Abstract
Background
Numerous studies have shown that there are links between obesity, liver fat, and the gut microbiome. However, there are mixed results on whether probiotics could impact the gut microbiome and/or help to decrease liver fat and obesity outcomes.
Objective
This study aimed to determine whether a probiotic supplement (VSL#3®) intervention altered gut microbiota and/or gut hormones associated with appetite regulation. The secondary aim of this study was to determine whether VSL#3® altered body composition and liver fat and fibrosis.
Methods
We conducted a double-blind, randomized placebo controlled trial in 19 obese Latino adolescents. The intervention consisted of three packets per day of VSL#3® or a matched placebo for 16 weeks. Pre- and post-intervention measures included gut microbial abundance, gut appetite regulating hormones, anthropometrics, body composition, liver fat and liver fibrosis. We conducted linear models to determine whether there were any significant differences in the changes in these outcomes following VSL#3® intervention.
Results
Compared to placebo, adolescents that received VSL#3 had significant increases in total adiposity (%) (+1.7±0.6 vs −1.3±0.5, p<0.01) and trunk adiposity (%) (+3.3 ± 0.8 vs −1.8 ± 0.8, p<0.01) with no significant effects on liver fat/fibrosis, insulin/glucose, gut microbial abundances or gut hormones. It is important to note that recruitment efforts were short of what was planned so this limitation must be considered.
Conclusion
VSL#3 supplementation may lead to increased adiposity in obese Latino adolescents with no significant detectable changes in gut microbiota, gut appetite-regulating hormones, liver fat and fibrosis and dietary intake.
Keywords: obesity, probiotics, gut microbiota, non-alcoholic fatty liver disease, body composition, Hispanic population, adolescents
INTRODUCTION
According to the Center for Disease Control and Prevention, the prevalence of adolescent obesity is 21.9% among Hispanic populations in the United States1. These high rates of obesity can lead to numerous co-morbidities including non-alcoholic fatty liver disease (NAFLD). NAFLD is a major chronic liver disease that can progress to non-alcoholic steatohepatitis (NASH) and is a risk factor for a variety of metabolic impairments such as type 2 diabetes2,3. While weight loss and dietary sugar restriction has been proposed for liver fat reduction, these strategies rely on volitional behavioral changes that may be difficult to sustain over the long-term. Thus, other approaches need to be identified to prevent NAFLD and related metabolic impairments among at-risk children and adolescents. Effective treatments are especially needed for obese Hispanic youth, who have a greater susceptibility to obesity and the accumulation of liver fat compared to other racial/ethnic groups4,5.
Emerging work in both animal and human models suggests a role for the gut microbiome in the etiology of liver fat accumulation and other metabolic abnormalities associated with obesity and type 2 diabetes6,7. It has been suggested that a obesogenic gut microbiota harvests more dietary energy, which leads to increased body mass8 and may have a negative impact on gut hormones7. Therefore, the gut microbiome presents a potential target for combating obesity and obesity-related diseases such as NAFLD.
Studies have shown that probiotic supplementation attenuated liver and abdominal fat accumulation in obese rats fed a high-fat diet9–12. In a study among Italian children with NASH, VSL#3 probiotic supplementation decreased BMI and NASH severity and increased circulating levels of the satiety hormone glucagon-like peptide-1 (GLP-1) compared to placebo13. In a recent meta-analysis of nine randomized controlled trials, probiotics were effective at improving blood lipids and insulin sensitivity among patients with NAFLD14. These findings highlight probiotic supplementation as a potential therapeutic strategy for reducing overall adiposity and liver fat in humans. However, to our knowledge, no studies have examined whether VSL#3 supplementation has the ability to reduce obesity, liver fat and fibrosis, metabolic markers and/or gut hormones among high-risk Hispanic youth. Furthermore, no studies have examined whether probiotic supplementation results in detectable changes in the relative abundance of bacteria in the gut in a population of Hispanic adolescents. Therefore, the aim of this study was to conduct a 16-week double-blind, randomized placebo controlled clinical trial in obese Hispanic adolescents in order to determine if VSL#3® treatment reduced adiposity, liver fat, and liver fibrosis or altered fasting levels of insulin, glucose, and/or gut hormones (e.g., GLP-1, ghrelin, and peptide YY).
METHODS
Study Design
Nineteen obese Hispanic adolescents (12–18 years of age) completed a 16-week parallel, double-blind and placebo-controlled trial examining the efficacy of probiotic supplementation in changing gut microbiota and gut-derived appetite regulating hormones as primary outcomes. Body composition, liver fat and liver fibrosis, plasma levels of insulin and glucose and food intake were also measured as secondary outcomes. We randomly assigned participants to one of two intervention groups: a treatment group that received daily supplementation of an active probiotic culture called VSL#3® (Probiotic, n=8), or a control group that received daily supplementation with a matched, inactive product (Placebo, n=11) (see Supplemental Methods). Participants consumed these supplements three times per day for 16 weeks. Non-caloric, sugar-free flavored drinks (VitaminWater Zero) were provided by the study team as the vehicle for consuming the supplements. Adherence and side effects were monitored via scheduled phone calls to the participants and via reporting logs. Outcomes measures and covariates were assessed at study entry and after 16–18 weeks of consuming the supplements. On occasions where participants could not make the 16-week visit, we scheduled their appointment within 2 weeks and the participants continued supplementation until that date; thus, the intervention lasted up to 18-weeks for some participants.
Participants
We recruited obese Hispanic adolescents from communities surrounding USC who met the following inclusion criteria: a) age 12–18 years, b) BMI percentile ≥95th for age and gender; c) self-identified Hispanic ethnicity; and d) post-puberty as determined by self-assessed of Tanner Stage ≥ 4. Participants were excluded for any of the following: a) diagnosis of any disease known to influence insulin action and secretion (including type 1 and type 2 diabetes); b) involvement in a weight loss, exercise or sports program within six months prior to participation; c) use of medication known to influence body composition or fat distribution, insulin resistance, gut function, or lipid profiles; d) history of renal/liver disease or any disease affecting liver fibrosis and steatosis; e) diagnosis/current treatment for celiac, inflammatory bowel syndrome, Crohn’s disease or other major gastrointestinal issues; f) any other disease that could compromise the immune system; g) current pregnancy; and h) current smoking (more than 1 cigarette in the past week or ≥200 cigarettes in lifetime) or use of other recreational drugs. Because antibiotics can impact the gut microbiome, we further excluded participants if they had used antibiotics within 30 days prior to study participation. Prescription of more than 1 week of antibiotics during the intervention also served as a withdrawal criterion.
Procedures
After an overnight fast for a minimum of 10 hours, participants arrived at the Diabetes and Obesity Research Institute at the University of Southern California (USC) between 7:00–9:00 am for their clinical visit. A registered physician conducted a medical exam to confirm participants’ eligibility and health status. The participants self-assessed pubertal development using a diagrammatic representation of Tanner staging adapted from Marshall and Tanner15. We conducted all study measures, see Supplemental Methods, at the beginning of the intervention to establish baseline and repeated measures at the end of the intervention to determine the effectiveness of the intervention. Briefly, a stool sample and fasting blood draw were collected to characterize the gut microbiota and measures fasting levels of insulin, glucose, and gut hormones. A DEXA scan was performed to assess body composition and a whole abdominal MRI scans was used to measure liver fat and fibrosis (see Supplemental Methods). Parental consent and child assent was obtained prior to any testing procedure. The University of Southern California’s (USC) Institutional Review Board approved all research. The trial was initially registered before the start of the trial under https://prstest.nlm.nih.gov which was a test site for clinicaltrials.gov. This trial is now registered at clinicaltrials.gov as NCT03115385.
Gut Microbiota Analysis
We collected stool samples using commercial collection kits developed by Second Genome (South San Francisco, CA) that contained a preservative and the samples was stored at −80°C immediately after receipt. If a participant was unable to provide the stool sample in person at the study visit, then the study team provided a prepaid envelope containing the collection kit so the participant could mail their sample to the lab within 1–2 days of the study visit; all samples were received at the lab within 5 days of the visit. The relative abundance of bacterial taxa was determined using 16S rRNA amplicon sequencing conducted by Second Genome (San Francisco, CA). Briefly, nucleic acid isolation was performed with the MoBio PowerMag® Microbiome kit (Carlsbad, CA) according to manufacturer’s guidelines and optimized for high-throughput processing. All samples were quantified via the Qubit® Quant-iT dsDNA High Sensitivity Kit (Invitrogen, Life Technologies, Grand Island, NY) to ensure that they met minimum concentration and mass of DNA. To enrich the sample for bacterial 16S V4 rDNA region, DNA was amplified utilizing V4 fusion primers described by Caporaso et al16. The complete sequences of the primers were: Foward – 5’ GTGCCAGCMGCCGCGGTAA 3’ and Reverse – 5’ GGACTACHVGGGTWTCTAAT 3’. Samples that met the post-PCR quantification minimum and were advanced for pooling and sequencing on the Illumina Miseq v3 sequencer platform. The 16S rDNA sequence reads were quality filtered, clustered into operational taxonomic units (OTUs) with a shared 97% identity by UPARSE (de novo OTU clustering), and a representative consensus sequence per de novo OTU was aligned against the Greengenes reference database (version 13.5)17 and assigned taxonomy to determine community profiles. The UPARSE clustering algorithm comprises a chimera filtering and discards likely chimeric OTUs. All non-strain sequences that passed the quality filtering were mapped to the representative consensus sequences to generate an abundance table for de novo OTUs. The raw 16S rRNA sequence reads used for this study are available on the NCBI Short Read Archive associated with the NCBI Bioproject PRJNA421553 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA421553).
Blood Assays and Dietary Recalls
Blood samples were collected after a 10-hour overnight fast. These samples were collected in green top tubes (glucose and insulin) and black top tubes (active and total ghrelin, active GLP-1 and PYY) that both contained spray-dried K2EDTA anticoagulant and proprietary protease inhibitors that perform similarly to a DPP-IV inhibitor (BD Biosciences, San Jose, CA, USA). Green top tubes were immediately centrifuged with black tops being centrifuged immediately after the green tops. The samples were centrifuged at 2,000g for 15 minutes at 4°C. Plasma was aliquoted into separate cryovials for each analyte. The plasma samples were then stored at −80°C until analysis in which the plasma was analyzed using ELISA kits from EMD Millipore Corp (St. Charles, MO, USA). Plasma glucose was assayed in duplicate using the glucose oxidase method and a Yellow Springs Instrument 2300 analyzer (YSI Inc., Yellow Springs, OH, USA). Insulin was assayed in duplicate using the Millipore ELISA kits.
To assess daily energy intake, 24-hour diet recalls were performed in duplicate (first in person, other by telephone) using the “multiple-pass” method and analyzed using the Nutrition Data System for Research software (NDSR) version 2014, developed by the Nutrition Coordinating Center, University of Minnesota.
Statistical Analysis
Distributions of trial outcomes were assessed for normality and when data were not normal we transformed the data to fit a normal distribution. Independent t-tests were used to confirm that both groups were matched on the primary study measures at baseline. Unless otherwise noted, all comparisons used the Welch t-test statistic (“unequal variances” t-test), which is considered the best approach for contrasting small, independent samples where unequal variances are assumed. Chi-square analysis was used to confirm that the groups did not significantly differ in gender distribution. For gut microbiota analysis, we log-normalized sequence read counts of each taxonomic group to account for varying sequencing depths across samples as well as meet the assumptions of normality using the following equation:
Taxa were retained if there was a total read count of at least 1,000 across samples and were present in at least 25% of samples. Changes in adiposity, metabolic measures and gut microbiota across the intervention were examined using multivariate linear models where the independent variable was treatment (VSL#3 or placebo). Baseline t-tests indicated that the groups significantly differed in BMI at the start of the study (p=0.04), so baseline BMI was included as a covariate in these analyses. Sex and change in energy intake was also used as covariates.
Linear models were used to assess the effect of probiotic vs. placebo supplementation on BMI, waist circumference, fat mass, fat free mass, adiposity (%), liver fat fraction, and liver fibrosis across the 16-week study period. We also assessed changes in the gut appetite regulating hormones and gut microbiota abundance using linear models. Results are presented as the mean change ± standard error (M ± SE) unless otherwise stated. All statistical analyses were performed in R statistical package version 3.3.1 with a priori significance level set at p < 0.0518.
RESULTS
Participant characteristics
Twenty-seven participants were recruited between March 2015 and November 2015 with 25 being randomized to either probiotic or placebo arm using an adaptive stratified block design (see Supplemental Methods). Of the 25 adolescents initially enrolled in this study, one participant was deemed ineligible due to a low Tanner development stage, one participant withdrew before completing the intervention, two participants were excluded for antibiotic-use, and one participant was excluded for probable diabetes (i.e., baseline fasting blood glucose > 126 mg/dl19). The final sample size was n=19 and the CONSORT diagram is shown in Figure 1. The participants experienced no severe adverse events during the intervention. The baseline participant characteristics are shown in Table 1. Independent t-tests confirmed that both groups were similar for age, weight, height, body composition, liver fat, liver fibrosis, fasting blood levels, and self-reported energy and macronutrient intake before beginning the intervention. Baseline BMI was significantly greater in the placebo group (p=0.04) compared to the probiotic group and, thus, was included as a covariate in the statistical analyses reported below. Chi-square confirmed that the groups did not significantly differ in gender distribution (p=0.58).
FIGURE 1. CONSORT flow chart for study procedure.
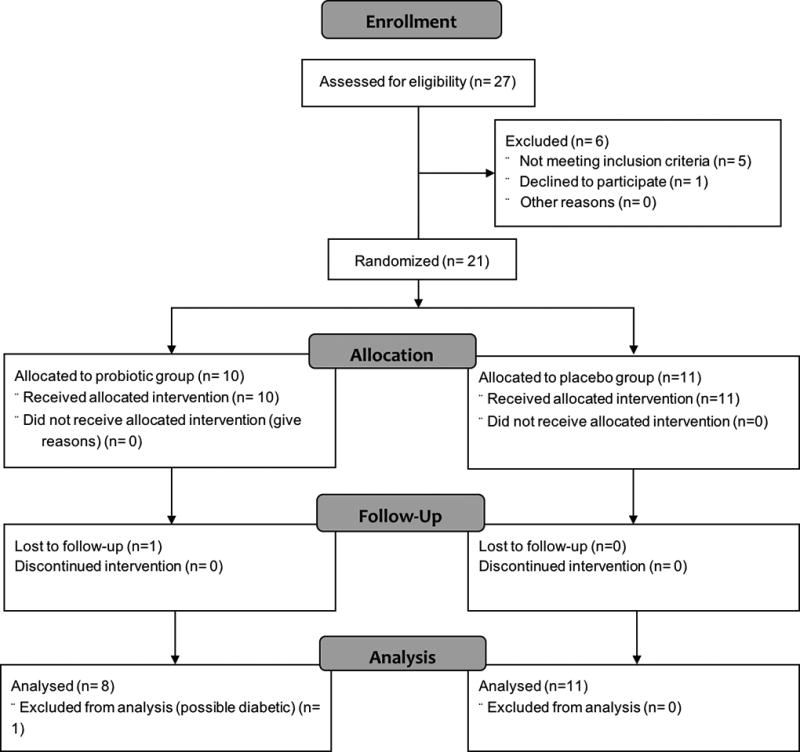
TABLE 1.
Baseline Characteristics
| Probiotic (n=9) | Placebo (n=11)a | |
|---|---|---|
| General characteristics | ||
| Age (years) | 14.4 (2.24) | 14.9 (1.81) |
| Sex (F/M) | 6/3 | 6/5 |
| Weight (kg) | 83.4 (16.0) | 90.8 (15.3) |
| Height (cm) | 164.0 (8.8) | 161.9 (5.7) |
| Body Composition: DXA and MRI | ||
| BMI (kg/m2) | 30.7 (3.3)* | 34.5 (4.3) |
| BMI Percentile | 97.3 (1.3) | 98.0 (1.7) |
| Waist Circumference | 96.9 (13.0) | 104.6 (13.7) |
| Total Fat Mass (kg) | 33.3 (6.3) | 37.3 (8.0) |
| Total Lean Mass (kg)b | 45.8 (10.1) | 49.0 (9.6) |
| Total Adiposity (%)c | 41.0 (3.4) | 42.2 (5.1) |
| Trunk Fat Mass (kg) | 15.6 (2.3) | 18.8 (5.1) |
| Trunk Lean Mass (kg) | 22.1 (4.4) | 23.6 (4.5) |
| Trunk Adiposity (%) | 40.8 (4.0) | 43.40 (5.8) |
| Visceral Adipose Tissue (liters) | 1.54 (0.7) | 1.91 (0.9) |
| Subcutaneous Adipose Tissue (liters) | 6.75 (1.8) | 7.86 (2.5) |
| Liver Condition | ||
| Liver Adiposity (%) | 4.50 (1.0) | 10.43 (9.3) |
| Liver Fibrosis by MRE (kPa) | 2.15 (0.2) | 2.31 (0.5) |
| Macronutrient Intake | ||
| Total Caloric Intake | 1644.2 (305.9) | 1878.8 (225.0) |
| Total Carbohydrates (%) | 52.7 (7) | 53.8 (10.3) |
| Total Sugar (%) | 23.6 (8.3) | 20.7 (5.2) |
| Added Sugar (%) | 11.7 (6.8) | 10.8 (5.3) |
| Total Fat (%) | 29.5 (4.1) | 28 (4.5) |
| Total Protein (%) | 17.8 (6) | 18.1 (6.6) |
Mean (SE) for the N=20 participants at baseline.
Group differences assessed with Welch t t-tests at *p<0.05. All comparisons were NS except BMI, which was included as a covariate in the subsequent data analyses.
Lean mass reflects lean tissue, excluding bone and water content.
Adiposity calculated as percent of fat mass (g) to total mass (g)
Effect of the Intervention on the Gut Microbiota, Gut hormones and Fasting Blood Measures
When comparing the microbiota of the placebo and probiotic treatment groups before intervention for each microbe and for overall diversity indices, there appeared to be significant (significance defined by a p < 0.05 and at a 10% false discovery rate) differences in the gut microbiota of the two groups at baseline. Because of pre-intervention differences, we performed linear models for each of the microbes adjusting for baseline abundance and baseline BMI. When we conducted a multidimensional scaling at the family-level taxonomic classifications using Bray-Curtis dissimilarity metric, we found that the intervention had no effect on the overall composition of the gut microbes or individual microbes at all taxonomic levels (Figure 2) (Table 2). There were no significant effects of treatment on changes in leptin, active or total GLP-1, total ghrelin, and peptide YY (Table 2). In addition, fasting glucose and insulin were not significantly affected by treatment (Table 2).
FIGURE 2. Gut microbial composition was not significant altered after intervention.
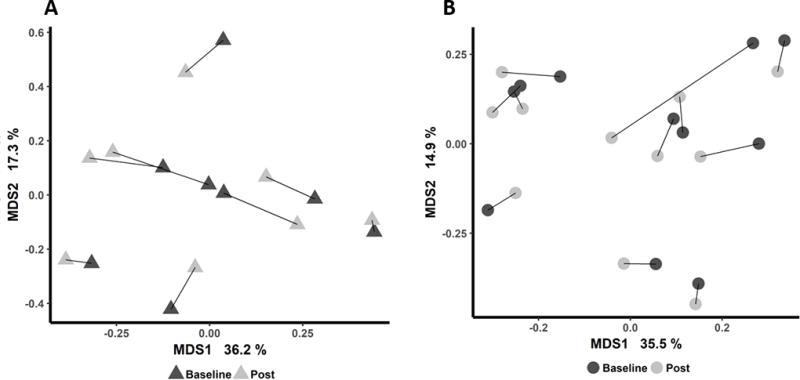
Multiple dimension scaling using Bray-Curtis dissimilarity metric for baseline and post-intervention family-level taxonomic classifications of 16S sequence reads for (a) probiotic and (b) placebo groups. Each participant’s baseline and post samples are connected with a line.
TABLE 2.
Change in Primary Outcome Variables Across Intervention
| Probiotic (n=8) | Placebo (n=11) | P-valuea | |
|---|---|---|---|
| Percent Change in Gut Hormones | M (SE) | M (SE) | |
| Leptin | 2.1 (9.7) | −1.9 (8.2) | 0.68 |
| GLP-1 Total | 4.3 (13.8) | −0.5 (7.2) | 0.74 |
| GLP-1 Active | 121.4 (106.7) | 59.2 (58.8) | 0.62 |
| Ghrelin | 4.3 (11.6) | 8.8 (7.8) | 0.56 |
| PYY | −6.1 (6.2) | 16.9 (15.5) | 0.08 |
| Percent Change in Blood Parameters | |||
| Glucose (mg/dl) | 2.8 (1.9) | −0.8 (1.6) | 0.13 |
| Insulin (µU/ml) | 2.3 (1.3) | 0.2 (1.3) | 0.36 |
| Percent Change in Diversity Indices | |||
| Shannon Index | −7.1 (4.6) | −6.3 (3.4) | 0.93 |
| Simpson Index | −2.9 (1.7) | −2.7 (1.5) | 0.87 |
| Inverse Simpson | −13.6 (19.6) | −20.3 (11.6) | 0.87 |
| Richness | −3.8 (3.4) | −3.2 (5.2) | 0.62 |
| Evenness | −6.7 (4.2) | −5.6 (3.2) | 0.8 |
| Percent Change in Phylum-level Microbiota Logged Abundance | |||
| Actinobacteria | −4.4 (4.4) | 2 (1.7) | 0.16 |
| Bacteroidetes | 3.1 (2) | 2.7 (1.3) | 0.74 |
| Cyanobacteria | −1.4 (9.2) | −0.9 (4.4) | 0.49 |
| Euryarchaeota | −6.9 (11.2) | −9.9 (8.1) | 0.56 |
| Firmicutes | −1 (0.8) | −1.1 (0.4) | 0.56 |
| Fusobacteria | −4.7 (5.1) | 13.7 (11.7) | 0.26 |
| Lentisphaerae | 0 (5.3) | −4.6 (6.6) | 0.86 |
| Proteobacteria | −2.5 (1.6) | 1.2 (2.9) | 0.62 |
| Tenericutes | −15 (10.8) | −5.7 (9.1) | 0.51 |
| Verrucomicrobia | 2.8 (25) | −5.9 (5.4) | 0.74 |
Data are unadjusted change scores, M (SE). P-value reflects the time by treatment interaction obtained from a linear mixed model controlling for baseline BMI.
Denotes significant difference in percent changes by intervention group at p < 0.05 using Welch t-tests
Effects of Intervention on Body Composition and Liver Measurements
The effects of the intervention on body composition outcomes are summarized in Table 3. As shown in Table 3, participants in the VSL#3® group exhibited a significant increase in adiposity across time, whereas the placebo group exhibited a decrease in adiposity. These findings were independent of baseline BMI, sex, and change in energy intake. Separate analyses of total body fat, trunk fat and lean mass confirmed that changes in fat percentage were specifically due to alterations in fat mass, which increased for participants in the VSL#3® group. These alterations in body composition were accompanied by an increase in BMI in the VSL#3® group relative to the placebo group. Waist circumference was not significantly dependent upon treatment. There were no significant effects of treatment on visceral or subcutaneous adiposity. There was no evidence that probiotic supplementation altered liver fat percent or liver fibrosis. We also observed that treatment with VSL#3® did not result in any significant changes in energy intake of dietary macronutrients.
TABLE 3.
Percent Change in Secondary Outcome Variables Across Intervention
| Probiotic (n=8) | Placebo (n=11) | P-valuea | |
|---|---|---|---|
| Change in Body Composition and Adiposity | M (SE) | M (SE) | |
| BMI (kg/m2) | 0.6 (0.2) | −0.07 (0.3) | 0.06 |
| Waist Circumference | 3.6 (1.7) | −0.3 (1.7) | 0.65 |
| Total Fat Mass (kg) | 2.1 (5.7) | −0.9 (0.6) | <0.01 |
| Total Lean Mass (kg) | −0.7 (0.7) | 1.3 (0.6) | 0.12 |
| Total Adiposity (%) | 1.7 (0.6) | −1.3 (0.5) | <0.01 |
| Trunk Fat Mass (kg) | 2.3 (0.8) | −0.2 (0.4) | <0.01 |
| Trunk Lean Mass (kg) | −0.1 (0.8) | 1.3 (0.5) | 0.14 |
| Trunk Adiposity (%) | 3.3 (0.7) | −1.8 (0.8) | <0.01 |
| VAT (liters) | 0.003 (0.4) | 0.07 (0.05) | 0.22 |
| SAT (liters) | 0.3 (0.4) | 0.04 (0.2) | 0.68 |
| Change in Liver Condition | |||
| Liver Adiposity (%) | 0.004 (0.4) | −0.8 (1.3) | 1.0 |
| Liver Fibrosis by MRE (kPa) | 0.3 (0.4) | 0.04 (1.2) | 0.46 |
| Change in Macronutrient Intake | |||
| Total Caloric Intake | 212.3 (296.0) | 108.7 (190.3) | 0.33 |
| Total Carbohydrates (%) | −0.2 (2.3) | 2.3 (3.7) | 0.79 |
| Total Sugar (%) | −2.4 (2.6) | −0.5 (2.2) | 0.42 |
| Added Sugar (%) | 1.4 (1.8) | 2.2 (2.2) | 0.66 |
| Total Fat (%) | 0.8 (1.8) | 0.36 (2.4) | 0.93 |
| Total Protein (%) | −0.6 (1.9) | −2.6 (2.4) | 0.66 |
Data are unadjusted percent change scores (relative to baseline), M (SE). P-value reflects the time by treatment interaction obtained from a linear mixed model controlling for baseline BMI.
VAT, visceral adipose tissue; SAT subcutaneous adipose tissue.
DISCUSSION
Previous studies suggest that VSL#3® supplementation may improve liver fat, NASH, adiposity, and alter gut hormones13. To our knowledge, no studies have examined the efficacy of VSL#3® supplementation among obese Hispanic adolescents who are at increased risk for obesity and NAFLD. Further, it is currently unknown how VSL#3® supplementation may impact gut microbial diversity and/or relative abundance. Therefore, the aim of this study was to determine if a probiotic supplementation with VSL#3® would alter the gut microbiota and/or obesity related outcomes, including liver fat, in obese Hispanic adolescents. Overall probiotic supplementation did not alter the gut microbiota or fasting glucose or insulin levels. However, VSL#3® supplementation resulted in an increase of total and trunk adiposity in the absence of any changes in reported dietary intake or gut hormones related to appetite.
To our knowledge, this is the first study to show that VSL#3® supplementation may lead to increased adiposity in an obese population. There has been various studies that have shown that treatment with various Lactobacillus species have been associated with weight gain in animals and lean adults20. Yet, previous studies in overweight or obese adults have shown that probiotics lead to weight loss or have no effects on body weight21–23. It is important to note that the current work was modeled after a 16-week probiotic supplementation intervention study in obese (≥85 BMI percentile) Italian children (ages 9–12) with NASH using the same VSL#3® formulation13. Results from the Alisi et al study showed that the severity of NASH and BMI was decreased in the probiotic compared to the placebo group. Their study also found that VSL#3® supplementation increased circulating levels of the satiety hormones glucagon-like peptide-1 (GLP-1) and activated GLP-1. While the study by Alisi et al, suggests that VSL#3® supplementation leads to decreases in adiposity, our study provides evidence that this there may be differential responses to probiotic supplementation across individuals as a function of age, obesity status, and/or race/ethnicity.
In the current study, VSL#3® compared to placebo did not alter the composition of the gut microbiota. Since there were no changes in the gut microbiota over the intervention, it is unlikely that changes in the gut bacterial abundance contributed to increased adiposity. However, it is possible that the functional potential of the gut microbiota was altered and future studies should perform untargeted metagenomics or metaproteomics in order to examine this possibility. Even further, we found that the 16S rRNA sequence reads assigned to the same taxonomic classification as the bacteria in VSL#3® (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum subsp. longum, Bifidobacterium longum subsp. infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. bulgarius) were only observed in the stool sample of one participant in the VSL#3® group. The lack of sequence reads that map to the bacteria in VSL#3® suggests that these bacteria was not at a detectable level to observe any changes after the intervention. The sequence reads that were used were extracted from the V4 region of the 16S rRNA gene and it this region has been shown to be highly sensitive in differentiating species-level bacteria in VSL#3®24.
Although this study is strengthened by its randomized controlled study design, detailed measures of body composition, fasting blood measures, and the gut microbiota, it is limited by its small sample size. Due to increases in adiposity and termination of funding, the intervention was ended prior to enrolling 20 participants in each arm. At our intended sample size, the power to detect intervention differences of 6.8% in liver adiposity would have been 81%. With our current sample size, this power is falls to 47% which can make us underpowered to detect differences in change of liver fat percentage. This could be a reason we do not detect a significant change in gut microbiota but this is not certain as we did not do a power analysis for changes in gut microbiota (there are no other studies that examine the impact of VSL#3® on the gut microbiota of youth so there is limited data for a power analysis for changes in gut microbiota). This study is also limited by the use self-reported dietary recalls, which may have limited our ability to detect small changes in energy intake that could may have contributed to increased adiposity over the course of the intervention. Although unlikely, it is possible that unmeasured decreases in physical activity contributed to the weight in the treatment group over the study period. Also, it must be noted that the participants used VitaminWater Zero as a means for delivery of probiotic or placebo up to three times a day. The beverage contains erythritol and stevia leaf extract as sweeteners. A study by Suez et. al.25 showed that consumption of artificial sweeteners (including saccharin-, sucralose- or aspartame-based) could increase glucose intolerance in mice and humans. Our beverage does contain the artificial sweeteners erythritol and stevia, and to our knowledge there are no studies examining the impact of these sweeteners on gut microbiota so little is known about how these sweeteners could affect gut microbiota in these adolescents. Despite these possible limitations, this is the first to examine the effects of probiotic supplementation in Hispanic adolescents, who are at increased risk for obesity and NAFLD. Importantly, results from this study suggest that this population may have undesired responses to probiotics, which should be investigated larger and more racially and ethnically diverse study populations.
In conclusion, results from this study show that a 16-week probiotic supplementation with VSL#3® significantly increased total adiposity among obese Hispanics adolescents in the absence of alterations in the gut microbiota. Additionally, VSL#3® supplementation also did not alter liver fat, gut hormones or fasting measures of glucose or insulin among minority youth at high-risk for NAFLD. Additional studies are needed to determine the mechanisms by which VSL#3® supplementation may lead to increased adiposity in this population and further research could help to guide what populations could benefit from the protective effects of probiotics against obesity.
Supplementary Material
Acknowledgments
Sources of support: L.K. Whittier Foundation Grant #003457-00001
Footnotes
Clinical trial registered at www.clinicaltrials.gov: NCT03115385
All authors have read and approved the manuscript as submitted. All authors declare no conflict of interest.
Authors’ contributions to manuscript
MIG and TLA designed the research; TLA, AAM, and BAG conducted research; DHH and SLP processed MRI images and assessed MRE; RBJ and AAM analyzed data and performed statistical analysis; RBJ, AAM and TLA wrote the paper; RBJ and MIG had primary responsibility for final content.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. [Accessed March 22, 2017];Key findings What was the prevalence of obesity among adults in 2011–2014? 2011 http://c.ymcdn.com/sites/www.acutept.org/resource/resmgr/Critical_EdgEmail/0216-prevalence-of-obesity.pdf.
- 2.Newton KP, Hou J, Crimmins NA, et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016;92123:e161971. doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh JA, Karpen S, Vos MB. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162(3):496–500. e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quirós-Tejeira RE, Rivera CA, Ziba TT, Mehta N, Smith CW, Butte NF. Risk for Nonalcoholic Fatty Liver Disease in Hispanic Youth With BMI ≥95th Percentile. J Pediatr Gastroenterol Nutr. 2007;44(2):228–236. doi: 10.1097/MPG.0b013e31802d4acc. [DOI] [PubMed] [Google Scholar]
- 6.Machado MV, Cortez-Pinto H. Diet, Microbiota, Obesity, and NAFLD: A Dangerous Quartet. Int J Mol Sci. 2016;17(4):481. doi: 10.3390/ijms17040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirpich IA, Marsano LS, Mcclain CJ. Gut–liver axis, nutrition, and non-alcoholic fatty liver disease ☆. Clin Biochem. 2015;48:923–930. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 9.Esposito E, Iacono A, Bianco G, et al. Probiotics Reduce the Inflammatory Response Induced by a High-Fat Diet in the Liver of Young Rats. J Nutr. 2009;139(5):905–911. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 10.Xu R, Wan Y, Fang Q, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2011;50(1):72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanida M, Shen J, Maeda K, et al. High-fat diet-induced obesity is attenuated by probiotic strain Lactobacillus paracasei ST11 (NCC2461) in rats. Obes Res Clin Pract. 2008;2(3):159–169. doi: 10.1016/j.orcp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-Y, Park J-H, Seok S-H, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta - Mol Cell Biol Lipids. 2006;1761(7):736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Alisi A, Bedogni G, Baviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39(11):1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Zhu Y, Wen Y, Liu G, Wan C. Efficacy of probiotics in nonalcoholic fatty liver disease in adult and children: A meta-analysis of randomized controlled trials. Hepatol Res. 2016 Feb; doi: 10.1111/hepr.12671. [DOI] [PubMed] [Google Scholar]
- 15.Marshall Wa, Tanner JM. Variations in the Pattern of Pubertal Changes in Boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. (Supplement 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Env Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Development Core Team. R Found Stat Comput Vienna Austria. Vol. 0. 2016. R: A Language and Environment for Statistical Computing. [DOI] [Google Scholar]
- 19.American Diabetes Association AD. Dabelea D, Rewers A, et al. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. (Supplement 1) [DOI] [PubMed] [Google Scholar]
- 20.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Agerholm-Larsen L, Raben A, Haulrik N, Hansen A, Manders M, Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. [Accessed April 17, 2017];Eur J Clin Nutr. 2000 54:288–297. doi: 10.1038/sj.ejcn.1600937. http://search.proquest.com/docview/219659539?pq-origsite=gscholar. [DOI] [PubMed] [Google Scholar]
- 22.Kadooka Y, Sato M, Imaizumi K, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64(6):636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 23.Woodard GA, Encarnacion B, Downey JR, et al. Probiotics Improve Outcomes After Roux-en-Y Gastric Bypass Surgery: A Prospective Randomized Trial. doi: 10.1007/s11605-009-0891-x. [DOI] [PubMed] [Google Scholar]
- 24.de Vrese M, Winkler P, Rautenberg P, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: A double blind, randomized, controlled trial. Clin Nutr. 2005;24(4):481–491. doi: 10.1016/J.CLNU.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


