Abstract
Vasoactive intestinal peptide (VIP) and its G protein-coupled receptors, VPAC1R and VPAC2R, are prominent in the immune system and regulate many aspects of T cell-dependent immunity. In mouse T cells, VPAC1R is expressed constitutively, whereas VPAC2R is induced by immune stimuli. VPAC2R-null (VPAC2R−/−) mice on a C57BL/6 background are shown here to have normal basic immune characteristics, including serum Ig concentrations, blood levels of all leukocytes, and spleen number of total T cells (CD3+) and T cells bearing CD4, CD8, and CD28. Hapten-evoked cutaneous delayed-type hypersensitivity (DTH) was significantly enhanced in VPAC2R-null mice compared with age- and sex-matched wild-type mice. In contrast, generation of IgE anti-hapten antibodies and active cutaneous anaphylaxis were ≥70% lower in VPAC2R-null mice than in wild-type controls. Cytokine production by splenic CD4+ T cells, stimulated with adherent anti-CD3 plus anti-CD28 antibodies, revealed higher levels of IL-2 (mean = 3-fold) and IFN-γ (mean = 3-fold), and lower levels of IL-4 (mean = one-fifth) in VPAC2R-null mice than wild-type controls. Loss of VIP-VPAC2R maintenance of the normal ratio of Th2/Th1 cytokines thus leads to a state of enhanced DTH and depressed immediate-type hypersensitivity, which may alter both host defense and susceptibility to immune-mediated diseases.
Keywords: immunity‖T cell‖neuropeptide‖cytokine‖IgE
Vasoactive intestinal peptide (VIP) is produced by cholinergic and sensory nerves, including those in thymus, spleen, and lymph nodes, and by T cells (1–3). VIP has potent effects on T cell differentiation, migration, and generation of diverse cytokines (4–12). Production of some cytokines by the two subsets of mouse helper T (Th) cells in vitro is regulated differentially by VIP. VIP inhibits release of IL-2 from mouse type 1 Th (Th1) cells, which mediate classical delayed-type cellular immunity, and from type 2 Th (Th2) cells and enhances release of IL-5 from mouse Th2 cells, which mediate acute and subacute hypersensitivity reactions, such as allergy. Effects of VIP on the generation of many other cytokines by Th cells, however, is variably dependent on the source and state of activation of the Th cells. Type I G protein-coupled VIP receptors (VPAC1Rs) are highly expressed constitutively by unstimulated Th cells in mouse blood and spleen, whereas the homologous VPAC2Rs are expressed at low levels or absent (13–18). However, VPAC2Rs are up-regulated to high levels and VPAC1Rs down-regulated by Th cell stimulation, suggesting that VPAC2Rs are the dominant transducer of effects of VIP on activated Th cells (19–22). Investigations of the capacity of VIP to suppress Th1-mediated delayed-type hypersensitivity (DTH) and to enhance Th2-dependent immediate-type hypersensitivity reactions in vivo through VPAC2Rs have been hampered by the lack of effective pharmacological agents. A transgenic (TG) mouse model has been developed in which normally inducible VPAC2Rs are constitutively expressed in CD4+ (helper-inducer) T cells of the Th1 cell-dominant C57BL/6 strain of mice, at levels similar to those observed at the peak of hypersensitivity responses (23). In this model, endogenous VIP decreases the ratio of Th1 cell-derived to Th2 cell-derived cytokines. As a result, these VPAC2R TG mice have elevated blood IgE, IgG1, and eosinophils with increased IgE antibody responses, which heighten cutaneous allergic reactions, but have depressed DTH. Here, we describe the complementary C57BL/6 mouse model, where VPAC2R-null mice were generated by targeted insertion of a mutation in exon 1 of the VPAC2R gene. In VPAC2R-null mice, endogenous VIP fails to suppress the ratio of Th1-/Th2-type cytokines, which results in enhanced DTH and reduced immediate-type hypersensitivity.
Materials and Methods
Generation of VPAC2R−/− (VPAC2R-Null) Mice.
The VPAC2R-null mice were created initially on a mixed C57BL/6 × 129ola genetic background. A lacZ-neoR cassette was inserted into the first coding exon of the VPAC2R gene by gene targeting in 129o1a embryonic stem cells. Mouse blastocysts were injected with four correctly targeted clones to produce chimeric mice, from which was obtained germ-line transmission of the mutant allele. After backcrossing with the C57BL/6 strain for six generations, heterozygous mice were mated to produce the VPAC2R−/− (VPAC2R-null) mice and littermate controls used in the present study.
Enumeration of Populations of Leukocytes in Blood and Spleen of Wild-Type and VPAC2R-Null Mice.
Each 100-μl aliquot of mouse tail vein blood was transferred to an EDTA microtainer (Becton-Dickinson) and introduced into a Hemavet 850 Mascot model blood cell counter (CDC Technologies, Oxford, CT), which had been calibrated with a standard mixture of mouse leukocytes. Suspensions of mononuclear leukocytes from blood and spleen also were labeled with a panel of fluorescent antibodies (BD-PharMingen) specific for CD3 (total) T cells, CD4 T cells, CD8 T cells, CD25 T cells, and CD28 T cells, as well as CD19 (B cells) for flow cytometric analyses (FACScan, BD-Biosciences).
Quantification of mRNA Encoding VPAC1R and VPAC2R in Mouse Immune Cells.
RNA was extracted from replicate 20-μg to 100-μg fragments of diverse tissues and suspensions of 2 to 5 × 106 immunomagnetically purified immune cells of different types (Miltenyi Biotec, Auburn, CA) by a standard TRIzol method (Life Technologies, GIBCO/BRL, Grand Island, NY) for oligo(dT)-primed synthesis of first-strand cDNAs (Superscript RT, Life Technologies). Real-time PCR amplification and analyses of mouse VPAC1R and VPAC2R, and of mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and other stable standards was performed in a Perkin–Elmer-Applied Biosystems PRISM 7700 Sequence Detector with a standard TaqMan PCR reagent kit(Applied Biosystems User Bulletin No. 2, PRISM 7700 Sequence Detection System, 1997). The mouse VPAC1R primers were 5′-AACTTTAAGGCCCAGGTGAAAAT-3′and 5′-CCTGCACCTCGCCATTG-3′, and the TaqMan probe was 5′-FAM-TTGTGGTGGCCATCCTCTACTGCTTCCATAMRA-3′. The mouse VPAC2R primers were 5′-TGAGCCCAAGATGAGGGC-3′ and 5′-GTTCACTCACCCGCACCAG-3′, and the TaqMan probe was 5′-FAM-TGACCTGCTACTGCTG-TAMRA-3′. The mouse GAPDH primers were 5′-TGCACCACCAACTGCTTAG-3′ and 5′-GGATGCAGGGATGATGTTC-3′, and the TaqMan probe was 5′-fluorescein-CAGAAGACTGTGGATGGCCCCTC-3′. The TaqMan probe end-labels were 5-(and 6-)carboxy-tetramethylrhodamine (TAMRA; quencher fluorochrome) conjugated to the 3′-terminal nucleotide and 6-carboxy-fluorescein (FAM) or fluorescein (reporter fluorochrome) linked to the 5′-terminal nucleotide (Integrated DNA Technologies, Coralville, IA). Values for the prevalence of expression of VPAC1R- and VPAC2R-encoding mRNA in different tissues and populations of immune cells were derived by subtracting the threshold cycle (CT) value for mouse GAPDH from the respective CT values for VPAC1R and VPAC2R. This CT difference then was expressed as a ratio relative to the CT difference for a reference tissue or cell, which here was liver for organ extracts and B cells for immune cell extracts, for which the levels were the lowest of each source category to be reliably quantified and thus were set at 1.0.
Determination of VPAC1R and VPAC2R Proteins by Western Blots.
Western blots of proteins extracted from tissues and purified sets of immune cells were performed as described (23, 24) and developed with rabbit polyclonal anti-amino-terminal peptide antibodies to VPAC1R (amino acids 122–134) and VPAC2R (amino acids 105–122; refs. 24 and 25).
Quantification of Serum Ig Concentrations.
All measurements were performed with two dilutions of serum from tail-vein blood of 8- to 12-week-old mice that had been anesthetized with methoxyflurane (Metofane, Schering-Plough). ELISA kits for total IgG (Cygnus Technologies, Plainville, MA), IgG1, IgG2a, IgA, IgM (Bethyl Laboratories, Montgomery, TX), and IgE (Crystal Chemical, Chicago, IL) were used as directed.
Isolation of Spleen Immune Cells and Assessment of Cytokine Generation.
Spleens were removed from groups of five to six VPAC2R-null and wild-type mice at 8–12 weeks of age. Splenic tissues were minced and pushed through 70-μm pore nylon filters (Falcon, Becton Dickinson Labware) to disaggregate cells in RPMI medium 1640 with 10% FBS, 100 units/ml of penicillin G, and 100 μg/ml of streptomycin (complete RPMI). Erythrocytes and granulocytes were removed by centrifugation through Ficoll-Hypaque (Amersham Pharmacia-Pharmacia, Biotec) at 20°C for 15 min at 300 × g, and adherent mononuclear leukocytes were removed by incubation in polystyrene Petri dishes at 37°C for 45 min in 5% CO2 in air. Each T cell-enriched suspension was washed three times and resuspended in 400 μl of 0.02 M sodium phosphate-buffered 0.12 M NaCl (pH 7.3) with 2 mM EDTA and 0.5 g/100 ml of fatty acid-free BSA [Calbiochem-Nova Biochem; immunopurification (IP) buffer]. Twenty micrograms per 107 cells of biotin-conjugated anti-CD4 monoclonal mouse antibody (BD-PharMingen) were added to each suspension, followed by incubation for 60 min at 8°C. After reaction with antibody, splenic cells then were washed twice, resuspended in 400 μl of IP buffer, and incubated with 25 μl/107 cells of streptavidin-conjugated metallic beads (Miltenyi Biotec) for 30 min at 4°C. Some splenic cell suspensions were incubated directly with anti-CD4 monoclonal antibody-bearing metallic beads for 60 min at 8°C. Antibody-labeled splenic cells were washed twice and resuspended in 1 ml of IP buffer for 2 cycles of magnetic column chromatography, which yielded CD4+ T cells at over 96% purity, as assessed by analytical flow cytometry. Replicate 0.5-ml aliquots of 3 × 105 CD4+ T cells in complete RPMI medium were preincubated with 10−9 M to 10−6 M purified synthetic VIP or VPAC1R- or VPAC2R-selective peptide analogs of VIP, and stimulated with 0.5 μg/well each of adherent anti-CD3 and anti-CD28 mouse monoclonal antibodies (BD-PharMingen). VIP, the VPAC1R-selective peptide [K15,R16,L27]VIP(1–7)/GRF(8–27), and the VPAC2R-selective peptide Ac-[E8,OCH3-Y10,K12,NL17,A19,D25,L26,K27,28]VIP cyclo (21–25) were synthesized and purified as described (26, 27). After 24 h and 96 h at 37°C, plates were centrifuged, and supernatant medium was harvested for ELISA assays of IL-2, IL-4, IL-10, and IFN-γ (Endogen, Cambridge, MA). Cytokine concentrations are provided as pg/ml or ng/ml, and as a percentage of the T cell receptor-stimulated CD4+ T cell positive control (100%). A composite index designed to describe relative changes in the ratio of IFN-γ (Th1 cells) to IL-4 (Th2 cells) generated by CD4+ T cells was calculated from values without and with exogenous VIP or a selective VPACR agonist (see Table 3). Thus, no change in IFN-γ/IL-4 would be 1.0, whereas a concurrent 50% decrease in IFN-γ and two-fold increase in IL-4 would lead to a change in ratio of 0.25.
Table 3.
Effects of VIP and VPAC2R-selective agonists on the ratio of Th1/Th2- cytokines generated by CD4+ T cells of VPAC2R-null and matched wild-type mice after T cell receptor stimulation (change in IFN-γ/IL-4 secretion)
| VIP
|
VPAC2R agonist, 10−6 M | VPAC1R agonist, 10−6 M | |||
|---|---|---|---|---|---|
| 10−9 M | 10−8 M | 10−7 M | |||
| VPAC2R-null | 1.02+ | 1.00+ | 1.08+ | 0.91+ | 1.35 |
| Wild type | 0.30 | 0.29 | 0.32 | 0.27 | 0.98 |
Each value is the mean of 96 h results for groups of six mice. Statistical significance of differences between the two groups was calculated and depicted as in Fig. 2.
Determination of Expression of Cutaneous Delayed-Type Hypersensitivity.
Groups of six 8- to 16-week-old wild-type and VPAC2R-null mice were immunized s.c. in two sites, one on each flank, by using 1 mg per site of 4-hydroxy-3-nitrophenylacetyl-hydroxysuccinimide ester (NP OSu; Biosearch) in 40 μl of dimethyl-sulfoxide, followed by 100 μl of 0.05 M sodium borate-buffered 0.1 M NaCl (pH 8.6) in the dorsal midline skin. Six days later, one rear footpad was challenged with 40 μg of NP OSu in 25 μl of PBS, and the opposite rear footpad received 25 μl of PBS alone. Footpad thickness was quantified before, and 24 h and 48 h after, the challenge injection, by using a calibrated digital read-out micrometer with 0.025 mm resolution (Fisher Scientific). After euthanasia, the rear paws were removed uniformly at the ankle joint and weighed. Each NP OSu-induced increase in footpad thickness was expressed as a percentage of that before injection and the increase in rear foot weight as a percentage of the weight of the PBS-injected foot. The significance of differences between swelling and weight evoked by NP OSu and by PBS alone in each group of mice was calculated with a standard Student's paired t test.
Assessment of Serum Concentration of IgE Anti-Trinitrophenyl (TNP) Antibodies and Active Cutaneous Anaphylaxis (ACA).
Groups of five 8-week-old VPAC2R-null mice and wild-type control mice were immunized i.p. with 10 μg of TNP-derivatized keyhole limpet hemocyanin (TNP-KLH) (Biosearch) adsorbed to 0.2 mg of Al(OH)3 (primary). Each mouse was boosted 14 days later i.p. with 10 μg of TNP-KLH (secondary). IgE anti-TNP levels in 14-day and 21-day tail vein sera were quantified by ELISA by using 0.5 μg of TNP-CGG (Biosearch) per well of 96-well plates, and then in sequence 0.1 ml of serum diluted 1/10, 1/30, and 1/100 and then 0.1 ml of a 1:20,000 dilution of horse radish peroxidase-conjugated goat anti-mouse IgE (Bethyl Laboratories), with five washes between steps, and standard development reagents. Values of absorbancy were converted to ng/ml by using a curve generated with monoclonal IgE anti-TNP (BD-PharMingen). Groups of mice then were challenged 7 days after primary and secondary immunization with 0.5 μg and 2.0 μg of TNP-CGG (Biosearch) in 10 μl of PBS in multiple cutaneous sites on the flanks and PBS alone in several sites, within 5 min after 0.4 ml of 0.5% (wt/vol) Evans blue intravenously. Mice were euthanized and flank skin reflected to allow measurements of the mean diameter of blue; the diameters of TNP-CGG-induced reactions were corrected by subtraction of that of PBS control sites.
Results
Characteristics of VPAC2R-Null Mice.
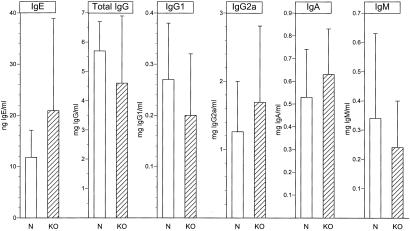
VPAC2R-null mice were normal in general health, social behavior, and breeding patterns. There have been no unexpected infections or inflammatory disorders. Blood levels of the major classes of leukocytes are no different from those of normal C57BL/6 mice housed in the same facility (Table 1). The size and basic lymphocyte composition of their spleens also are indistinguishable from normal (Table 2). Serum Ig levels of unchallenged VPAC2R-null mice are identical to those of normal C57BL/6 mice (Fig. 1).
Table 1.
Levels of blood leukocytes in VPAC2R-null and matched wild-type control C57BL/6 mice (×1,000 per microliter)
| Total Leuk | Neutr | Lymph | Mono | Eos | Bas | |
|---|---|---|---|---|---|---|
| Wild type | 4.22 ± 1.63 | 0.69 ± 0.29 | 3.10 ± 1.10 | 0.28 ± 0.17 | 0.12 ± 0.08 | 0.03 ± 0.02 |
| VPAC2R−/− | 4.20 ± 1.07 | 0.64 ± 0.19 | 3.25 ± 0.88 | 0.21 ± 0.06 | 0.07 ± 0.03 | 0.02 ± 0.01 |
Each value is the mean ± SD of the results of leukocyte counts from tail vein blood of five 8-week-old mice in each group. Total Leuk, total of all types of leukocytes; Neutr, neutrophils; Lymph, all types of lymphocytes; Mono, monocytes; Eos, eosinophils; and Bas, basophils.
Table 2.
Distribution of splenic T cells from wild-type and VPAC2R-null mice (mean % of total viable mononuclear leukocytes; n = 6)
| CD3+ (Total T Cells) | CD4+ | CD8+ | CD28+ | |
|---|---|---|---|---|
| Wild type | 45 | 20 | 30 | 39 |
| VPAC2R-null | 47 | 21 | 32 | 40 |
Figure 1.
Serum Ig concentrations in VPAC2R-null (KO) mice and sex- and age-matched wild-type control (N) mice. Each bar symbol depicts the mean and SD of the results of ELISAs for sera of 28 wild-type and 20 VPAC2R-null mice. There are no significant differences between the groups.
TaqMan real-time PCR analyses of mouse VPAC2R detected no message in splenic mixed T cells, CD4+ T cells or CD8+ T cells of VPAC2R-null mice before or after activation by incubation on adherent anti-CD3 plus anti-CD28 antibodies for 4 days, as for subsequent studies of cytokine generation. In contrast, the expected mean relative copy number of mouse VPAC2R was detected in T cells of wild-type mice and was increased significantly by incubation on adherent anti-CD3 plus anti-CD28 antibodies for 4 days (23). Real-time PCR determination of expression of mouse VPAC1R by splenic T cells showed no significant differences between the mean relative copy numbers for VPAC2R-null mice (0.98) and wild-type C57BL/6 mice (1.00).
Delayed-Type and Immediate-Type Hypersensitivity.
Transgenic C57BL/6 mice expressing human VPAC2 receptors constitutively and selectively in CD4+ T cells have high levels of IgE and eosinophils, mount enhanced immediate-type hypersensitivity reactions, and manifest depressed DTH (23). It was anticipated therefore that the VPAC2R-null mice might show diminished immediate-type hypersensitivity and greater than normal DTH. When groups of mice were sensitized intradermally and challenged 6 days later in rear footpads with the hapten NP OSu, swelling measured by two methods was significantly greater at 24 h and 48 h in the VPAC2R-null mice than in wild-type C57BL/6 mice (Fig. 2). For neither type of mice was there detectable swelling in the rear footpads after challenge with PBS alone. In contrast, both the serum level of IgE anti-TNP antibodies and the magnitude of immediate cutaneous reactions to TNP-CGG induced by immunization with TNP-derivatized keyhole limpet hemocyanin were lower in VPAC2R-null mice than wild-type C57BL/6 mice (Fig. 3).
Figure 2.
Greater expression of delayed-type hypersensitivity by VPAC2R-null (KO) mice than matched wild-type control (N) mice. The left-hand value of each pair is the result of quantification by digital micrometry and the right-hand value is that determined by weighing the rear paws after euthanasia. Each value is the mean ± SD of data from studies of 10 mice in each group. The ranges of paw thicknesses before injections were 2.29 mm to 2.81 mm for normal mice and 2.26 mm to 2.53 mm for the KO mice (0% base for calculation of NP OSu-induced increases) and the ranges of weights of PBS-injected paws were 115.3 mg to 132.5 mg for normal mice and 108.3 mg to 143.3 mg for KO mice at 24 h (0% base for NP OSu-induced increases). Statistical significance was evaluated by Student's t test. +, P < 0.05; *, P < 0.01.
Figure 3.
Lower serum concentrations of IgE anti-TNP antibody and lesser active cutaneous anaphylactic reactions to TNP in VPAC2R-null (KO) mice than sex- and age-matched wild-type control (N) mice. Each bar symbol in both frames is the mean ± SD of the results for two groups of six mice. Statistical analyses and symbols for significance are the same as in Fig. 2.
Deviation of the Normal Pattern of Th1/Th2 Cytokines.
The principal basis for enhancement of immediate-type hypersensitivity in T cell-selective VPAC2R transgenic C57BL/6 mice is lower generation of IFN-γ and higher production of IL-4 and Il-5 by stimulated CD4+ T cells than by CD4+ T cells of wild-type mice (23). The production of cytokines by splenic CD4+ T cells activated with adherent anti-CD3 plus anti-CD28 antibodies was compared for VPAC2R-null mice and wild-type C57BL/6 mice. Secretion of the critical cytokine IL-2 by splenic stimulated Th1 and, in lesser amounts, Th2 CD4+ T cells of VPAC2R-null mice was 3.6-fold and 4.7-fold higher after 24 h and 96 h, respectively, than that from wild-type mice (Fig. 4). The higher level of IL-2 attained by CD4+ T cells of VPAC2R-null mice than of wild-type mice is presumably because of loss of VPAC2R-mediated suppression of IL-2 production by endogenous VIP in the former group and sustained VPAC2R-mediated suppression in the latter group. This result also implies a lack of effective compensatory suppression through the normal level of VPAC1Rs in the VPAC2-null mice.
Figure 4.
Higher production of IL-2 by TCR-stimulated CD4+ T cells of VPAC2R-null (KO) than sex- and age-matched wild-type control (N) mice. Each bar symbol depicts the mean ± SD of data from three studies of groups of six mice. Statistical analyses and symbols for significance are the same as in Fig. 2.
It was next determined whether the lower level of IL-2 from wild-type splenic CD4+ T cells, attributed to prolonged suppression by the VPAC2R-endogenous VIP axis, could be further diminished by exogenous VIP. As expected, the VPAC2R-selective cyclic peptide agonist further reduced IL-2 secretion by CD4+ T cells of wild-type mice, but not of VPAC2R-null mice (Fig. 4). In contrast, the VPAC1R-selective agonist, at a concentration equal to that of the VPAC2R-selective agonist, inhibited IL-2 secretion by the two populations of CD4+ T cells indistinguishably. The effects of physiologically attainable levels of native VIP reflected principally its action through the VPAC2R. Native VIP at 10−9 M and 10−8 M significantly suppressed IL-2 release from stimulated wild-type CD4+ T cells, but not from stimulated VPAC2R-null CD4+ T cells at 24 h and 96 h (Fig. 4). The suppression of IL-2 release from stimulated VPAC2R-null and wild-type CD4+ T cells by the pharmacological level of 10−7 M VIP was similarly significant, presumably because of sufficient mediation by an equivalent number of VPAC1Rs (Fig. 4).
Investigations of secretion of two Th subtype-defining cytokines by splenic stimulated CD4+ T cells of VPAC2R-null mice revealed substantial deviation from the pattern observed for wild-type C57BL/6 mice (Fig. 5). The mean levels of IL-4 attained by stimulated VPAC2R-null CD4+ T cells were only 62% and 25% of those observed with wild-type CD4+ T cells at 24 h and 96 h, respectively. The correspondingly higher mean concentrations of IFN-γ achieved by stimulated VPAC2R-null CD4+ T cells at 24 h and 96 h were 360% and 676%, respectively, of those from wild-type CD4+ T cells (Fig. 5). In two of the studies, IFN-γ enzyme-linked immunospot (ELISpot) assays (R & D Systems) of the stimulated CD4+ T cells from VPAC2R-null mice and wild-type mice for intracellular IFN-γ after 96 h revealed a mean of 21.6% and 19.8% positive, respectively (P > 0.1). Thus, endogenous exposure to VIP failed both to enhance IL-4 and to inhibit IFN-γ production and secretion by CD4+ T cells of VPAC2R-null mice, in contrast to the effects observed in wild-type C57BL/6 mice. Thus, the ratios of IFN-γ to IL-4 secreted by CD4+ T cells of VPAC2R-null mice were higher than those for CD4+ T cells of wild-type C57BL/6 mice, with values of 5.8 at 24 h and 27 at 96 h, compared with normalized values of 1.0 at both times for wild-type mice.
Figure 5.
Differences in production of IL-4 and IFN-γ by TCR-stimulated CD4+ T cells of VPAC2R-null (KO) and sex- and age-matched wild-type control (N) mice. Each bar and bracket depicts the mean ± SD of data from three studies of groups of six mice. Statistical analyses and symbols for significance are the same as in Fig. 2.
As for IL-2 secretion, the differences in ratios of IFN-γ to IL-4 secreted by stimulated CD4+ T cells of VPAC2R-null mice and of wild-type mice deviated further after in vitro introduction of exogenous native VIP or a VPAC2R-selective agonist. VIP at 10−9 M to 10−7 M significantly reduced IFN-γ secretion from splenic stimulated CD4+ T cells of wild-type mice after 96 h, relative to only marginal suppression of IFN-γ secretion from the CD4+ T cells of VPAC2R-null mice. At these same concentrations, VIP enhanced IL-4 secretion from stimulated CD4+ T cells of wild-type mice more than that from VPAC2R-null mice. Thus, the changes in ratio of IFN-γ to IL-4 were lower for fully responsive CD4+ T cells of wild-type mice than for dysregulated CD4+ T cells of VPAC2R-null mice at all concentrations of VIP (Table 3). The predominant dependence of effects of native VIP on VPAC2Rs was confirmed in the same studies by the capacity of a VPAC2R-selective agonist to reduce IFN-γ secretion and increase IL-4 secretion from CD4+ T cells of wild-type mice more than from those of VPAC2R-null mice (Table 3). In contrast, the lack of significant effect of a VPAC1R-selective agonist on IFN-γ and IL-4 secretion in the same studies were not significantly different for the two sets of stimulated CD4+ T cells, presumably reflecting the equivalent number and activity of VPAC1Rs.
Discussion
The importance of VIP in immunity was suggested initially by the high concentrations established in immune organs from sources in regional nerves and some T cells, and by the breadth of potent effects on T cell development and functions (2, 3, 12, 19). This possibility was supported by finding independent and opposite regulation of expression of constitutive VPAC1Rs and inducible VPAC2Rs on T cells by Th2 cell-derived cytokines and by stimulation of T cell antigen receptors (TCRs; ref. 12, 19, and 22). That VIP may even act as a Th2 cell cytokine has been considered based both on Th2 cells being the principal immune source of VIP and on the greater sensitivity of Th2 cells than Th1 cells to some effects of VIP (28). Higher responses of Th2 cells than Th1 cells to VIP have been demonstrated in studies of inhibition of their terminal differentiation and of VIP protection from activation-induced apoptosis (28). The differentially greater susceptibility of Th2 cells than Th1 cells to effects of VIP led to the prediction that shifts in the balance of production of Th1/Th2 cytokines would be one critical immune consequence of alterations in expression of VPAC2Rs on Th cells. Such modifications in the ratio of Th1/Th2 cytokines are fundamental to the distinctively different immune phenotypes of both VPAC2R-null and TG mice.
There are three possible reasons why modifying the capacity of Th cells to detect and respond to a single endogenous neuropeptide results in strikingly altered immune phenotypes. The first is the quantitative predominance of VIP in peptidergic neurons supplying immune organs, with especially dense representation in T cell corridors and T cell-rich follicles, and the preferential generation of VIP by Th cells (2, 17, 19, 29). Interstitial fluids and secretions reflecting these sources frequently manifest VIP concentrations of up to 10 nM (17). The second is the high density of VPACRs in respiratory and gastrointestinal mucosa-associated lymphoid tissues and newly established DTH reactions, where up to 70% of T cells manifest expression of VPAC1Rs or VPAC2Rs as contrasted with 1% to 3% of blood T cells (17, 30). The third apparent reason is the magnitude, persistence, and Th cell subset specificity of the effects of VIP on cytokines critical for the regulation of Th1 to Th2 balance. Deletion of the inducible T cell VPAC2R-VIP axis in the present knock-out (KO) model thus increases the ratio of Th1/Th2 cell activities, which is reflected in a higher IFN-γ/IL-4 ratio in products of TCR-stimulated splenic CD4+ T cells (Fig. 4, 5). The augmented Th1/Th2 profile of VPAC2R-null mice leads to heightened DTH and diminished immediate-type hypersensitivity (Fig. 2, 3). In contrast, isolated elevation of expression of CD4+ T cell VPAC2Rs, to levels observed after immune activation of CD4+ T cells, in a TG model in the same strain of mice promotes functional dominance of Th2 cells, as reflected in a higher IL-4 and IL-5/IFN-γ ratio (23). The immune phenotypic consequences for VPAC2R TG mice are enhanced allergic reactions and depressed DTH responses (23). The combined impact of the level of VPAC2R expression and of persistent effects of endogenous VIP is documented by the observation that most of the maximal deviation of Th1/Th2 cytokines secreted by CD4+ T cells in vitro is attributable to prior sustained in vivo exposure to native VIP rather than to that added in culture (Fig. 4, 5).
Results of prior in vitro studies of effects of VIP on T cell functions would permit predictions of only some of the immune abnormalities observed in the VPAC2R-null mice and VPAC2R TG mice. The capacity of VIP to inhibit IL-2 generation at a transcriptional level is well-established (4). Therefore, loss of the major inducible VPAC2R in VPAC2R-null mice would be expected to enhance production of IL-2 and some solely Th1 cytokines and thereby DTH reactions. That IFN-γ secretion by CD4+ T cells also is increased in VPAC2R-null mice would not be simply predictable, as levels in vitro have been reported to be increased, decreased, or unchanged, depending on many aspects of the test system, including the variable expression of VPAC2Rs in differently stimulated sets of mouse splenic T cells (12). Such increases in IFN-γ in VPAC2R-null mice are consistent with observed decreases in IFN-γ generation by similarly stimulated CD4+ T cells of VPAC2R TG mice (23). Increases in IFN-γ in VPAC2R-null mice may be secondary to the 57% to 68% mean decreases in IL-10 generation by anti-CD3 plus anti-CD28 antibody-stimulated splenic CD4+ T cells documented in two studies of groups of five wild-type and VPAC2R-null mice. The prominently lower production of IL-4 by stimulated CD4+ T cells of VPAC2R-null mice (Fig. 5) may be attributable to the unmasked dominance of a normal level of VPAC1Rs, known to transduce suppression of IL-4 and IL-10, rather than the loss of VPAC2Rs, for which less is known of isolated effects on Th2-type cytokine generation by mouse CD4+ T cells.
There are many unanswered questions relevant both to mechanisms and biological implications of altered levels of activity of the VPAC2R-VIP axis in T cells. Those which can be approached simply with existing technologies include delineation of the neural and T cell sources of VIP in each organ and definition of the types of immune responses affected by VPACRs. The relative roles of native VPAC1Rs and VPAC2Rs in immune responses may be defined partially with the available selective agonists and after studies of mouse VPAC1R KO and TG models. However, more definitive understanding may require development of selective and bioavailable antagonists for VPAC1Rs and VPAC2Rs. The value of pursuing these lines of investigation is already supported by current demonstrations of major immune system perturbations mediated solely through one subfamily of neuropeptide receptors on subsets of T cells. It cannot yet be predicted, however, whether such neuroregulatory effects are of sufficient magnitude to alter the courses of infections, inflammation, and autoimmune diseases in which T cell-dependent effector pathways have a major role.
Acknowledgments
We thank Amy Choi for assistance with mouse studies and Robert Chan for expert graphics. This research was supported by Grant AI 29912 from the National Institutes of Health.
Abbreviations
- VIP
vasoactive intestinal peptide
- VPAC1 and VPAC2
types I and II G protein-coupled receptors for VIP
- CT
threshold cycle
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Th cells
helper T cells
- TNP
trinitrophenyl
- TNP-CGG
TNP-derivatized chicken gamma globulin
- NP OSu
4-hydroxy-3-nitrophenylacetyl-hydroxysuccinimide ester
- KO
knock-out or null
- TCR
T cell receptor
- DTH
delayed-type hypersensitivity
- TG
transgenic
References
- 1.Said S I, Mutt V, editors. Ann NY Acad Sci. 1988;527:1–691. [Google Scholar]
- 2.Gomariz R P, Leceta J, Garrido E, Garrido T, Delgado M. Regul Pept. 1994;50:177–184. doi: 10.1016/0167-0115(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger D L, Lorton D, Brouxhon S, Felten S, Felten D L. Adv Neuroimmunol. 1996;6:5–27. doi: 10.1016/s0960-5428(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 4.Mathew R C, Cook G A, Blum A M, Metwali A, Felman R, Weinstock J V. J Immunol. 1992;148:3572–3577. [PubMed] [Google Scholar]
- 5.Sun L, Ganea D. J Neuroimmunol. 1993;48:59–66. doi: 10.1016/0165-5728(93)90059-8. [DOI] [PubMed] [Google Scholar]
- 6.Johnston J A, Taub D D, Lloyd A R, Conlon K, Oppenheim J J, Kevlin K V. J Immunol. 1994;153:1762–1768. [PubMed] [Google Scholar]
- 7.Martinez C, Delgado M, Gomariz R P, Ganea D. J Immunol. 1996;156:4128–4136. [PubMed] [Google Scholar]
- 8.Xia M, Leppert D, Hauser S L, Sreedharan S P, Nelson P J, Krensky A M, Goetzl E J. J Immunol. 1996;156:160–167. [PubMed] [Google Scholar]
- 9.Goetzl E J, Banda M J, Leppert D. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- 10.Hernanz A, Tato E, de la Fuente M, de Miguel E, Arnalich F. J Neuroimmunol. 1996;71:25–30. doi: 10.1016/s0165-5728(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 11.Pankhaniya R, Jabrane-Ferrat N, Gaufo G O, Sreedharan S P, Dazin P, Kaye J, Goetzl E J. FASEB J. 1998;12:119–127. doi: 10.1096/fasebj.12.1.119. [DOI] [PubMed] [Google Scholar]
- 12.Dorsam G, Voice J, Kong Y, Goetzl E J. Ann NY Acad Sci. 2001;952:79–91. doi: 10.1111/j.1749-6632.2000.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 13.Sreedharan S P, Patel D R, Xia M, Ichikawa S, Goetzl E J. Biochem Biophys Res Commun. 1994;203:141–148. doi: 10.1006/bbrc.1994.2160. [DOI] [PubMed] [Google Scholar]
- 14.Sreedharan S P, Huang J-X, Cheung M-C, Goetzl E J. Proc Natl Acad Sci USA. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay M, Fantes J, Scherer S, Boyle S, West K, Tsui L-C, Belloni E, Lutz L, Van Heyningen V, Harmar A J. Genomics. 1996;37:345–352. doi: 10.1006/geno.1996.0569. [DOI] [PubMed] [Google Scholar]
- 16.Delgado M, Martinez C, Johnson M C, Gomariz R P, Ganea D. J Neuroimmunol. 1996;68:27–38. doi: 10.1016/0165-5728(96)00063-x. [DOI] [PubMed] [Google Scholar]
- 17.Kaltreider H B, Ichikawa S, Byrd P K, Ingram D A, Kishiyama K L, Sreedharan S P, Warnock M L, Beck J M, Goetzl E J. Am J Resp Cell Mol Biol. 1997;16:133–144. doi: 10.1165/ajrcmb.16.2.9032120. [DOI] [PubMed] [Google Scholar]
- 18.Harmar, A. J., Arimura, A., Gozes, I., Journot, L., Laburthe, M., Pisegna, J. R., Rawlings, S. R., Robberecht, P., Said, S. I., Sreedharan, S. P., et al. (1998) Pharmacol. Rev.50, 265–270. [PMC free article] [PubMed]
- 19.Ganea D. Adv Neuroimmunol. 1996;6:61–74. doi: 10.1016/s0960-5428(96)00007-1. [DOI] [PubMed] [Google Scholar]
- 20.Delgado M, Martinez C, Leceta J, Gomariz R P. Neuroimmunomodulation. 1999;6:97–107. doi: 10.1159/000026369. [DOI] [PubMed] [Google Scholar]
- 21.Metwali A, Blum A M, Li J, Elliott D E, Weinstock J V. FASEB J. 2000;14:948–954. doi: 10.1096/fasebj.14.7.948. [DOI] [PubMed] [Google Scholar]
- 22.Lara-Marquez M L, O'Dorisio M S, O'Dorisio T M, Shah M H, Karacay B. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 23.Voice, J. K., Dorsam, G., Lee, H., Kong, Y. & Goetzl, E. J. (2001) FASEB J., in press. [DOI] [PubMed]
- 24.Goetzl E J, Patel D R, Kishiyama J L, Smoll A C, Turck C W, Law N M, Rosenzweig S A, Sreedharan S P. Mol Cell Neurosci. 1994;5:145–152. doi: 10.1006/mcne.1994.1016. [DOI] [PubMed] [Google Scholar]
- 25.Jabrane-Ferrat N, Pollock A S, Goetzl E J. Biochemistry. 2000;39:9771–9777. doi: 10.1021/bi0008783. [DOI] [PubMed] [Google Scholar]
- 26.Xia M, Sreedharan S P, Bolin D R, Gaufo G O, Goetzl E J. J Pharmacol Exp Ther. 1997;281:629–633. [PubMed] [Google Scholar]
- 27.Gourlet P, Vandermeers A, Vertongen P, Rathe J, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Peptides. 1997;18:1539–1545. doi: 10.1016/s0196-9781(97)00228-3. [DOI] [PubMed] [Google Scholar]
- 28.Delgado M, Ganea D. J Immunol. 2001;166:2907–2912. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa S, Sreedharan S P, Goetzl E J, Owen R L. Regul Pept. 1994;54:385–395. doi: 10.1016/0167-0115(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 30.Ichikawa S, Sreedharan S P, Owen R L, Goetzl E J. Am J Physiol. 1995;268:L584–L588. doi: 10.1152/ajplung.1995.268.4.L584. [DOI] [PubMed] [Google Scholar]