Abstract
Objective
This observational pilot study examined objective circadian phase and sleep timing in chronic migraine (CM) and healthy controls (HC) and the impact of circadian factors on migraine frequency and severity.
Background
Sleep disturbance has been identified as a risk factor in the development and maintenance of CM but the biological mechanisms linking sleep and migraine remain largely theoretical.
Methods
Twenty women with CM and 20 age-matched HC completed a protocol that included a seven-day sleep assessment at home using wrist actigraphy followed by a circadian phase assessment using salivary melatonin. We compared CM versus HC on sleep parameters and circadian factors. Subsequently, we examined associations between dim-light melatonin onset (DLMO), the midpoint of the sleep episode, and the phase angle (time from DLMO to sleep midpoint) with the number of migraine days per month and the migraine disability assessment scale (MIDAS).
Results
CM and HC did not differ on measures of sleep or circadian phase. Within the CM group, more frequent migraine days per month was significantly correlated with DLMO (r = .49, p = .039) and later sleep episode (r = .47, p = .037). In addition, a greater phase angle (i.e., circadian misalignment) was significantly correlated with more severe migraine-related disability (r = .48, p = .042). These relationships remained significant after adjusting for total sleep time.
Conclusions
This pilot study revealed that circadian misalignment and delayed sleep timing are associated with higher migraine frequency and severity which was not better accounted for by the amount of sleep. These findings support the plausibility and need for further investigation of a circadian pathway in the development and maintenance of chronic headaches. Specifically, circadian misalignment and delayed sleep timing could serve as an exacerbating factor in chronic migraines when combined with biological predispositions or environmental factors.
Keywords: Circadian rhythms, sleep, migraine
Introduction
Chronic migraine (CM) is a debilitating condition that affects approximately 2% of the general population with a prevalence 2.5 to 6.5 times higher in women than in men.1 Sleep disturbance has been identified as a key risk factor in the development and maintenance of CM 2,3 but the biological mechanisms linking sleep and migraine remain unclear. Studies have found that both increased and decreased sleep time are associated with chronic headaches 3–5 and daytime naps are a common strategy used to relieve headaches.6–8 While most studies have examined the duration or quality of sleep, relatively less attention has been given to examining the timing of sleep or circadian factors in migraine sufferers.
Circadian rhythms play an important role in sleep/wake regulation, and emerging evidence suggests that circadian factors might play a role in the etiology of migraine. One animal study found mutations in a component of the molecular circadian clock in connection to migraine. 9 Human studies conducted on migraine sufferers have found a delay in circadian phase 10–12 and lower levels of plasma melatonin, a key circadian hormone.10,13–15 Furthermore, the administration of melatonin has been shown to prevent or reduce headaches.11,12,16,17 While previous studies have used invasive measures to assess circadian rhythms, a reliable and non-invasive method of measuring circadian phase is available through saliva collection to examine dim-light melatonin onset (DLMO).18 DLMO is a biomarker of circadian phase that can be used with other sleep measures to provide information on the alignment between the circadian rhythm and the sleep episode. Circadian misalignment has been investigated in other pain conditions 19,20 but has not yet been investigated in people with migraine. Therefore, salivary melatonin collection could be a particularly useful method to complement standard sleep assessments to examine both sleep and circadian factors in headache sufferers.
The purpose of this study was to investigate objectively-measured circadian phase and sleep timing in women with CM compared to age-matched healthy controls (HC). The first aim was to compare CM and HC on sleep timing and circadian factors. The second aim was to examine the impact of the circadian factors on migraine frequency and severity within the CM group. We hypothesized that the CM group would have a delayed circadian phase relative to HC and that greater circadian misalignment would be associated with worse migraine symptoms.
Methods
Study Design
The SANDMAN (Sleep Assessment across Night and Day in Migraine And Non-migraine individuals) project is an observational pilot study consisting of an assessment of habitual sleep at home and an assessment of sleep and circadian phase in a controlled laboratory environment in women with CM and age-matched controls. The overall project aims were to examine the variability of sleep behaviors (e.g., timing of bedtimes and rise times) between CM and HC, to characterize the neurobiological correlates of sleep regulation/dysregulation in CM, and to examine the relationship between variability in sleep behaviors, neurobiological correlates, and headache pain. This report is a subset of the SANDMAN project focusing on circadian phase and sleep timing by examining data collected from the circadian assessment in the laboratory and the sleep assessment at home during the seven days prior to the circadian assessment.
Participants
Participants were females between 18 and 41 years of age with regular menstrual cycles. All CM participants met the International Classification of Headache Disorders (ICHD-3) criteria 21 with ≥ 15 headache days per month for at least three months and symptoms meeting criteria for migraine (with or without aura) on at least eight days per month. HC participants were age-matched (± three years) to a CM participant and reported no history of any headache disorders and no more than one headache per month during the past 12 months. Other exclusion criteria for both CM and HC were: (1) unstable medical condition that required immediate treatment, (2) other pain conditions such as chronic low back pain and fibromyalgia, (3) psychiatric condition that required immediate treatment or interfered with the study protocol, (4) daily use medications known to affect melatonin (e.g., beta blockers, melatonin pills), (5) use of illicit substances (e.g., narcotics, hallucinogens), (6) women who were pregnant or nursing, and (7) travel across more than three time zones in the month prior to the study protocol.
Participants were recruited through posted advertisements, flyers in neurology and primary care clinics, and a women’s health registry list. Screening for eligibility involved a telephone interview followed by an in-person interview that included a review of medical history, the Structured Diagnostic Interview for Headache-Revised (SDIH-R) 22 to assess for headache symptoms, the Structured Diagnostic Interview for DSM-IV (SCID) 23 to assess for psychiatric conditions, and the Duke Structured Interview Schedule for Sleep Disorders 24 to assess for sleep disorders. Diagnosis of migraine was confirmed by a medical exam with the study neurologist (MP). The study was approved by the Institutional Review Board at Rush University Medical Center and all participants provided written informed consent prior to participation.
Measures
Self-Report Data
Several self-report measures were collected during the screening visit and the laboratory assessment to characterize the sample. Demographic data were collected during the screening visit. The migraine disability assessment scale (MIDAS),25 a five-item questionnaire that measures migraine-related disability over a three-month period, was used to measure migraine severity. The number of migraine days per month (30 days) from the SDIH-R was used to quantify migraine frequency. The Center for Epidemiological Studies Depression Scale (CES-D) 26 was used to assess depressive symptoms and the State-Trait Anxiety Inventory-Trait (STAI-T) 27 was used to assess trait-levels of anxiety. Finally, the Morningness-Eveningness Questionnaire (MEQ) 28 was used to measure chronotype, the behavioral manifestation of circadian rhythms based on the individual’s preferred schedule.
Daily Headache Ratings
An 11-point numerical rating scale (0 = no headache, 10 = extremely painful headache) was used to capture headache frequency and severity 22,29. From this scale, a score ≥ 2 (at least slightly painful) was used as a cut-off for a “headache day.” These ratings were completed daily through the study website as part of the sleep assessment. In addition, participants completed a headache rating at the end of the circadian assessment to determine the headache rating during the day of the circadian assessment.
Wrist Actigraphy
Wrist actigraphy (Actiwatch Spectrum, Phillips Respironics, Bend, OR) was used to measure objective sleep/wake patterns. Participants wore actigraphs for seven days immediately prior to the in-lab assessment, and concurrently completed daily diaries of sleep and headache ratings for the same seven days. Actigraphy is commonly used to assess longitudinal sleep/wake patterns and has been well validated as an objective measure of sleep.30,31 Participants were instructed to wear the actigraph on the non-dominant wrist during the day and night, using the event marker on the device to indicate bed time (i.e., lights out) and rise time (i.e., lights on). These indicators were used to establish the time in bed (TIB) interval. When no valid event markers were available, any available data (e.g., self-report sleep diary data) were used to establish the time in bed. Data were analyzed in one minute epochs using medium sensitivity for determining wakefulness with Respironics Actiware version 6.0.
Dim Light Melatonin Onset (DLMO)
As a biomarker of circadian phase, the DLMO from the saliva collection (see procedures below) was calculated for each participant using linear interpolation as the clock time when melatonin concentration levels exceeded a threshold of the mean of three consecutive low daytime values (baseline value) plus twice the standard deviation of these three values.32,33 In addition, the melatonin levels were required to remain above the threshold for at least two hours. DLMO is used extensively in human sleep research as a reliable marker of circadian phase.18,34
Procedures
Sleep assessment
Approximately seven to 10 days prior to the laboratory (circadian) assessment each participant received an orientation to explain the study procedures. This included instructions for wearing the actigraph 24 hours per day and making daily entries using on-line sleep diaries for the seven days prior to the circadian assessment. Since the goal was to record habitual sleep/wake patterns, participants were asked to maintain their usual sleep/wake routines during this time. To prepare for the laboratory assessment, participants were also given specific instructions to abstain from certain foods (e.g., candy, bananas, popsicles), substances (e.g., lipstick), and medications (e.g., beta blockers) known to interfere with melatonin production or analysis of saliva. This included abstaining from caffeine (>200mg) or alcohol in the 24 hours before the laboratory assessment and abstaining from nonsteroidal anti-inflammatory drugs (NSAIDs) in the 72 hours before the laboratory assessment. Participants were also instructed to avoid napping on the day of the laboratory assessment.
Circadian assessment
Participants arrived at the sleep laboratory eight hours prior to their habitual bed time, which was determined by calculating the average bed time from the previous seven days. Upon arrival, participants were screened for alcohol using a breathalyzer. Screening for nicotine, narcotics, and other common drugs of abuse were conducted using a urine drug screen kit. None of the participants tested positive for alcohol or drugs. Beginning six hours prior to habitual bedtime, participants remained in dim light < 5 lux, as measured by a light meter (Extech EasyView 31 Light Meter, Nashua, NH, USA), since light exposure can suppress melatonin. Saliva samples were collected every 30 minutes using Salivettes (Sarstedt, Newton, NC, USA). Participants were seated or reclined during the six-hour sampling period but were not allowed to nap. They were allowed to stand or use the restroom in dim light between samples as needed until 10 minutes before each sample collection. Participants in the migraine group were allowed to take acetaminophen, if needed. Research staff was present to monitor adherence to the protocol throughout the circadian assessment. This protocol follows standard procedures for the collection of DLMO to control factors (i.e., light exposure, substances, and daytime naps) known to impact melatonin. 35,36 Saliva samples were centrifuged immediately following collection and then frozen before they were packed in dry ice and shipped to Solidphase, Inc (Portland, ME) for analysis. Saliva samples were assayed for melatonin through direct radioimmunoassay using standard ALPCO kits with an assay sensitivity of 0.5 pg/ml, intra and interassay CV < 7.5% at 3 pg/ml. Following the last saliva collection, participants were allowed to sleep at their habitual bedtime in the laboratory.
Data Analyses
For each participant, means across the seven-day sleep assessment were used to derive sleep parameters and sleep timing. Technical problems with the actigraph device led to incomplete data for six participants (three HC, three CM). The means across four days (n = 1), five days (n = 3), and six days (n = 2) were used for these participants. Sleep parameters derived from the actigraph included sleep onset latency, wake after sleep onset, early morning awakenings, total sleep time, and time in bed (using the method described above). Additionally, sleep efficiency was calculated as the percentage of total sleep time during the period of time in bed. For timing of the sleep phase, the midpoint of the sleep episode was calculated by taking the midpoint of the interval from sleep onset (bed time + sleep onset latency) to the rise time. The sleep midpoint does not take into account any time awake within this interval (i.e., wake after sleep onset, early morning awakenings). For circadian misalignment, we calculated a phase angle for each participant equal to the time interval from DLMO (circadian phase) to the sleep midpoint (sleep phase). The phase angle has been used as a measure of the circadian misalignment between the DLMO and the midpoint of sleep.37
Prior to conducting the main analyses, preliminary inspection of the sample characteristics was conducted using descriptive statistics, one-way analysis of variance (ANOVAs), and chi-squared tests (see sample characteristics below and Table 1). In addition, assumptions for parametric tests were verified using histograms and Levene’s test for homogeneity of variances. For the main comparisons between CM and HC, we conducted a series of one-way ANOVAs on self-report measures, sleep parameters, sleep timing, and DLMO. For analyses on the relationship between circadian factors and migraine symptoms, we conducted Pearson correlations followed by post-hoc linear regressions for the CM group. All analyses used two-tailed testing and p < .05 as the level for statistical significance. No statistical power analysis was conducted prior to the study. The sample size was selected based on the availability of the resources from the funding mechanism for this pilot study. All analyses were conducted using SPSS version 24 for Windows.
Table 1.
Participant Characteristics.
| CM (n=20) | HC (n=20) | p value | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 32.7 (5.8) | 32.2 (6.2) | .803 |
| Ethnicity (% Hispanic) | 10% | 5% | .548 |
| Race (%) | .501 | ||
| White | 55% | 70% | |
| Black | 25% | 25% | |
| More than 1 Race | 15% | 5% | |
| American Indian | 5% | 0% | |
| Years of Education | |||
| Mean (SD) | 15.8 (3.7) | 17.1 (2.2) | .197 |
| Relationship Status (%) | .219 | ||
| Partnered | 65% | 60% | |
| Single | 30% | 40% | |
| Divorced/Separated | 5% | 0% | |
| Employment (%) | .970 | ||
| Full-Time | 65% | 65% | |
| Part-Time | 10% | 5% | |
| Unemployed | 5% | 5% | |
| Caregiver | 10% | 15% | |
| Student | 10% | 10% | |
| BMI, kg/m2 | .283 | ||
| Mean (SD) | 29.06 (8.51) | 26.36 (6.82) | |
| Medications (n) | - | ||
| OTC analgesics | 9 | 0 | |
| NSAIDs | 7 | 4 | |
| Triptans | 9 | 0 | |
| Antihistamines | 7 | 3 | |
| Antidepressants | 6 | 0 | |
| Opiates | 4 | 0 | |
| Benzodiazepines/Hypnotics | 4 | 0 | |
| Antihypertensives | 3 | 0 | |
| Antiepileptics | 2 | 0 | |
| Other OTC | 14 | 7 | |
| Use of Contraceptives (%) | 65% | 45% | .267 |
| MIDAS | <.001* | ||
| Mean (SD) | 58.3 (34.4) | 0.0 (0.0) | |
| CES-D | .001* | ||
| Mean (SD) | 11.3 (10.9) | 1.9 (2.5) | |
| STAI-T | .002* | ||
| Mean (SD) | 35.2 (7.5) | 28.0 (5.7) | |
| MEQ | .162 | ||
| Definitely Morning type | 10% | 0% | |
| Moderately Morning type | 10% | 35% | |
| Neither | 65% | 55% | |
| Moderately Evening type | 15% | 10% | |
| Definitely Evening type | 0% | 0% | |
Abbreviations: CM = chronic migraine; HC = healthy control; BMI = body mass index; OTC = over-the-counter; NSAID=nonsteroidal anti-inflammatory drug; MIDAS = Migraine Disability Assessment; CES-D = Center for Epidemiological Studies Depression Scale; STAI-T = State-Trait Anxiety Inventory-Trait; MEQ = Morningness-Eveningness Questionnaire.
Note. For medications, some participants were taking more than 1 medication. As a result, the total does not equal the sample size. Other OTC = vitamins or supplements. No comparisons were made for medications. For relationship status, partnered = married, engaged, or lives with partner. For employment, full-time = 32 hours or more of work per week; full-time caregiver is for children or other relatives. One-way ANOVAs were conducted on continuous variables (age, years of education, BMI, MIDAS, CES-D, and STAI). Chi-squared tests were conducted on categorical variables (ethnicity, race, relationship status, employment, use of contraceptives, and MEQ).
p<.05 for between-group comparisons between CM vs HC.
Results
A total of 65 potential participants were screened for eligibility with 24 CM and 20 HC who qualified for the study. Two CM participants did not complete the assessment protocol and no age-matched controls were available for two CM participants. Therefore, the final sample size was 20 CM and 20 HC. Two CM did not have a sufficient rise in melatonin using the method described above for calculating DLMO; these participants were excluded from analyses with DLMO and phase angle.
Sample Characteristics
The average age for the entire sample was 32.4 years, with no significant difference between CM and HC. No significant differences were found on other demographic variables, including ethnicity, race, years of education, relationship status, and employment status (see Table 1). As expected, CM used a number of pain medications including abortive medications for migraines (e.g., triptans), analgesics, NSAIDs and opiates. There were no significant differences between groups on use of contraceptives or BMI. Data from the SDIH-R revealed that CM participants reported an average of 23.9 headache days per month with a mean of 18.0 migraine days per month (standard deviation [SD] = 6.2 days) and 5.9 other headache days per month (e.g. tension headache; SD = 8.2). Typical pain intensity of a migraine was 7.60 (SD = 1.74) on a scale from 0 (no pain) to 10 (extremely painful). The median number of months with migraines was 60.0 (mean = 74.3 months). Ten CM participants met criteria for migraine with aura and 10 had migraine without aura. The CM group reported significantly higher scores on the MIDAS compared to the HC group, F(1,38) = 57.61, p < .001. The CM group also reported more symptoms of depression on the CES-D, F(1,38) = 14.18, p < .01, and more trait-level anxiety on the STAI, F(1,38) = 11.66, p < .01, compared to HC. Also, 20% (n = 4) of the migraine group reported CES-D ≥ 16, a score which indicates clinically elevated symptoms of depression. Using a chi-square test, we found no significant differences between CM and HC on the MEQ, with the majority of participants in both groups endorsing neither morning nor evening preferences. Headache ratings during the sleep assessment revealed a mean rating of 3.95 (SD = 1.92) for the CM group and a mean rating of 0.21 (SD = 0.40) for the HC group (median = 0, IQR = 0.00 – 0.42). Using a cut-off of headache rating ≥ 2 to indicate a headache day, the CM group reported a mean of 5.15 (SD = 1.46) headache days (76.6% of days reported). The HC group reported a median of 0 headache days (IQR = 0.00 – 0.43; 5.5% of days reported). During the circadian assessment, 17 out of 20 (85%) CM participants reported a headache rating ≥ 2, with a mean rating of 4.48 (SD = 2.72).
Sleep and Circadian Measures
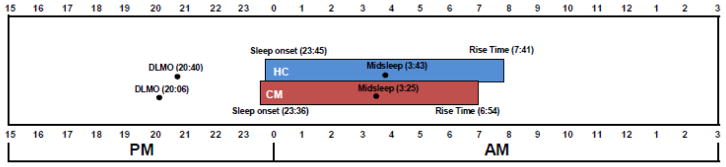
CM and HC were not significantly different on sleep parameters during the 7-day sleep assessment period (see Table 2). During this time, the CM group had an average of 405 minutes of sleep per night compared to 418 minutes of sleep per night in the HC group, but this difference was not significant (p = .392, η2 = .019). Sleep efficiency (p = .879, η2 = .001), sleep onset latency (p = .574, η2 = .008), wake after sleep onset (p = .701, η2 = .004), and early morning awakenings (p = .661, η2 = .005) were all similar across groups, indicating no significant differences in sleep disturbance between these two groups and small effect sizes. No significant differences were found in the time of sleep onset (CM = 23:36 vs HC = 23:45, p = .727, η2 = .003), sleep midpoint (CM = 3:25 vs HC = 3:43, p = .416, η2 = .017), or rise time (CM = 6:54 vs HC = 7:41, p = .122, η2 = .062). For circadian phase, the CM group had an average DLMO occurring at 20:06, which was 34 minutes earlier than the HC group, but this was not statistically significant (p = .209, η2 = .043). The phase angle was not significantly different between the two groups (7.18 vs 7.06 hours), indicating no significant differences in circadian misalignment (p = .707, η2 = .004). See Figure 1 for the relative timing of the circadian and sleep phase.
Table 2.
Sleep and Circadian Measures.
| CM | HC | p value | η2 | |
|---|---|---|---|---|
| Sleep Parameters, in minutes Mean (SD) |
||||
| Total Sleep Time | 405.38 (52.20) | 418.12 (40.10) | .392 | .019 |
| Time in Bed | 472.03 (50.68) | 485.57 (43.38) | .370 | .021 |
| Sleep Efficiency | 85.81 (6.91) | 86.09 (4.43) | .879 | .001 |
| Sleep Onset Latency | 15.59 (17.06) | 12.96 (11.71) | .574 | .008 |
| Wake after Sleep Onset | 37.20 (15.91) | 39.21 (16.95) | .701 | .004 |
| Early Morning Awakening | 13.85 (9.89) | 15.10 (7.87) | .661 | .005 |
| Sleep Timing | ||||
| Sleep Onset Time | 23:36 | 23:45 | .727 | .003 |
| Sleep Midpoint Time | 3:25 | 3:43 | .416 | .017 |
| Rise Time | 6:54 | 7:41 | .122 | .062 |
| DLMO | 20:06 | 20:40 | .209 | .043 |
| Phase Angle, hours Mean (SD) |
7.18 (1.01) | 7.06 (0.97) | .707 | .004 |
Abbreviations: DLMO = dim-light melatonin onset.
Note: Sleep parameters and sleep timing variables are averages based on the 7 days of at-home actigraphy assessment immediately prior to the in-lab assessment. Phase Angle is calculated as the time (in hours) from DLMO to sleep midpoint. η2 = Eta-squared
Figure 1.
Circadian Phase and Sleep Timing.
This figure illustrates the relative time of the dim-light melatonin onset (DLMO) and sleep episode for the chronic migraine (CM) and healthy control (HC) groups. The average time of sleep onset, midsleep, and rise time were measured from actigraphy during the 7-day sleep assessment. The phase angle was calculated as the time from DLMO to midsleep.
Relationship between Circadian Factors and Migraine Symptoms
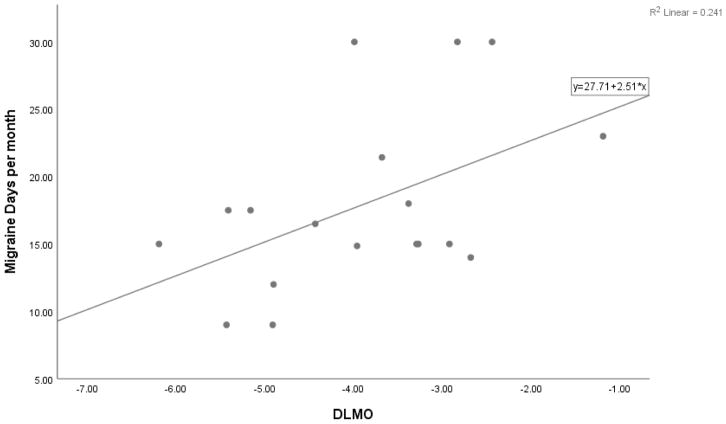
Within the CM group, the relationship between DLMO and number of migraine days per month was examined with a Pearson coefficient. A significant positive correlation (r = .49, n = 18, p = .039) was found, accounting for 24.1% of the shared variance. This indicates that a later melatonin onset was associated with more frequent migraine days per month (see Figure 2). A significant positive correlation was also found between the sleep midpoint and number of migraine days per month (r = .47, n = 20, p = .037), accounting for 21.9% of the shared variance. This indicates that a later sleep episode was also associated with more frequent migraine days per month. Finally, a significant positive correlation was found between phase angle and MIDAS (r = .48, n = 18, p = .042), accounting for 23.4% of the shared variance. This indicates that a greater misalignment between DLMO and sleep phase is associated with greater levels of migraine-related disability. The magnitude of these correlations are considered medium to large when using Cohen’s 38 recommendations for interpreting correlation coefficients (r = .50 for large effect).
Figure 2.
Scatterplot of Dim-light Melatonin Onset (DLMO) and Migraine Days per Month for the Chronic Migraine Group.
This figure depicts the relationship between DLMO (as measured in hours from midnight) and migraine days per month (out of 30 days). Pearson r = 0.49, n = 18, p = .039.
To determine if these associations could be accounted for by the amount of sleep, we conducted post-hoc linear regressions for the significant correlations above, adjusting for total sleep time measured by actigraphy during the sleep assessment. DLMO was significantly associated with migraine days per month (β = .517, p = .028, ΔR2 = .265). The sleep midpoint was significantly associated with migraine days per month (β = .552, p = .013, ΔR2 = .290). Phase angle was also significantly associated with MIDAS scores (β = .479, p = .041, ΔR2 = .230). These results indicate that the associations among circadian phase, sleep timing, and migraine symptoms were not better accounted for by the quantity of objectively-measured sleep. To examine the potential influence of depressive symptoms, we repeated the post-hoc linear regressions adjusting this time for total CES-D scores. Although the magnitude of the relationships (standardized beta) were mostly similar, the association between DLMO and migraine days per month (β = .523, p = .055, ΔR2 = .219), sleep midpoint and migraine days per month (β = .461, p = .057, ΔR2 = .191), and phase angle and MIDAS scores (β = .475, p = .055, ΔR2 = .220) were not significant at the p < .05 level.
Discussion
This pilot study investigated the plausibility of circadian dysregulation as a potential factor in the exacerbation of chronic migraines. We did not find support for the hypothesis that individuals with CM would have a delayed circadian phase relative to HC. However, we found that delayed timing of sleep and circadian phase were associated with the frequency of migraines in the CM group, which was consistent with our hypotheses. Specifically, later DLMO and later sleep timing were associated with more migraine days per month and the magnitude of the relationship was in the moderate to large range. Moreover, these associations were present when adjusting for objectively-measured total sleep time, indicating that the relationship was not better accounted for by the amount of nocturnal sleep. We also found that greater circadian misalignment, as measured by the phase angle between DLMO and the sleep midpoint, was related to higher scores on the MIDAS, with the magnitude of the correlation considered to be medium to large. Together, these findings indicate that circadian misalignment could play a role in the pathophysiology of CM.
Previously, we proposed a biobehavioral model of chronic headaches and insomnia to explain how coping behaviors for headaches can precipitate and perpetuate sleep disturbances, thereby serving as one potential pathway for increasing the propensity for future headache episodes and the transformation to chronic headaches.39 Disturbances in the circadian system was proposed as one potential mechanism of sleep disturbance but there was a dearth of studies that assessed circadian phase in migraine sufferers. The present findings provide preliminary evidence for the role of circadian factors in the biobehavioral model, raising the possibility that circadian misalignment could be an exacerbating factor in chronic migraines. Given that headache sufferers often use sleep as a means of coping with headache pain,6 the palliative use of sleep could lead to irregular sleep times and misalignment with the circadian phase. Furthermore, migraine sufferers might prefer environments with dim light due to photophobia, which could also dysregulate circadian rhythms. Finally, circadian dysregulation could serve as an underlying mechanism that accounts for the frequent comorbidity between chronic migraine and other conditions such as insomnia and mood disorders. In the post-hoc analyses, depressive symptoms accounted for some of the variance between circadian phase and migraine symptoms and previous studies have found that delayed circadian phase is associated with an exacerbation of depressive symptoms.40,41 Taken together, the present findings serve as a first step in refining the biobehavioral model by revealing a potential circadian pathway which could have an additive effect on migraine frequency and severity when combined with biological predispositions (e.g., genetics) and/or environmental factors (e.g., stress, sensory stimuli, certain foods).
No significant differences and small effect sizes were found between CM and HC on indices of objectively-measured sleep disturbance, including total sleep time, sleep efficiency, sleep onset latency, and wake after sleep onset. Previous findings in the literature have been inconsistent when using objective measures of sleep in chronic headaches.39 Our sample consisted of only women with CM who were age-matched to HC, so it is possible that sex and age moderate the relationship between sleep disturbances and headaches. Another possible explanation is that circadian misalignment rather than sleep disturbance per se might better account for the mechanisms linking sleep and chronic headache. Further research is needed to elucidate the mechanisms of sleep disturbance in chronic headache and to distinguish the impact of circadian factors from the impact of sleep disturbances on migraines.
Our findings also have potential clinical implications for the management of migraines. Migraine sufferers with a delayed sleep phase may benefit from an intervention aimed at phase advancement. In a proof-of-concept study using 10 women with fibromyalgia, Burgess et al19 demonstrated that bright light treatment in the morning (i.e., phototherapy) produced phase advances in circadian timing that were associated with an increase in pain tolerance. For migraine sufferers, circadian phase advancement could be achieved using exogenous melatonin or behavioral modification of sleep schedules (i.e., chronotherapy). Previous studies administering oral melatonin have found some success in reducing migraine frequency and severity.12,17 Our findings suggest that the mechanism of action in these studies could involve advancement of the circadian phase to an earlier time. Recently, home-based assessment of salivary DLMO has become available and appears to yield reliable results, 35,42 with the advantages of minimizing time and cost for patients in clinical settings. Given that circadian assessment has been recommended as part of the assessment of headaches 16 home-based DLMO measures could enhance the precision of delivering chrono-therapeutic treatments for migraine patients.
Strengths of this study include using objective measures of sleep and circadian phase and controlling for the effects of age by matching CM with HC. To our knowledge, this is the first study to compare migraine and age-matched controls on circadian phase and sleep timing using salivary DLMO, a reliable biomarker for circadian phase. Our findings appear congruent with previous findings of delayed sleep phase using plasma melatonin in CM,10,12 supporting the benefits of using salivary DLMO as a non-invasive biomarker for circadian phase in CM.
Some limitations should be considered when interpreting the findings of this study. First, we sought to minimize potential confounds (e.g., age-related changes) by including only women between the ages of 18 and 41. However, this limits the ability to generalize these findings to men with CM or to women in other age groups. In addition, we only selected those who reported at least 15 headache days per month to minimize the variability in migraine occurrence during the protocol. This limits the ability to generalize these findings to those who suffer episodic migraines and precluded analyses on ictal versus interictal states. Given the exploratory nature of our study, we did not adjust for multiple comparisons, which raises the possibility of Type I error. Therefore, our findings should be interpreted as preliminary and further research is needed to examine the reproducibility of these findings. Finally, these findings revealed associations between circadian factors and migraine; however, causation and directionality of the relationship cannot be determined with these data. It is possible that more severe or frequent migraines might contribute to a delay in sleep phase and circadian misalignment.
Conclusion
Our findings provide new data that raises the possibility of sleep timing and circadian phase as a potential factor in the pathophysiology of chronic migraine. Given the preliminary nature of these findings, future studies should seek to replicate these findings in a larger sample and conduct hypothesis-driven studies to elucidate the role of circadian factors in migraine. For example, experimental testing of the impact of circadian phase shifting (i.e., re-alignment) on migraine frequency and severity would provide more compelling evidence to demonstrate the connection between circadian rhythms and migraines. Since sleep disturbance is only one factor that can exacerbate migraines, future studies should also examine the potential link between circadian dysregulation and other known exacerbating factors of chronic migraine (e.g., obesity, mood disorders).
Acknowledgments
We would like to thank Toni Iurcotta and Athanasios Kondilis for their help in data acquisition and management. This study was funded by an NIH grant (NS081088) awarded to the first author. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement:
Jason C. Ong: No Conflict
Hannah L. Taylor: No Conflict
Margaret Park: No Conflict
Helen J. Burgess: Scientific Advisor for Natrol, LLC.
Rina Fox: No Conflict
Sarah Snyder: No Conflict
Jeanetta C. Rains: No Conflict
Colin Espie: Co-founder of and a shareholder in Big Health Inc.
James K. Wyatt: No Conflict
References
- 1.Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30(5):599–609. doi: 10.1111/j.1468-2982.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 2.Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006;46(9):1334–1343. doi: 10.1111/j.1526-4610.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45(7):904–910. doi: 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- 4.Paiva T, Farinha A, Martins A, Batista A, Guilleminault C. Chronic headaches and sleep disorders. Archives of Internal Medicine. 1997;157(15):1701–1705. [PubMed] [Google Scholar]
- 5.Kikuchi H, Yoshiuchi K, Yamamoto Y, Komaki G, Akabayashi A. Does sleep aggravate tension-type headache? An investigation using computerized ecological momentary assessment and actigraphy. BioPsychoSocial medicine. 2011;5:10. doi: 10.1186/1751-0759-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong JC, Stepanski EJ, Gramling SE. Pain coping strategies for tension-type headache: possible implications for insomnia? Journal of clinical sleep medicine. 2009;5(1):52–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Bag B, Karabulut N. Pain-relieving factors in migraine and tension-type headache. International journal of clinical practice. 2005;59(7):760–763. doi: 10.1111/j.1368-5031.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 8.Blau JN. Resolution of migraine attacks: sleep and the recovery phase. Journal of neurology, neurosurgery, and psychiatry. 1982;45(3):223–226. doi: 10.1136/jnnp.45.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan KC, Bates EA, Shapiro RE, et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5(183):183ra156, 181–111. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peres MF, Sanchez del Rio M, Seabra ML, et al. Hypothalamic involvement in chronic migraine. Journal of neurology, neurosurgery, and psychiatry. 2001;71(6):747–751. doi: 10.1136/jnnp.71.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claustrat B, Brun J, Geoffriau M, Zaidan R, Mallo C, Chazot G. Nocturnal plasma melatonin profile and melatonin kinetics during infusion in status migrainosus. Cephalalgia. 1997;17(4):511–517. doi: 10.1046/j.1468-2982.1997.1704511.x. discussion 487. [DOI] [PubMed] [Google Scholar]
- 12.Nagtegaal JE, Smits MG, Swart AC, Kerkhof GA, van der Meer YG. Melatonin-responsive headache in delayed sleep phase syndrome: preliminary observations. Headache. 1998;38(4):303–307. doi: 10.1046/j.1526-4610.1998.3804303.x. [DOI] [PubMed] [Google Scholar]
- 13.Claustrat B, Loisy C, Brun J, Beorchia S, Arnaud JL, Chazot G. Nocturnal plasma melatonin levels in migraine: a preliminary report. Headache. 1989;29(4):242–245. doi: 10.1111/j.1526-4610.1989.hed22904242.x. [DOI] [PubMed] [Google Scholar]
- 14.Brun J, Claustrat B, Saddier P, Chazot G. Nocturnal melatonin excretion is decreased in patients with migraine without aura attacks associated with menses. Cephalalgia. 1995;15(2):136–139. doi: 10.1046/j.1468-2982.1995.015002136.x. discussion 179. [DOI] [PubMed] [Google Scholar]
- 15.Murialdo G, Fonzi S, Costelli P, et al. Urinary melatonin excretion throughout the ovarian cycle in menstrually related migraine. Cephalalgia. 1994;14(3):205–209. doi: 10.1046/j.1468-2982.1994.014003205.x. [DOI] [PubMed] [Google Scholar]
- 16.Rovers J, Smits M, Duffy JF. Headache and sleep: also assess circadian rhythm sleep disorders. Headache. 2014;54(1):175–177. doi: 10.1111/head.12217. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves AL, Martini Ferreira A, Ribeiro RT, Zukerman E, Cipolla-Neto J, Peres MF. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87(10):1127–1132. doi: 10.1136/jnnp-2016-313458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14(3):227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 19.Burgess HJ, Park M, Ong JC, Shakoor N, Williams DA, Burns J. Morning Versus Evening Bright Light Treatment at Home to Improve Function and Pain Sensitivity for Women with Fibromyalgia: A Pilot Study. Pain Medicine. 2016:pnw160. doi: 10.1093/pm/pnw160. [DOI] [PubMed] [Google Scholar]
- 20.Burgess HJ, Swanson GR, Keshavarzian A. Endogenous melatonin profiles in asymptomatic inflammatory bowel disease. Scand J Gastroenterol. 2010;45(6):759–761. doi: 10.3109/00365521003749818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 22.Andrew ME, Penzien DB, Rains JC, Knowlton GE, McAnulty RD. Development of a computer application for headache diagnosis: the Headache Diagnostic System. Int J Biomed Comput. 1992;31(1):17–24. doi: 10.1016/0020-7101(92)90050-3. [DOI] [PubMed] [Google Scholar]
- 23.First M, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - Patient edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 24.Edinger JD, Wyatt JK, Stepanski EJ, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Archives of general psychiatry. 2011;68(10):992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- 25.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 27.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 28.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 29.Smitherman TA, Penzien DB, Rains JC, Nicholson RA, Houle TT. Advances in Psychotherapy: Evidence-Based Practice. Boston: Hogrefe; 2015. Headache. [Google Scholar]
- 30.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 32.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12(5):457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 33.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28(8):714–718. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17(2):181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 35.Burgess HJ, Wyatt JK, Park M, Fogg LF. Home Circadian Phase Assessments with Measures of Compliance Yield Accurate Dim Light Melatonin Onsets. Sleep. 2015;38(6):889–897. doi: 10.5665/sleep.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical power analysis for the behavioral sciences. Hilsdale, NJ: Lawrence Earlbaum Associates; 1988. p. 2. [Google Scholar]
- 39.Ong JC, Park M. Chronic headaches and insomnia: Working toward a biobehavioral model. Cephalalgia. 2012;32(14):1059–1070. doi: 10.1177/0333102412455709. [DOI] [PubMed] [Google Scholar]
- 40.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168(3):259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 2010;178(1):205–207. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess HJ, Park M, Wyatt JK, Fogg LF. Home dim light melatonin onsets with measures of compliance in delayed sleep phase disorder. J Sleep Res. 2016;25(3):314–317. doi: 10.1111/jsr.12384. [DOI] [PubMed] [Google Scholar]