50 μg) into mPFC significantly decreased ethanol intake in a dose-related manner in dependent mice but did not affect moderate drinking in nondependent mice. In a third study, viral-mediated overexpression of BDNF in mPFC prevented escalation of drinking in dependent mice but did not alter intake in nondependent mice. Collectively, these results provide evidence that adaptations in cortical (mPFC) BDNF activity resulting from chronic ethanol exposure play a role in mediating excessive ethanol drinking associated with dependence.
Keywords: ethanol dependence, BDNF, medial prefrontal cortex, ethanol drinking, mice
1. Introduction
Alcohol use disorder constitutes a significant public health problem. Alcoholism is a chronic relapsing disease and heavy (excessive) levels of drinking can lead to dependence. Chronic alcohol (ethanol) exposure triggers neuroadaptations that contribute to escalation and maintenance of sustained excessive ethanol consumption associated with dependence (Becker, 2012; Hansson et al., 2008; Koob, 2013; Koob and Le Moal, 2008; Vengeliene et al., 2008). Several animal models have been developed that demonstrate dependence-related excessive levels of ethanol consumption (Becker, 2013; Becker and Ron, 2014; Vendruscolo and Roberts, 2014). For example, we developed a mouse model of ethanol dependence that involves repeated cycles of chronic intermittent ethanol (CIE) exposure and results in escalation of voluntary ethanol consumption (Becker and Lopez, 2004; Griffin et al., 2014; Griffin et al., 2009a; Lopez and Becker, 2005). Use of these preclinical models has been critical for identifying neuroadaptive changes that underlie dependence and promote excessive levels of drinking.
We previously conducted a study to profile brain regional changes in gene expression associated with our mouse model of ethanol dependence and relapse drinking (Melendez et al., 2012). Analysis of the gene microarray results revealed a robust down-regulation in Brain- Derived Neurotrophic Factor (Bdnf) mRNA expression in medial prefrontal cortex (mPFC) following CIE exposure. This transcriptional change was confirmed by quantitative real-time polymerase chain reaction (RT-PCR) analysis (Melendez et al., 2012). This finding is congruent with a growing body of evidence implicating a role for BDNF in regulation of various ethanol- related behaviors.
BDNF is a member of the neurotrophin family of growth factors that play an important role in the development and maintenance of the nervous system. BDNF is widely distributed and highly expressed in mammalian brain, and it is secreted from neurons in an activity- dependent manner, interacting with TrkB receptors and low-affinity p75NT receptors to regulate a multitude of cellular processes (Sandhya et al., 2013). BDNF has been implicated in a number of neuropsychiatric disorders (Nagahara and Tuszynski, 2011; Ninan, 2014), including substance use disorders (Ghitza et al., 2010; Russo et al., 2009). In particular, preclinical and clinical studies have implicated BDNF in alcohol drinking, dependence, and relapse (Davis, 2008). For example, in humans, a polymorphism in the Bdnf gene (Val66Met) is functionally significant in that the allele encoding Val66 was shown to confer higher risk for relapse (Wojnar et al., 2009) while the 66Met variant was associated with earlier onset and greater severity of alcohol dependence (Matsushita et al., 2004).
Studies in animals also show a relationship between BDNF and various ethanol-related behaviors. For example, several genetic rodent models demonstrate an inverse relationship between propensity to drink and BDNF expression levels in the brain (Hensler et al., 2003; McGough et al., 2004; Moonat et al., 2011; Pandey et al., 2004; Prakash et al., 2008; Yan et al., 2005). Moderate levels of ethanol consumption were reported to increase Bdnf mRNA expression in hippocampus (Stragier et al., 2015) and dorsolateral striatum (Bahi and Dreyer, 2013; Jeanblanc et al., 2009; Logrip et al., 2009). Conversely, prolonged ethanol intake produced decreased Bdnf mRNA expression in frontal cortex (Darcq et al., 2014; Logrip et al., 2009; Orru et al., 2016; Tapocik et al., 2014). Further, viral-mediated decreases in BDNF expression in dorsal striatum increased ethanol drinking (Jeanblanc et al., 2009), while increasing BDNF expression in this brain region reduced ethanol consumption, effects mediated by BDNF-TrkB receptor interaction (Jeanblanc et al., 2006; Jeanblanc et al., 2013; Logrip et al., 2008).
Few studies have examined BDNF expression in prefrontal cortex and how changes in cortical BDNF levels influence ethanol consumption in the context of dependence. Here we report that repeated cycles of CIE exposure produce a lasting reduction in BDNF protein expression in mPFC. Replenishing this deficit by directly infusing BDNF into the mPFC selectively reduced excessive drinking in dependent mice without altering more moderate levels of intake in nondependent subjects. Further, viral-mediated overexpression of BDNF in mPFC protected against the escalation of ethanol intake observed after repeated CIE exposure.
2. Material and methods
2.1. Subjects
Adult (9 weeks old) male C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) were individually housed under a 12-hr light/dark cycle (lights on at 0200 hr) in an environmentally controlled facility, with food and water continuously available at all times. All work was approved by the Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2. General Study Design
A model of ethanol dependence and relapse drinking developed in our laboratory (Becker and Lopez, 2004; Griffin et al., 2014; Griffin et al., 2009a; Lopez and Becker, 2005) was utilized for all studies. Briefly, mice were first trained to drink ethanol (15% v/v) in a 2-bottle choice limited access (2 hr/day) paradigm. Once stable baseline ethanol intake was established (~4 weeks), mice were separated into two groups (equated for baseline level of ethanol intake). Mice received either chronic intermittent ethanol (CIE) vapor (CIE group) or control air (CTL group) exposure in inhalation chambers (16 hr/day for 4 days), as detailed below. After a 72 hr abstinence period, all mice were given the opportunity to drink ethanol for 5 consecutive days under the limited access conditions as before. This pattern of weekly CIE (or air) exposure alternated with weekly limited access Test drinking sessions was repeated for several cycles (Figure 1).
Figure 1: Schematic of general study design for ethanol dependence and relapse drinking model.
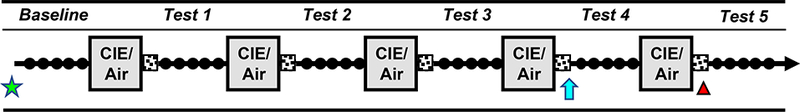
Stable baseline ethanol intake was established during daily limited access (2 hr/day) sessions (filled circles). Mice were then separated into groups that received chronic intermittent ethanol (CIE) vapor or Air exposure in inhalation chambers 16 hr/day for 4 days, followed by a 72-hr forced abstinence period (stippled squares). Weekly CIE/Air exposure treatment periods alternated with 5-daily limited access Test drinking sessions for several cycles. In Study 1, mice were sacrificed at 0, 8, or 72 hr following a 5th CIE/Air exposure cycle (denoted by red triangle). In Study 2, mice received bilateral microinjection of BDNF (0, 0.25, 0.50 μg/side) into the mPFC 24-hr prior to the first drinking session of Test cycles 4, with alternative doses given prior to Tests 5 or 6 (denoted by blue arrow). In Study 3, mice received infusions of active (AAV-BDNF) or control (AAV-GFP) virus into the mPFC 2 weeks prior to the start of study (denoted by green star). See Methods for procedural details.
2.3. Limited Access Drinking Procedure
Mice were given limited access to ethanol (15% v/v, with tap water as the alternative fluid) in the home cage, as previously described (Becker and Lopez, 2004; Lopez and Becker, 2005). Briefly, the 2-hr drinking sessions started 30 min before the beginning of the dark cycle. Solutions (prepared daily) were presented at room temperature in 15 ml graduated tubes, and the position of the ethanol and water bottles was alternated daily to avoid side preferences. The amount of ethanol and water consumed (± 0.1 ml) was recorded daily and body weights were recorded weekly. Mice were not food or water deprived at any time during the experiments.
2.4. Chronic Intermittent Ethanol (CIE) Exposure
Mice were exposed to chronic intermittent ethanol vapor or air in inhalation chambers, as previously described (Becker and Lopez, 2004; Griffin et al., 2014; Griffin et al., 2009a; Lopez and Becker, 2005). Chamber ethanol concentrations were monitored daily and air flow was adjusted to maintain ethanol concentrations within a range that yielded stable blood ethanol levels throughout exposure (200–250 mg/dl). Ethanol concentration in the inhalation chambers and blood ethanol levels in CIE and CTL mice were determined as previously described (Becker and Hale, 1993; Lopez and Becker, 2005).
2.5. Study Procedures
Study 1:
This study examined the effects of CIE exposure on BDNF protein expression in the mPFC. Mice were treated in the ethanol dependence and relapse drinking model. Following a 5th CIE (or air) exposure cycle, separate groups of CIE (N= 7/group) and CTL (N= 57/group) mice were sacrificed at 0-hr, 8-hr, or 72-hr time points. Brain tissue samples were collected for BDNF protein measurement.
Study 2:
This study examined whether microinjection of BDNF into the mPFC reduces escalated ethanol consumption associated with CIE exposure. After recovery from stereotaxic surgery, mice were treated in the ethanol dependence and relapse drinking model. CIE (N= 8–15/group) and CTL (N= 9–13/group) mice received bilateral microinjection of BDNF (0, 0.25, 0.50 μg/side) into the mPFC 24 hr prior to the first drinking session of Test Cycles 4, 5, or 6. Microinjections were administered in a quasi-randomized manner, with no animal receiving more than two infusions and no animal receiving both BDNF doses.
Study 3:
This study examined whether viral-mediated overexpression of BDNF in the mPFC prevents the development of escalated drinking associated with CIE exposure. Mice received stereotaxic-guided infusions of active (AAV-BDNF) or control (AAV-GFP) virus into the mPFC 2 weeks prior to the start of study. Ethanol intake during four weeks of baseline and then Test Cycles 1–4 was monitored in CIE (N= 9–10/group) and CTL (N= 10–11/group) mice. Brain (mPFC) tissue was collected at the end of Test 4 for measurement of BDNF protein (ELISA) and immunohistochemical validation of viral placements.
2.6. Brain Tissue Collection
Following decapitation, brains were rapidly extracted and quickly frozen in a mixture of isopentane and dry ice, and then stored at −80° C until dissection. Coronal sections were made on ice at 4 mm and 6 mm rostral to the interpeduncular fossa, which was used as a reference point (~−3.5 mm to Bregma) (Franklin and Paxinos, 2008). This 2 mm section was used to isolate dorsomedial prefrontal cortex using ice-cold stainless steel 1 mm punches (Ted Pella, Inc.). Bilateral tissue samples were collected for protein analysis and samples were homogenized (brief sonication) in lysis buffer and stored (−80° C) until the assay performed.
2.7. BDNF Protein Measurement
BDNF protein levels in brain tissue samples were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Human BDNF DuoSet). The ELISA kit used has a wide linear range (0 to 1500 pg/ml) and a limit of detection, determined in our laboratory, of 15 pg/ml. Standards (8 concentrations) were run in triplicate and brain samples (100 μl aliquots) assayed in duplicate. The manufacturer’s instructions were followed with the exception that the lysis buffer for tissue homogenization was made using Protease Inhibitor Cocktail (Cal Biochem) and Igepal and glycerol (Sigma). An aliquot (50 μl) of the tissue sample homogenate was used to quantify total protein concentration (Bradford method) using a 600nm Protein Assay (Pierce).
2.8. Preparation of Adeno-Associated Virus (AAV) for BDNF Overexpression
A pAAV-IRES-hrGFP plasmid containing the cytomegalovirus (CMV) promotor and the human BDNF sequence (NCBI Reference Sequence: NG_011794.1) was provided by D. Ron. The plasmid was amplified in DH5a E. coli cells and purified using the Promega PureYield Midiprep kit. An aliquot of the plasmid was sent to the UNC Viral Vector Laboratory and packaged into Adeno-Associated virus (AAV, serotype 5) with a titer of 4.1 ×10Λ12 viral particles (AAV-BDNF). The control virus used for the sham group was AAV5-hSYN-GFP (AAV-GFP) obtained from Addgene (50465-AAV5) at a titer of 3×10Λ12 viral particles.
2.9. Stereotaxic Surgical Procedures
Basic procedures for stereotaxic surgery and implanting guide cannulae in mice were similar to that previously described (Griffin et al., 2014; Griffin et al., 2009b). Under isoflurane anesthesia, mice had the bilateral guide cannulae (Plastics One, Inc) implanted targeting the mPFC (AP: +2 mm, ML: ±0.4 mm, DV: −1.2 mm, Bregma was used as a reference point) (Franklin and Paxinos, 2008). Bilateral microinjections were delivered using dual syringe Model 11 Pumps (Harvard Apparatus) in a volume of 0.25 μl/side administered over 2 min, with the injector left in place for an additional 2 min to allow diffusion of the injected agent. Recombinant human BDNF (rhBDNF, R&D Systems) was prepared in 1xPBS (Boston Bioproducts) immediately prior to microinjection. For BDNF overexpression studies, 0.25 μl of either AAV- BDNF or AAV-GFP was bilaterally infused into the mPFC (AP: +2 mm, ML: ±0.4 mm, DV: −1.7 mm) using a 0.5 μl, 33-gauge Hamilton Neuros syringe (Reno, NV) at a rate of 0.05 μl per minute. After a 5 min diffusion period, the syringe was slowly retracted over an additional 5 min period. All animals undergoing surgery were given 1–2 weeks recovery time before experiments commenced.
2.10. Histological Procedures
Mice with guide cannulae were deeply anesthetized using urethane (3 g/kg), transcardially perfused with normal saline (10 ml) followed by 10% Formalin® (10 ml) and fixed brains were sectioned (40 μm) and stained using Cresyl Violet. Placements were determined by reference to a mouse stereotaxic atlas (Franklin and Paxinos, 2008). Only data from mice with correct bilateral microinjector placements were included in data analyses. A subset of BDNF-overexpressing and GFP-expressing mice were deeply anesthetized using urethane (3 g/kg), transcardially perfused with normal saline (10 ml) followed by 4% paraformaldehyde (10 ml) and fixed brains were sectioned (40 μm), placed in 1x phosphate buffered saline (PBS) then mounted with Prolong Diamond® prior to imaging. The native GFP tag in AAV-BDNF and AAV- GFP expressing tissue was imaged on an EVOS FL microscope (ThermoFisher, Rochester, NY). The remaining mice were used for measurement of BDNF levels in mPFC by ELISA. Only data from mice with correct bilateral GFP expression or elevated BDNF levels were included in data analyses.
2.11. Statistical Analyses
Study 1:
Ethanol consumption (g/kg) was analyzed with a 2-way repeated measures ANOVA, with Group (CIE, CTL) as a between-subjects factor and Test Cycle (Baseline and Tests 1–4) as a repeated measure. BDNF protein expression data were analyzed by 2-way ANOVA, with Group and Time (0, 8, 72 hr) as factors.
Study 2:
Ethanol intake prior to BDNF microinjection was analyzed by repeated measures ANOVA, with Group and Test Cycle as factors. Daily intake after BDNF microinjection was analyzed by 3-way ANOVA, with Group (CIE, CTL) and Dose (0, 0.25 and 0.50 μg BDNF) as between-subject factors and Days as a repeated measure. Separate 2-way ANOVAs (Group x Days) were conducted for each BDNF dose, and average ethanol intake during the final test cycle (Test 4) was analyzed by 2-way ANOVA (Group x Dose).
Study 3:
Average weekly ethanol intake over the 4-week baseline period was analyzed by ANOVA, with Group (CTL-GFP, CTL-BDNF, CIE-GFP, CIE-BDNF) as a between-subjects factor and Weeks as a repeated measure. Drinking data during the last week of baseline and the four test cycles was analyzed by 2-way ANOVA (Group x Test Cycle), and separate analyses were also conducted for mice that received AAV-GFP and those that received AAV- BNDF treatment. Intake during Test 4 for all groups was analyzed by 1-way ANOVA, a similar analysis was performed on BDNF levels in mPFC from ELISA assay.
For all studies, statistical analyses were carried out using Statistica (TIBCO) software package. Post-hoc comparisons were performed when appropriate (Newman-Keuls), with p< 0.05 considered statistically significant.
3. Results
3.1. Effect of Chronic Intermittent Ethanol Exposure on BDNF Protein Expression in mPFC
Chronic ethanol exposure in inhalation chambers was relatively consistent across exposure cycles with resultant BEC values in the 200–250 mg/dl range (mean ± s.e.m. for CIE groups sacrificed at 0-hr, 8-hr, and 72-hr post-exposure = 213.6 ± 11.1, 205.7 ± 5.6, and 229.0 ± 7.9 mg/dl, respectively). As expected, CIE mice exhibited a progressive increase in ethanol consumption over successive test cycles. This was supported by ANOVA, which indicated a significant Group x Test Cycle interaction between [F(4,192)=14.82, p< 0.0001]. Post-hoc comparisons indicated that CIE mice consumed significantly more ethanol during Tests 3 and 4 compared to their baseline level of intake (ps< 0.001), and ethanol intake was greater in CIE mice compared to CTL mice during Test 3 (p< 0.001) and Test 4 (p< 0.001). In contrast, ethanol intake remained relatively stable over test cycles in CTL mice (Figure 2A).
Figure 2: Repeated cycles of chronic intermittent ethanol exposure produces escalation of voluntary ethanol drinking and reduces BDNF protein content in mPFC.
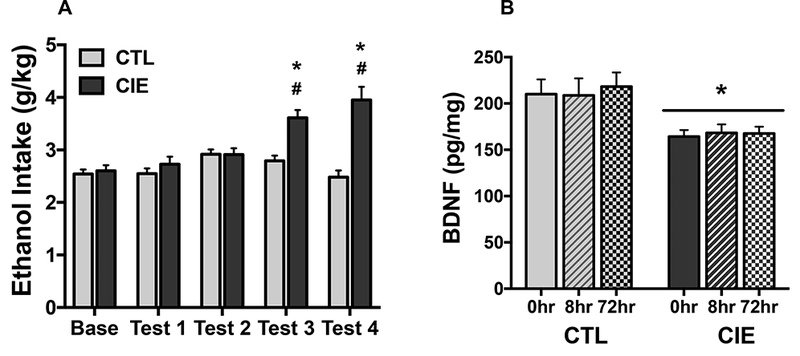
(A) Data are presented as weekly average ethanol intake (g/kg) for CIE (N= 21) and CTL (N= 19) groups during the last week of baseline and four test cycles. Ethanol dependent (CIE-exposed) mice increased ethanol intake during Test 3 and Test 4 compared to their Baseline level (#; ps< 0.001), and consumed more ethanol compared to nondependent (CTL) mice during these same test cycles (*; ps< 0.001). (B) BDNF protein content in mPFC measured by ELISA at 0-hr, 8-hr, or 72-hr following the final (5th) CIE/air exposure cycle in CIE (N= 7/time point) and CTL (N= 5– 7/time point) groups of mice sacrificed. CIE exposure produced a significant reduction in BDNF protein content in mPFC compared to controls (* p< 0.01). Values are mean ± s.e.m.
CIE and CTL mice were sacrificed at 0, 8, or 72 hr following a fifth CIE exposure cycle for determination of BDNF protein content in mPFC. ANOVA revealed a significant main effect of Group [F(1,34)= 8.60, p< 0.01] but no Group x Time interaction [F(2,34)< 1.0], indicating that CIE exposure produced a significant reduction in BDNF protein expression in mPFC at all time points evaluated (Figure 2B). Thus, relative to nondependent animals, decreased mPFC BDNF protein content immediately following CIE exposure persisted for at least 3 days into withdrawal.
3.2. Effect of BDNF Infusion Into mPFC on Ethanol Drinking in Dependent and Nondependent Mice
Chronic ethanol exposure during inhalation treatment was relatively stable across all cycles of exposure, yielding BEC values in the range of 200–250 mg/dl (mean ± s.e.m.= 213.0 ± 6.4 mg/dl). Ethanol consumption during the last week of Baseline was similar for CIE mice and CTL mice. Over repeated test cycles prior to BDNF treatment, ethanol intake significantly increased in CIE mice over Baseline levels, while intake in CTL mice remained relatively stable. This was supported by a significant Group x Test Cycle interaction [F(3,108)= 5.37, p< 0.005], and subsequent analyses indicated that CIE-exposed mice consumed more ethanol during Test 2 and 3 compared to their baseline level of intake as well as in comparison to CTL mice (ps< 0.005) (Table 1).
Table 1.
Ethanol consumption (g/kg) in CIE and CTL groups of mice prior to BDNF treatment (Study 2)
| Group | Baseline | Test 1 | Test 2 | Test 3 |
|---|---|---|---|---|
| CTL | 2.42 ± 0.12 | 2.26 ± 0.09 | 2.62 ± 0.09 | 2.47 ± 0.09 |
| CIE | 2.36 ± 0.11 | 2.54 ± 0.12 | 3.19 ± 0.20* | 3.34 ± 0.18* |
Values are mean ± s.e.m. for CTL (N= 18) and CIE (N= 20) groups.
significantly differs from baseline level of intake and corresponding CTL group intake (p< 0.05)
At 48 hr following the fourth, fifth, or sixth ethanol vapor/air exposure cycle (24 hr prior to the start of Test Cycles 4, 5, or 6), CIE and CTL groups received bilateral microinjections of BDNF (0, 0.25, 0.50 μg) in a quasi-randomized fashion. ANOVA revealed no significant effect of order of treatment, so data were combined for presentation and further analyses. Analysis of daily ethanol intake during the treatment test periods revealed a significant main effect of Group [F(1,58)= 41.18, p< 0.0001] and a Group x Dose interaction [F(2,58)= 11.73, p< 0.0001], but the Group x Dose x Day interaction did not achieve significance [F(8,232)< 1.0]. Separate ANOVA of vehicle-treated mice indicated a significant main effect of Group [F(1,26)= 68.08, p< 0.0001], and the lack of a significant Group x Day interaction [F(4,104)< 1.0] supports the observation that the elevated ethanol intake in CIE mice compared to CTL mice was consistent across all days of the testing period (Figure 3A). A similar pattern of results was evident in mice that received microinjection of the lower BDNF dose (0.25 μg). That is, CIE mice consumed significantly more ethanol than corresponding CTL mice treated with this dose of BDNF [F(1, 15)= 19.45, p< 0.001] (Figure 3B). In contrast, ethanol consumption did not differ between CIE and CLT mice that received intra-mPFC infusion of 0.50 μg BDNF [F(1,17)< 1.0] (Figure 3C).
Figure 3: BDNF infusion into mPFC selectively reduces escalated drinking in ethanol dependent (CIE-exposed) mice.
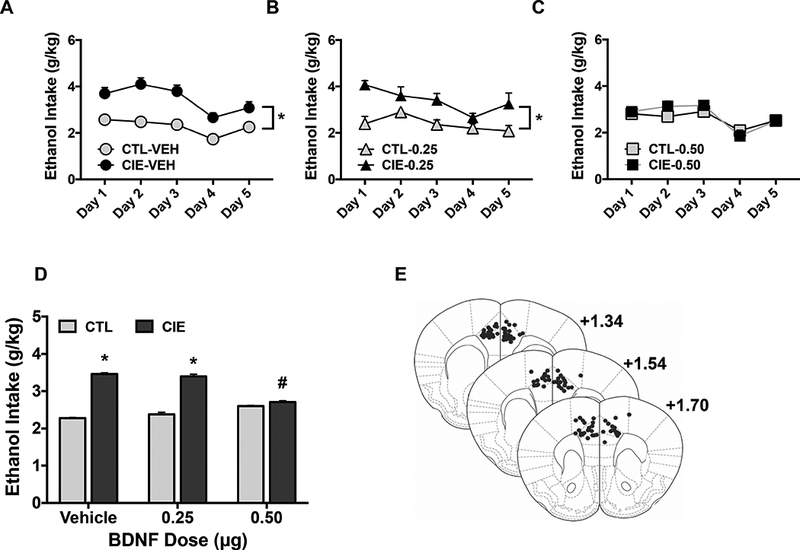
CIE and CTL mice received bilateral infusions of BDNF (0.25 or 0.50 μg/side) or vehicle (PBS) into the mPFC 48 hr following the 4th, 5th, or 6th CIE/air inhalation exposure cycle in a quasi-randomized order. Data are presented as ethanol intake (g/kg) as a function of test drinking day in (A) CIE and CTL mice injected with vehicle (N= 13–15/group); (B) CIE and CTL mice injected with 0.25 μg BDNF (N= 8–9/group); and (C) CIE and CTL mice injected with 0.50 μg BDNF (N= 9–10/group). CIE-exposed mice consumed significantly more ethanol compared to CTL mice across all test days when treated with vehicle (*, p< 0.001) or 0.25 μg BDNF (*, p< 0.001), but this difference was eliminated in mice treated with 0.50 μg BDNF. (D) Average ethanol intake (g/kg) over 5-day test period for all BDNF dose groups. Ethanol intake was significantly greater in CIE compared to CTL mice that received vehicle or 0.25 μg BDNF (*, p< 0.001), and 0.50 μg BDNF significantly reduced ethanol intake in CIE mice compared to vehicle treated CIE mice (#, p< 0.001). All values are mean ± s.e.m. (E) Representative placements (black dots) of microinjection sites within the mPFC.
Analysis of ethanol intake averaged over the 5-day test period following intra-mPFC infusions indicated that BDNF significantly reduced ethanol intake compared to vehicle-treated CIE mice while not altering intake in CTL mice (Group x Dose interaction: [F(2,58)= 11.73, p< 0.0001]). Post-hoc analysis indicated that ethanol consumption was significantly greater in dependent (CIE) mice compared to nondependent (CTL) mice that received vehicle (p< 0.001) or 0.25 μg BDNF (p< 0.001), but this group difference was eliminated in mice treated with the 0.50 μg BDNF dose. Further, ethanol intake was significantly lower in CIE mice that received microinjection of 0.50 μg BDNF compared to vehicle (p< 0.001). The lower BDNF (0.25 μg) dose did not significantly alter intake in CIE mice and neither BDNF dose altered intake in CTL mice (Figure 3D). Microinjector placements for subjects included in data analyses, determined by inspection of Cresyl Violet stained sections, are depicted schematically using a mouse stereotaxic atlas template (Franklin and Paxinos, 2008) (Figure 3E). Collectively, these results indicate that BDNF infusion into mPFC eliminates CIE-induced escalated drinking by selectively reducing ethanol consumption in dependent mice.
3.3. Effect of BDNF Overexpression in mPFC on Ethanol Drinking in Dependent and Nondependent Mice
Analysis of ethanol intake during the baseline phase of the study indicated no significant effect of Group [F(3,36)= 1.41, p> 0.10] or Group x Week interaction [F(3,108)= 3.26, p> 0.50], suggesting that BDNF overexpression in the mPFC did not alter moderate ethanol consumption (Table 2). Following this baseline period, mice were treated in the ethanol dependence and relapse drinking model. CIE exposure produced stable BECs in the 200–250 mg/dL range across all exposure cycles (mean ± s.e.m. for CIE-GFP and CIE-BDNF groups = 217.5 ± 7.4 and 213.4 ± 3.6 mg/dl, respectively).
Table 2.
Ethanol consumption (g/kg) during baseline phase in CIE-GFP, CIE-BDNF, CTL-GFP, and CTL-BDNF groups of mice (Study 3)
| Group | (N) | Baseline 1 | Baseline 2 | Baseline 3 | Baseline 4 |
|---|---|---|---|---|---|
| CTL-GFP | (10) | 2.14 ± 0.14 | 1.87 ± 0.13 | 2.10 ± 0.16 | 1.90 ± 0.08 |
| CTL-BDNF | (11) | 1.87 ± 0.14 | 1.70 ± 0.13 | 1.78 ± 0.15 | 1.74 ± 0.14 |
| CIE-GFP | (10) | 1.88 ± 0.12 | 1.71 ± 0.09 | 2.13 ± 0.21 | 1.86 ± 0.16 |
| CIE-BDNF | (9) | 1.68 ± 0.17 | 1.52 ± 0.15 | 1.77 ± 0.20 | 1.69 ± 0.17 |
Values are mean ± s.e.m.
ANOVA of average weekly ethanol intake during the last week of Baseline and Test Cycles 1–4 indicated a significant main effect of Group [F(3,36)= 10.15, p< 0.0001] and a significant Group x Test Cycle interaction [F(12,144)= 2.33, p< 0.005]. Separate analysis of mice expressing AAV-GFP revealed a Group x Time interaction [F(4,72)= 5.01, p< 0.005]. Post- hoc analysis indicated that ethanol intake significantly increased over Baseline levels during Tests 1–4 in CIE-GFP mice (ps< 0.01), while ethanol consumption remained relatively stable across all test cycles in CTL-GFP mice. Further, ethanol intake was significantly greater in CIE- GFP mice compared to CTL-GFP mice during Tests 2, 3, and 4 (ps< 0.001) (Figure 4A). Similar analysis in AAV-BDNF groups BDNF indicated no significant effect of Group [F(1,18)< 1.0] or Group X Test Cycle interaction [F(4,72)< 1.0], suggesting that over-expression of BDNF in the mPFC blocked escalation of intake observed after CIE exposure (Figure 4B). Analysis of Test 4 data for all groups revealed a significant effect of Group [F(3,36)= 17.65, p< 0.0001], with post- hoc comparisons indicating significantly greater ethanol intake in the CIE-GFP group compared to all other groups (ps< 0.001) (Figure 4C). Together, these findings indicate that overexpression of BDNF in mPFC selectively reduced dependence-driven escalation of drinking while not altering ethanol consumption in non-dependent mice.
Figure 4: BDNF overexpression in mPFC selectively reduces escalated drinking in ethanol dependent (CIE-exposed) mice.
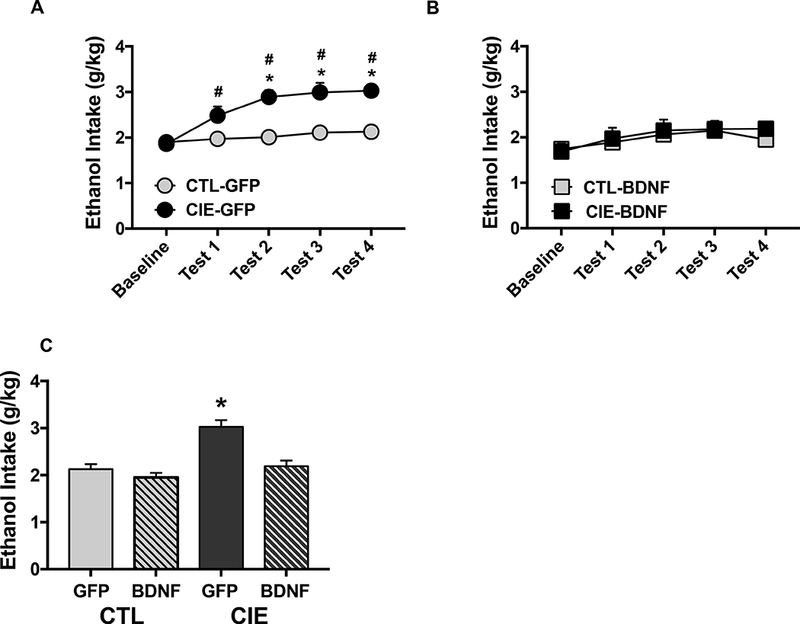
Active (AAV-BDNF) and control (AAV-GFP) virus was infused into the mPFC 2 weeks prior to baseline ethanol drinking. Data are presented as average weekly ethanol intake (g/kg) during the last week of baseline and four test cycles for (A) GFP-expressing CIE (N= 10) and CTL (N= 10) mice, and (B) BDNF-expressing CIE (N= 9) and CTL (N= 11) mice. Ethanol intake during Tests 1–4 increased over baseline level of intake in CIE-GFP mice (#, p< 0.01) and intake was greater in CIE-GFP compared to CTL-GFP mice during Tests 2, 3, and 4 (*, p< 0.001). (C) Ethanol intake (g/kg) for all groups during Test 4; ethanol consumption in CIE-GFP mice was greater than intake for all other groups (*, p< 0.001). Values ate mean ± s.e.m.
At the end of Test Cycle 4, mice were sacrificed to confirm viral expression or BDNF levels within the mPFC. A subset of mice from each group (N= 4–6/group) were evaluated to confirm location and extent of GFP fluorescence in mPFC (Figure 5A). The remaining mice were sacrificed, brains flash-frozen, and mPFC tissue samples punched from sections (Figure 5B) for later BDNF analysis (ELISA). Both CIE and CTL mice treated with the AAV-BDNF virus evidenced elevated BDNF levels in mPFC. ANOVA supported this impression, indicating a significant main effect of Group [F3,16)= 6.44, p< 0.005], and post-hoc tests indicated significantly higher BDNF levels in CIE-BDNF and CTL-BDNF groups compared to CIE and CTL mice treated with the control virus (ps< 0.05) (Figure 5C).
Figure 5: Verification of viral-mediated BDNF overexpression in mPFC.
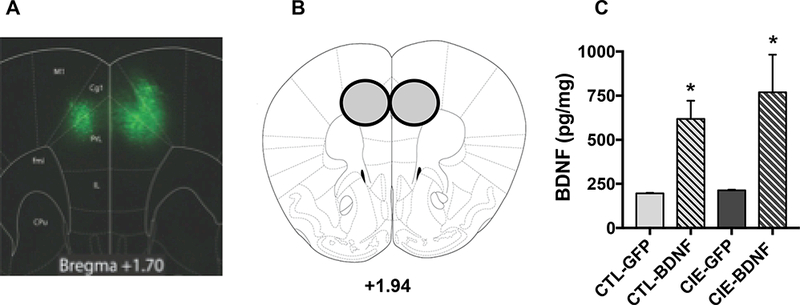
(A) Representative viral expression within the mPFC visualizing the native GFP tag. (B) Schematic of tissue punch for mPFC samples analyzed for BDNF protein levels. (C) BDNF protein content (pg/mg) in mPFC samples measured by ELISA. BDNF protein expression was significantly greater in CIE-BDNF and CTL-BDNF groups of mice compared to those infused with control virus (*, p< 0.05).
4. Discussion
Results of the present study show that ethanol dependence involving repeated cycles of CIE exposure produces a persistent reduction in BDNF protein content in mPFC. Replenishing this deficit by directly administering BDNF into the mPFC blocked escalated drinking already established in dependent mice without significantly altering more moderate levels of intake in nondependent mice. Furthermore, viral-mediated overexpression of BDNF in the mPFC prevented the development of escalated drinking ordinarily observed over successive, repeated cycles of CIE exposure. Collectively, these results implicate an important role for prefrontal cortical BDNF in mediating and/or promoting excessive levels of ethanol consumption associated with dependence.
Reduced BNDF protein expression in mPFC produced by CIE exposure is in general agreement with other studies demonstrating an inverse relationship between BDNF expression in the brain and propensity to consume ethanol. However, the relationship between BDNF and ethanol consumption is complex, varying with brain region and length of ethanol exposure. For example, acute or moderate levels of ethanol consumption have been shown to increase Bdnf mRNA and BDNF expression in dorsal striatum (Bahi and Dreyer, 2013; Jeanblanc et al., 2009; Logrip et al., 2009; McGough et al., 2004) and hippocampus (Stragier et al., 2015). In contrast, chronic ethanol exposure was reported to reduce BDNF mRNA and protein expression in forebrain structures such as hippocampus (Hauser et al., 2011) and cortex (Orru et al., 2016), but not nucleus accumbens (Darcq et al., 2014; Logrip et al., 2009; Tapocik et al., 2014). In some cases, reduced BDNF expression (in sub-nuclei of the amygdala) was only observed after withdrawal from chronic ethanol treatment (Pandey et al., 2008), while no changes in BDNF expression was observed in dorsal striatum even though this brain region was very sensitive to moderate ethanol exposure (Logrip et al., 2009).
To our knowledge, results from the present study are the first to report that direct injection of BDNF into the mPFC reduces ethanol consumption in mice with a history of dependence. The fact that this effect was only observed in dependent mice suggests that changes in cortical BDNF activity as a function of chronic ethanol exposure contribute, at least in part, to escalated drinking that is associated with dependence in this model. Since intra- mPFC injection of BDNF did not significantly alter moderate levels of ethanol consumption in nondependent mice, it is unlikely that the reduced intake in dependent animals was a nonspecific effect.
Similar to our microinjection findings, exogenous application of BDNF into the central and medial (but not the basolateral) nuclei of the amygdala reduced voluntary ethanol intake in rats. Further, antisense inhibition of endogenous BDNF in these regions increased ethanol drinking and behavioral measures of anxiety, with both effects reversed by co-infusion of BDNF (Pandey et al., 2006; Pandey et al., 2008). Interestingly, BDNF microinjection in the dorsolateral striatum was effective in reducing moderate ethanol consumption but did not reduce intake after long-term binge-like consumption (Darcq et al., 2016). Additionally, our results are generally consistent with those reporting that BDNF injection into the mPFC reduces cocaine seeking behavior (Barry and McGinty, 2017; Go et al., 2016; Sun et al., 2014; Whitfield et al., 2011). This suggests that manipulation of BDNF activity within the cortex influences addiction-related behaviors for ethanol and other substances of abuse.
Our finding that increased expression of BDNF in the mPFC prevents escalation of drinking associated with dependence further implicates an important role for the neurotrophic factor in the addiction process. It is interesting that viral-mediated overexpression of BDNF in the mPFC did not alter baseline level of ethanol intake, even though increased levels of BDNF protein content was observed within 2 weeks following injection of the virus (unpublished data). Further, overexpression of BDNF in the mPFC did not alter intake in nondependent mice but ethanol consumption was significantly reduced in dependent mice compared to CIE-exposed mice that received the control virus. This suggests that mitigating CIE-induced deficits in BDNF activity within the mPFC effectively prevents ethanol consumption from increasing above moderate levels of intake displayed by nondependent animals.
Other studies have similarly shown that viral-mediated increases in BDNF expression alter ethanol consumption, with effects dependent on the target brain region. For example, viralmediated increased BDNF expression in dorsal striatum reduced ethanol consumption while interference with BDNF production and its signaling effects via TrkB receptor activation increased ethanol drinking (Jeanblanc et al., 2009; Jeanblanc et al., 2006; Jeanblanc et al., 2013; Logrip et al., 2008; McGough et al., 2004). It has also been shown that excessive ethanol drinking in mice expressing the Met68BDNF polymorphism, which decreases activity-dependent BDNF release throughout the brain, can be reversed by overexpression of the Val68BDNF allele specifically in ventromedial prefrontal cortex (Warnault et al., 2016). These latter data complement our findings and further support the role of BDNF within the mPFC in the regulation of ethanol consumption.
The mechanism by which BDNF influences ethanol consumption and, in particular, excessive drinking associated with dependence is not fully understood. BDNF signaling through the TrkB receptor has been shown to play an important role in the regulation of ethanol drinking. For example, pharmacological antagonism of ERK/MAP kinase signaling down-stream of the TrkB receptor has been shown to reverse the suppressive effect of BDNF infusion in the dorsolateral striatum on ethanol intake (Jeanblanc et al., 2013). Similar studies have shown ERK/MAP kinase signaling down-stream of TrkB to mediate the suppressive effect of intra-mPFC BDNF on the reinstatement of cocaine seeking behavior (Barry and McGinty, 2017; Whitfield et al., 2011). Furthermore, activation of BDNF-TrkB receptor signaling is known to induce various transcription factors that not only regulate BDNF transcription itself, but also influence various other gene targets that are known to influence ethanol self-administration behavior, such as dopamine D3 receptors and neuropeptides (e.g., dynorphin, neuropeptide Y) (Jeanblanc et al., 2006; Jeanblanc et al., 2013; Logrip et al., 2008; Pandey et al., 2006; Pandey et al., 2008). While much of this ethanol-related work has focused on BDNF activity in subcortical structures (e.g., striatum, amygdala), future studies will need to determine whether similar signaling mechanisms underlie the ability of intra-mPFC BDNF treatment to selectively reduce escalated drinking associated with ethanol dependence.
5. Conclusion
In summary, a model of ethanol dependence involving repeated cycles of chronic intermittent ethanol exposure produced significant reductions in BDNF protein expression in mPFC that accompanied escalated ethanol consumption. To reverse this cortical deficit in BDNF, microinjection of BDNF into the mPFC selectively reduced excessive ethanol intake in dependent mice without altering more moderate and stable drinking in nondependent mice. Further, mitigation of chronic ethanol-induced BDNF reductions in mPFC by prior viral-mediated overexpression of BDNF in the mPFC prevented the escalation of drinking in dependent mice while not altering intake in nondependent mice. Collectively, these results indicate that chronic ethanol exposure produces adaptations in cortical BDNF, which play a significant role in contributing to excessive ethanol drinking associated with dependence. As such, these results have important clinical relevance given the increased interest in BDNF as a therapeutic target for various psychiatric disorders, including addiction (Nagahara and Tuszynski, 2011).
Highlights.
Ethanol dependence-related escalated drinking is accompanied by reduced BDNF protein expression in mPFC
Microinjection of BDNF into mPFC reversed ethanol dependence-related escalated drinking
Viral-mediated BDNF overexpression in mPFC prevented ethanol dependence-related escalated drinking
BDNF activity in mPFC may be a therapeutic target for reducing excessive drinking associated with ethanol dependence
Acknowledgements
The authors would like to thank Melissa Overstreet for her technical assistance. This research was supported by the Department of Veterans Affairs Medical Research grant (BLR&D BX000813) to HCB, and NIH/NIAAA grants (P50 AA010761, U01 AA014095, U24 AA020929, T32 AA007474) to HCB and grant R37 AA01684 to DR.
Footnotes
Declarations of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahi A, Dreyer JL, 2013. Striatal modulation of BDNF expression using microRNA124a- expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci 38, 2328–2337. [DOI] [PubMed] [Google Scholar]
- Barry SM, McGinty JF, 2017. Role of Src Family Kinases in BDNF-Mediated Suppression of Cocaine-Seeking and Prevention of Cocaine-Induced ERK, GluN2A, and GluN2B Dephosphorylation in the Prelimbic Cortex. Neuropsychopharmacology 42, 1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, 2012. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res 34, 448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, 2013. Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci 13, 355–377. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL, 1993. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res 17, 94–98. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, 2004. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Becker HC, Ron D, 2014. Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol 48, 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Hamida SB, Wu S, Phamluong K, Kharazia V, Xu J, Lombroso P, Ron D, 2014. Inhibition of striatal-enriched tyrosine phosphatase 61 in the dorsomedial striatum is sufficient to increased ethanol consumption. J Neurochem 129, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Morisot N, Phamluong K, Warnault V, Jeanblanc J, Longo FM, Massa SM, Ron D, 2016. The Neurotrophic Factor Receptor p75 in the Rat Dorsolateral Striatum Drives Excessive Alcohol Drinking. J Neurosci 36, 10116–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MI, 2008. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther 118, 36–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 2008. The Mouse Brain in Stereotaxic Coordinates, Third Edition. San Diego: Academic Press Third Edition. [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L, 2010. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev 35, 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go BS, Barry SM, McGinty JF, 2016. Glutamatergic neurotransmission in the prefrontal cortex mediates the suppressive effect of intra-prelimbic cortical infusion of BDNF on cocaine-seeking. Eur Neuropsychopharmacol 26, 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, 2014. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Becker HC, 2009a. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res 33, 1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC, 2009. b. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 201, 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M, 2008. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci 27, 1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y, 2011. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE, 2003. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem 85, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D, 2009. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci 29, 13494–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, Ron D, 2006. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci 26, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Logrip ML, Janak PH, Ron D, 2013. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci 37, 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2013. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 4, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008. Addiction and the brain antireward system. Annu Rev Psychol 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D, 2008. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J 22, 2393–2404. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D, 2009. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem 109, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC, 2005. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181, 688–696. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Kimura M, Miyakawa T, Yoshino A, Murayama M, Masaki T, Higuchi S, 2004. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol Clin Exp Res 28, 1609–1612. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D, 2004. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci 24, 10542–10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC, 2012. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol 17, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC, 2011. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol 16, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH, 2011. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10, 209–219. [DOI] [PubMed] [Google Scholar]
- Ninan I, 2014. Synaptic regulation of affective behaviors; role of BDNF. Neuropharmacology 76 Pt C, 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru A, Caffino L, Moro F, Cassina C, Giannotti G, Di Clemente A, Fumagalli F, Cervo L, 2016. Contingent and non-contingent recreational-like exposure to ethanol alters BDNF expression and signaling in the cortico-accumbal network differently. Psychopharmacology (Berl) 233, 3149–3160. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T, 2004. Partial deletion of the cAMP response elementbinding protein gene promotes alcohol-drinking behaviors. J Neurosci 24, 5022–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K, 2006. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxietylike behaviors. J Neurosci 26, 8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K, 2008. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci 28, 2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC, 2008. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res 32, 909–920. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ, 2009. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 56 Suppl 1, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A, Nanjappa V, Narayana J, Somani BL, Mukherjee KK, Pandey A, Christopher R, Prasad TS, 2013. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal 7, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier E, Massart R, Salery M, Hamon M, Geny D, Martin V, Boulle F, Lanfumey L, 2015. Ethanol-induced epigenetic regulations at the Bdnf gene in C57BL/6J mice. Mol Psychiatry 20, 405–412. [DOI] [PubMed] [Google Scholar]
- Sun WL, Eisenstein SA, Zelek-Molik A, McGinty JF, 2014. A single brain-derived neurotrophic factor infusion into the dorsomedial prefrontal cortex attenuates cocaine self-administration-induced phosphorylation of synapsin in the nucleus accumbens during early withdrawal. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, King C, Heilig M, 2014. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci 34, 4581–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ, 2014. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R, 2008. Neuropharmacology of alcohol addiction. Br J Pharmacol 154, 299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, Longo FM, Ron D, 2016. The BDNF Valine 68 to Methionine Polymorphism Increases Compulsive Alcohol Drinking in Mice That Is Reversed by Tropomyosin Receptor Kinase B Activation. Biol Psychiatry 79, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW Jr., Shi X, Sun WL, McGinty JF, 2011. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci 31, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M, 2009. Association between Val66Met brain-derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res 33, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QS, Feng MJ, Yan SE, 2005. Different expression of brain-derived neurotrophic factor in the nucleus accumbens of alcohol-preferring (P) and -nonpreferring (NP) rats. Brain Res 1035, 215–218. [DOI] [PubMed] [Google Scholar]


