Abstract
Objective
To quantify the prevalence of self-reported major mobility disability (SR-MMD) and its association with mortality in a nationally-representative sample of cancer survivors.
Materials and Methods
This study included patients with a history of cancer who participated in the National Health and Nutrition Examination Survey 1999─2010. SR-MMD was defined as self-reported difficulty or inability to walk a quarter of a mile. Vital status through December 15, 2011 was ascertained from the United States National Center for Health Statistics. Multivariable-adjusted Cox regression models were used to quantify the hazard ratio (HR) and 95% confidence interval (CI) between SR-MMD and mortality.
Results
The study included 1,458 cancer survivors who averaged 67.1 years of age. At baseline, 201 (13.7%) participants had SR-MMD. During a median follow-up of 4.7 years, 434 (29.8%) participants died. SR-MMD was independently associated with a higher risk of all-cause mortality [Hazard Ratio (HR): 2.15 (95% Confidence Interval (CI): 1.56 2.97); P<0.001] and cancer-specific mortality [HR: 2.49 (95% CI: 1.53 4.07); P<0.001]. The association between SR-MMD and all-cause mortality was not modified by age, sex, time since cancer diagnosis, body mass index, or comorbid health conditions.
Conclusion
SR-MMD is an easily ascertainable metric of physical function that is associated with a higher risk of mortality among cancer survivors. Integrating measures of physical function may help to guide clinical decision-making and improve long-term prognostication in this population. Interventions that prevent the development of SR-MDD, such as physical activity, should be evaluated in this population.
Keywords: Physical function, Ambulation, Patient reported outcome, Survival, Physical activity
INTRODUCTION
Physical function is an important determinant of health-related quality-of-life among cancer survivors.1 After a diagnosis of cancer, physical function deteriorates at an accelerated rate compared to that of age-matched cancer-free individuals.2 It is hypothesized that cancer and its treatments impair the cardiovascular, pulmonary, neurologic, and musculoskeletal systems that are required to sustain adequate physical function.3,4 Consequently, 57–66% of cancer survivors report at least one functional limitation, and many of these limitations persist for years after completing cancer treatment.5–7
The ability to walk 400 meters (m, approximately a quarter of a mile) is an objective measure of functional independence,8 and predicts the ability to safely ambulate in the community.9 Major mobility disability (MMD) is defined as the inability to walk 400 m without the use of a walker (use of a cane is acceptable).10 MMD is prognostic of several important outcomes in older adults, including all-cause mortality and incident cardiovascular disease.11 Despite the importance of objectively defined MMD, implementing the 400 m walk in practice may not be feasible given the need for a walking course of considerable length and dedicated staff time for in-person supervision.12 To circumvent this issue, self-reported difficulty or inability to walk a quarter of a mile was validated as a proxy for objectively defined MDD.13,14
Given the unique functional consequences of cancer and its treatments, and the validation of self-reported MDD (SR-MMD), this study aimed to achieve four objectives using a nationally-representative sample of cancer survivors. The first goal was to estimate the prevalence of SR-MMD among community-dwelling cancer survivors in the United States (US). Second, we sought to quantify the association between SR-MMD and all-cause and cancer-specific mortality. Third, we aimed to quantify the association between SR-MMD and all-cause mortality within specific subgroups. Our fourth and final goal was to assess whether there is evidence of a dose-response between self-reported degree of difficulty walking one quarter of a mile and all-cause and cancer-specific mortality.
MATERIALS and METHODS
Study Design
The National Health and Nutrition Examination Surveys (NHANES) are a series of consecutive cross-sectional studies designed to provide health information on a nationally-representative sample of non-institutionalized United States civilians. Participants reside in counties across the country, fifteen of which are visited annually. The current analysis used six consecutive cycles of NHANES data from 1999 to 2010. The study protocol was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. All participants provided written informed consent prior to participating in any study related activities.
Study Participants
NHANES participants included were males and females, aged ≥21 years, with a self-reported history of cancer (excluding non-melanoma skin cancer). Participants were also required to have the requisite measure necessary to define SR-MMD (described below).
Self-Reported Major Mobility Disability
SR-MMD was defined using a single question that asked participants to report “By yourself and without any special equipment, how much difficulty do you have walking for a quarter of a mile (that is about 2 or 3 blocks)”? Participants were not explicitly advised what modalities are included in the term “special equipment.” Possible responses included: “no difficulty”, “some difficulty”, “much difficulty”, or “unable to do”. The two responses of “much difficulty” and “unable to do” is validated to define the presence of SR-MMD.13
Mortality Outcome
The primary outcome of this study was all-cause mortality, defined as the time from assessment of SR-MMD to death from any cause. The secondary study outcome was cancer-specific mortality, defined as the time from assessment of SR-MMD to death attributable to cancer. Vital status was identified using the National Death Index database on December 31, 2011. Participants were linked to the National Death Index database using a probabilistic matching algorithm that included 12 identifiers, such as Social Security Number, sex, date of birth, race, state of residence, and marital status.15
Covariates
Demographic information including date of birth and sex, race, annual household income, and clinical information, including type of cancer, date of cancer diagnosis, and smoking history were ascertained from standardized participant questionnaires. Body mass index (BMI; kilograms (kg) per meter (m) squared; kg/m2) was calculated using participant height (m) and weight (kg), as measured by a study technician, and then categorized as underweight, normal weight, overweight, and obese using the World Health Organization definitions.16 Participation in any physical activity was defined as self-reported engagement in at least one bout of moderate- or vigorous-intensity physical activity of ≥10 minutes in duration within the past month. Self-rated health status was assessed using the first question of the SF-36 questionnaire.17 The presence of comorbid health conditions was ascertained from participant responses to the question of whether a doctor had ever told them that they had any of the following: type 2 diabetes mellitus, myocardial infarction, stroke, and/or congestive heart failure.
Statistical Analysis
Descriptive variables are presented as means and standard errors for continuous variables and percentages for categorical variables. We fit multivariable logistic regression models to estimate the Odds Ratio (OR) and 95% Confidence Interval (CI) to determine demographics and clinical characteristics that were associated with cohort inclusion. We fit Cox proportional hazards regression models to estimate the Hazard Ratio (HR), and 95% CI for SR-MMD and the time-to-death outcomes. Models were first adjusted for sex and age (model 1) and then fully adjusted for demographic, behavioral, and clinical characteristics (model 2). The assumption of proportional hazards was confirmed using log-log plots. We incorporated a statistical interaction term into the regression models to determine if the observed associations were modified by certain a priori designated patient and clinical characteristics, with these results presented as subgroup analyses to facilitate interpretation. Sample weights were integrated into all statistical analyses to account for nonresponse bias, multistage sampling probabilities, and the subpopulation of participants that were included in this analysis. Sensitivity analyses were conducted to quantify the strength that an unmeasured confounder would have to exert to explain the observed associations.18 P<0.05 (two-sided) was considered to indicate statistical significance. Stata/SE v.15.1 statistical software was used for all analyses.
RESULTS
Participant Characteristics
A total of 2,992 adults aged ≥21 years self-reported a prior diagnosis of cancer, and sufficient information to define SR-MMD was available on 1,458 (48.7%). The 1,458 cancer survivors in this analysis were older [OR: 1.08 (95% CI: 1.06 1.09); P<0.001], less likely to have melanoma [OR: 0.54 (95% CI: 0.33 0.88); P=0.013], less likely to report a myocardial infarction [OR: 0.69 (95% CI: 0.48 0.99); P=0.045], and less likely to report congestive heart failure [OR: 0.50 (95% CI: 0.33 0.76); P=0.002] when compared to the 1,534 cancer survivors who were excluded from this analysis. No other reported variables were predictive of participant inclusion.
Among 1,458 cancer survivors, age ranged from 21 to 85 years (Table 1). Most participants reported a history of breast (23.7%), genitourinary (22.7%), gynecologic (15.7%) or gastrointestinal (10.9%) cancer. Time since cancer diagnosis ranged from zero to 78 years. Approximately one-third of participants were within five years of their cancer diagnosis (33.7%). Most participants were overweight (40.9%) or obese (28.4%). BMI ranged from 15.2 to 65.5 kg/m2.
Table 1.
Demographic and clinical characteristics (N=1,458)
| Characteristic | Mean ± Standard Error or N (%) |
|---|---|
| Age, years | 67.1±0.5 |
| Sex | |
| Male | 41.7% |
| Female | 58.3% |
| Race and ethnicity | |
| Non-Hispanic White | 84.5% |
| Non-Hispanic Black | 7.4% |
| Other | 8.1% |
| Annual household income | |
| <$25,000 | 31.2% |
| ≥$25,000 $74,999 | 46.5% |
| ≥$75,000 | 11.3% |
| Refused, unknown, missing | 11.0% |
| Type of cancer | |
| Breast | 23.7% |
| Gastrointestinal | 10.9% |
| Genitourinary | 22.7% |
| Gynecologic | 15.7% |
| Lung/Thoracic | 5.6% |
| Hematologic | 4.4% |
| Melanoma | 7.8% |
| Other/Don’t know | 9.3% |
| Time since cancer diagnosis, years | |
| Mean (continuous) | 10.8±0.4 |
| <5 | 33.7% |
| 5─10 | 25.3% |
| ≥10 | 41.0% |
| Body mass index, kg/m2 | |
| Mean (continuous) | 28.1±0.2 |
| <18.5 | 2.4% |
| 18.5─24.9 | 28.3% |
| 25.0─29.9 | 40.9% |
| ≥30.0 | 28.4% |
| Smoking | |
| Never | 41.6% |
| Former | 43.5% |
| Current | 14.8% |
| Physical activity, past month | 34.7% |
| Self-rated health status | |
| Excellent | 7.6% |
| Very good | 27.4% |
| Good | 41.5% |
| Fair | 18.4% |
| Poor | 5.1% |
| Conditions | |
| Type 2 diabetes | 13.3% |
| Myocardial infarction | 8.7% |
| Stroke | 7.2% |
| Congestive heart failure | 6.0% |
Association Between Self-Reported Major Mobility Disability and Mortality
At baseline, 201 (13.7%) participants had SR-MMD. During a median follow-up of 4.7 years [interquartile range 2.7 7.9], 434 (29.8%) participants died from all-causes, and 174 died from cancer (11.9%).
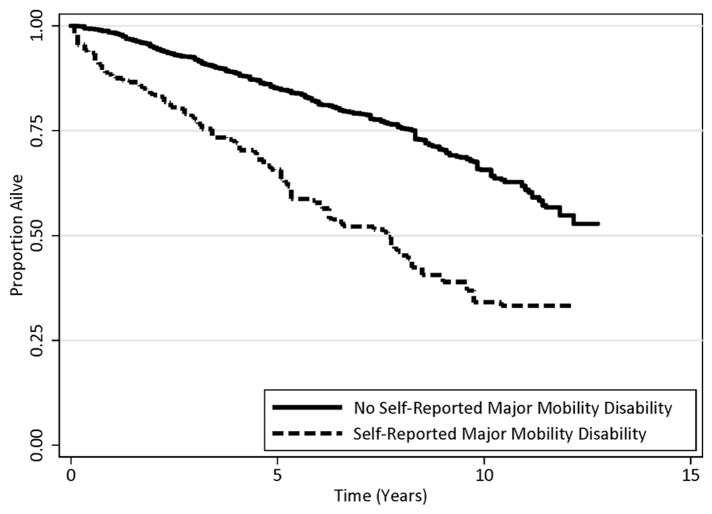
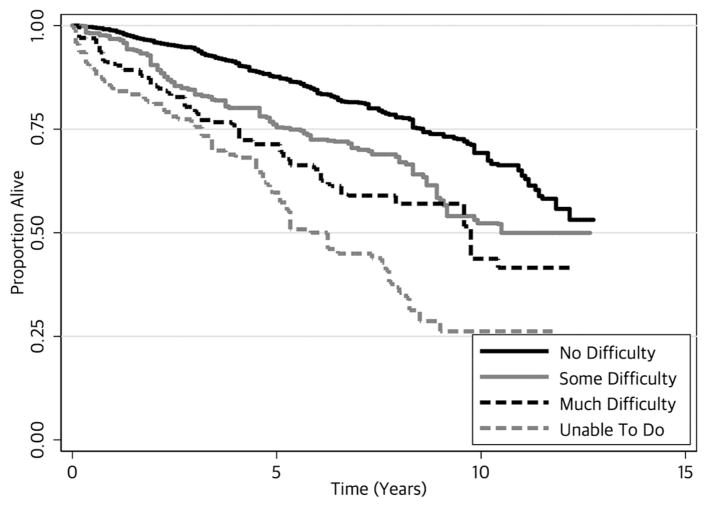
SR-MMD was independently associated with a higher risk of all-cause mortality [HR: 2.15 (95% CI: 1.56 2.97); P<0.001; Table 2; Figure 1]. In sensitivity analysis, the minimum strength of association, on the HR scale, independent of all other variables, that an unmeasured confounder must have with SR-MMD and all-cause mortality to fully attenuate the observed association with SR-MMD would be 2.78. There was a dose-response association between the degree of difficulty reported in walking one quarter of a mile and all-cause and all-cause mortality (Ptrend<0.001; Figure 2).
Table 2.
Association of walking difficulty and self-reported major mobility disability with mortality
| No. of Events/No. at Risk | Death Rate per100 Person-Years | Age & Sex Adjusted | Multivariable Adjusteda | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| All-Cause Mortality | ||||||
| Self-Reported Major Mobility Disability | ||||||
| No | 329/1,257 | 3.7 (3.3─4.3) | 1.00 | — | 1.00 | — |
| Yesb | 105/201 | 9.5 (7.4─12.1) | 2.74 (2.08─3.61) | <0.001 | 2.15 (1.56─2.97) | <0.001 |
| Walking for a quarter mile difficulty | ||||||
| No difficulty | 238/975 | 3.3 (2.8─3.9) | 1.00 | — | 1.00 | — |
| Some difficulty | 91/282 | 5.5 (4.3─7.2) | 1.80 (1.34─2.41) | <0.001 | 1.59 (1.16─2.17) | 0.004 |
| Much difficulty | 47/106 | 7.5 (5.2─11.0) | 2.72 (1.90─3.90) | <0.001 | 2.34 (1.52─3.60) | <0.001 |
| Unable to do | 58/95 | 11.8 (8.7─16.1) | 3.64 (2.36─5.51) | <0.001 | 2.70 (1.68─4.34) | <0.001 |
| Cancer-Specific Mortality | ||||||
| Self-Reported Major Mobility Disability | ||||||
| No | 134/1,257 | 1.6 (1.3─2.0) | 1.00 | — | 1.00 | — |
| Yesb | 40/201 | 4.0 (2.7─6.0) | 2.76 (1.87─4.10) | <0.001 | 2.49 (1.53─4.07) | <0.001 |
| Walking for a quarter mile difficulty | ||||||
| No difficulty | 100/975 | 1.3 (1.0─1.7) | 1.00 | — | 1.00 | — |
| Some difficulty | 34/282 | 2.6 (1.7─4.1) | 2.15 (1.39─3.32) | 0.001 | 2.07 (1.30─3.28) | 0.002 |
| Much difficulty | 24/106 | 4.1 (2.5─7.1) | 3.52 (2.15─5.77) | <0.001 | 3.60 (1.97─6.56) | <0.001 |
| Unable to do | 16/95 | 3.9 (2.1─7.6) | 3.24 (1.72─6.11) | <0.001 | 2.98 (1.50─5.94) | 0.002 |
HR, hazard ratio; CI, confidence interval.
Multivariable adjusted for age, sex, race, annual household income, type of cancer, time since cancer diagnosis, body mass index, physical activity, self-rated health status, type 2 diabetes, myocardial infarction, stroke, and congestive heart failure.
Defined as walking for a quarter mile difficulty as “much difficulty” or “unable to do”
Figure 1.
Kaplan-Meier all-cause survival estimates, by self-reported major mobility disability
Figure 2.
Kaplan-Meier all-survival estimates, by self-reported degree of difficulty walking one quarter of a mile.
SR-MMD was independently associated with cancer-specific mortality [HR: 2.49 (95% CI: 1.53 4.07); P<0.001]. In sensitivity analysis, the minimum strength of association, on the HR scale, independent of all other variables, that an unmeasured confounder must have with SR-MMD and cancer-specific mortality to fully attenuate the observed association with SR-MDD would be 3.15. There was a dose-response association between degree of difficulty walking one quarter of a mile and cancer-specific mortality (Ptrend<0.001).
Subgroup Analyses
In pre-planned exploratory subgroup analyses, age, sex, time since cancer diagnosis, BMI, and comorbid health conditions did not modify the association between SR-MMD and all-cause mortality (Table 3).
Table 3.
Association of self-reported major mobility disability with all-cause mortality, by subgroup
| Subgroup | Age & Sex Adjusted HR (95% CI) | P | P interaction |
|---|---|---|---|
| Age, years | 0.921 | ||
| ≤70 | 2.79 (1.51 5.15) | 0.001 | |
| >70 | 2.75 (1.97 3.82) | <0.001 | |
| Sex | 0.865 | ||
| Male | 2.69 (1.78 4.08) | <0.001 | |
| Female | 2.80 (1.92 4.08) | <0.001 | |
| Time since cancer diagnosis, years | 0.414 | ||
| ≤7 | 2.91 (2.56 3.32) | <0.001 | |
| >7 | 2.54 (1.68 3.84) | <0.001 | |
| Body mass index, kilograms per meter squared (kg/m2) | 0.380 | ||
| <30 | 2.87(2.17 3.80) | <0.001 | |
| ≥30 | 2.23 (1.02 4.89) | <0.001 | |
| Conditions | 0.060 | ||
| 0 | 2.81 (2.03 3.88) | <0.001 | |
| ≥1 | 4.02 (2.39 6.77) | <0.001 |
HR, hazard ratio; CI, confidence interval.
DISCUSSION
In this nationally-representative sample, more than one-in-seven cancer survivors had SR-MMD. SR-MMD was associated with a higher risk of all-cause and cancer-specific mortality. There was a dose-response association between difficulty walking a quarter of a mile and mortality, such that as reported difficulty increases, mortality risk increases proportionally. These data support the hypothesis that self-reported physical function represents vital information that may be useful in clinical decision-making and prognostication.19
These findings are consistent with prior studies that have demonstrated that self-reported physical function and frailty predict mortality in cancer survivors.5,20–22 In a cohort of 428 cancer survivors, self-reported disability (defined as having ≥3 functional limitations in activities of daily living) was associated with a significantly slower measured gait speed ( 0.28 m/s) and independently associated with a 76% higher risk of death when compared to participants without self-reported disability.5 Physical function is a central determinant of quality-of-life,1 and predicts chemotherapy toxicity in older adults.23–26 Prior studies of cancer survivors observe maximal cardiopulmonary capacities that are 27% below age-matched healthy sedentary controls,27 and it is estimated that 10% have cardiopulmonary capacities that are insufficient for independent functioning.28 Similar patterns have been observed for muscle strength and muscle mass.29 The accumulation of these and other physiologic impairments among cancer survivors likely underlie many of the functional impairments observed in this population.
Despite the importance of preserving physical function in cancer survivors, few therapeutic options exist and there is no standard of care for its management.30,31 Participation in physical activity is one of the strongest predictors of physical function among older adults without a history of cancer.32,33 In a phase II randomized clinical trial among 428 older adults at risk for mobility disability, a structured moderate-intensity physical activity program significantly improved surrogate measures of MMD, including short physical performance battery score and gait speed.34 The confirmatory phase III clinical trial among 1,635 older adults demonstrated that physical activity reduced the incidence of MMD by 18% over a median of 2.6-years.35 Evidence from randomized clinical trials in cancer survivors demonstrates that slowly-progressive weight lifting exercise prevents the definitive deterioration of self-reported physical function by 51% over 12-months.36 A combination of dietary modification, weight loss, and aerobic exercise was shown to attenuate the rate of decline in physical function in older overweight and obese survivors of colorectal, breast, and prostate cancer.37,38
The main strength of this study is the complex probability sample, which makes our inferences generalizable to the population of community-dwelling cancer survivors living within the United States. There are also several limitations to this study. We did not have data on stage of cancer at the time of diagnosis and the receipt of any cancer-directed treatments, such as surgery, chemotherapy, and radiation. It is possible that the inclusion of these variables would shift our effect size estimates towards the null. Our sensitivity analyses suggested that an unmeasured confounder would need to be large (HR of 2.9–3.3) to explain away the observed associations. Further, though it is possible that SR-MMD may differentially associate with mortality by cancer site, we did not have sufficient sample size to conduct meaningful subgroup analyses stratified by primary cancer site. Also, SR-MMD was a single cross-sectional measurement, therefore, we were unable to determine the onset of SR-MMD, and it is possible that some participants had SR-MMD prior to their cancer diagnosis. Nonetheless, these data suggest the SR-MMD is relatively common and prognostic of poor outcomes among cancer survivors.
In conclusion, our study supports SR-MMD as a predictor of mortality among cancer survivors. We observed a dose-response association between reported difficulty walking a quarter of a mile and mortality, such that as difficulty increases, mortality risk increases proportionally. This simple, easily ascertainable metric may be useful to guide clinical prognostication, and justifies the design of prospective randomized trials to prevent the development of MMD in this vulnerable and expanding population.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Author Contributions
Study concepts (JCB, MOH, MNH), study design (JCB, MOH, MNH), data acquisition (JCB, MOH), quality control of data and algorithms (JCB, MOH, MNH), data analysis and interpretation (JCB, MOH, MNH), statistical analysis (JCB, MOH), manuscript preparation (JCB), manuscript editing (MOH, MNH), manuscript review (JCB, MOH, MNH).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on Core Patient-Reported Outcomes in Cancer Clinical Trials: Symptomatic Adverse Events, Physical Function, and Disease-Related Symptoms. Clin Cancer Res. 2016;22(7):1553–1558. doi: 10.1158/1078-0432.CCR-15-2035. [DOI] [PubMed] [Google Scholar]
- 2.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. Functional status declines among cancer survivors: Trajectory and contributing factors. Journal of geriatric oncology. 2014;5(4):359–367. doi: 10.1016/j.jgo.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of the American Geriatrics Society. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10(6):598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 5.Brown JC, Harhay MO, Harhay MN. Patient-reported versus objectively-measured physical function and mortality risk among cancer survivors. Journal of geriatric oncology. 2016;7(2):108–115. doi: 10.1016/j.jgo.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 7.Schootman M, Aft R, Jeffe DB. An evaluation of lower-body functional limitations among long-term survivors of 11 different types of cancers. Cancer. 2009;115(22):5329–5338. doi: 10.1002/cncr.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang M, Cohen-Mansfield J, Ferrucci L, et al. Incidence of loss of ability to walk 400 meters in a functionally limited older population. J Am Geriatr Soc. 2004;52(12):2094–2098. doi: 10.1111/j.1532-5415.2004.52570.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoxie RE, Rubenstein LZ. Are older pedestrians allowed enough time to cross intersections safely? Journal of the American Geriatrics Society. 1994;42(3):241–244. doi: 10.1111/j.1532-5415.1994.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 10.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66(11):1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 12.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Rejeski WJ, Gill TM, et al. A Comparison of Self-report Indices of Major Mobility Disability to Failure on the 400-m Walk Test: The LIFE Study. The Journals of Gerontology: Series A. 2017 doi: 10.1093/gerona/glx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 15.Lochner K, Hummer RA, Bartee S, Wheatcroft G, Cox C. The public-use National Health Interview Survey linked mortality files: methods of reidentification risk avoidance and comparative analysis. Am J Epidemiol. 2008;168(3):336–344. doi: 10.1093/aje/kwn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization WH. Obesity: preventing and managing the global epidemic. World Health Organization; 2000. [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 health survey: manual and interpretation guide. Quality Metric Inc; 2000. [Google Scholar]
- 18.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 19.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown J, Harhay M, Harhay M. Physical function as a prognostic biomarker among cancer survivors. British journal of cancer. 2015;112(1):194–198. doi: 10.1038/bjc.2014.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JC, Harhay MO, Harhay MN. The Prognostic Importance of Frailty in Cancer Survivors. J Am Geriatr Soc. 2015;63(12):2538–2543. doi: 10.1111/jgs.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng JA, Clough-Gorr K, Cabral HJ, Silliman RA. Predicting 5- and 10-year survival in older women with early-stage breast cancer: self-rated health and walking ability. J Am Geriatr Soc. 2015;63(4):757–762. doi: 10.1111/jgs.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu T, Chen R, Lin SC, et al. Pilot of three objective markers of physical health and chemotherapy toxicity in older adults. Curr Oncol. 2015;22(6):385–391. doi: 10.3747/co.22.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol. 2011;78(2):138–149. doi: 10.1016/j.critrevonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med. 2009;43(5):342–346. doi: 10.1136/bjsm.2007.044800. [DOI] [PubMed] [Google Scholar]
- 29.Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25(5):947–958. doi: 10.1093/annonc/mdt551. [DOI] [PubMed] [Google Scholar]
- 30.Burg MA, Adorno G, Lopez ED, et al. Current unmet needs of cancer survivors: analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II. Cancer. 2015;121(4):623–630. doi: 10.1002/cncr.28951. [DOI] [PubMed] [Google Scholar]
- 31.Hurria A, Li D, Hansen K, et al. Distress in older patients with cancer. J Clin Oncol. 2009;27(26):4346–4351. doi: 10.1200/JCO.2008.19.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchner DM, Beresford SA, Larson EB, LaCroix AZ, Wagner EH. Effects of physical activity on health status in older adults. II. Intervention studies. Annu Rev Public Health. 1992;13(1):469–488. doi: 10.1146/annurev.pu.13.050192.002345. [DOI] [PubMed] [Google Scholar]
- 33.Wagner EH, LaCroix AZ, Buchner DM, Larson EB. Effects of physical activity on health status in older adults. I: Observational studies. Annu Rev Public Health. 1992;13(1):451–468. doi: 10.1146/annurev.pu.13.050192.002315. [DOI] [PubMed] [Google Scholar]
- 34.Investigators LS. Pahor M, Blair SN, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 35.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. Journal of Clinical Oncology. 2015;33(19):2184–2189. doi: 10.1200/JCO.2014.57.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. Jama. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]