Abstract
Background
Clinical trials of baked milk (BM) introduction have demonstrated accelerated resolution of milk allergy.
Objective
Long-term data regarding real world introduction of BM is lacking. We sought to characterize our experience of BM introduction.
Methods
We performed a retrospective chart review of consecutive BM oral food challenges performed in our clinic from 2009 – 2014, with a minimum follow-up of 24 months.
Results
Of the 206 patients challenged, 99 (48%) passed and 187 were sent home with detailed instructions to incorporate BM into their diets. After a median of 49 months of follow-up, 43% of the 187 had progressed to direct milk, 20% to less-cooked forms of milk, 10% remained ingesting BM, and 28% were strictly avoiding milk. Higher milk-IgE levels were associated with decreased odds of passing a BM challenge and advancing to less-cooked forms of milk. Predictors of progressing to less-cooked forms of milk were passing the challenge and younger age. There were 79 reported milk reactions involving 68 patients (33% of total) during follow-up. Of these, 78% were classified as mild, 14% severe, and 6 patients developed eosinophilic esophagitis. Of 11 severe reactions, 4 were accidental exposures, 3 were planned escalations, and 4 occurred with previously tolerated doses.
Conclusions
The majority of patients who underwent a BM challenge, including those who failed their challenge, were able to progress to direct or less-cooked forms of milk. However, adverse reactions were common, and even a successful BM challenge does not guarantee future tolerance of BM or preclude later reactions, even to previously tolerated doses.
Keywords: milk allergy, baked milk, oral food challenge
Introduction
Cow’s milk allergy is the most common food allergy in infants and young children. Successful introduction of baked milk (BM) to milk allergic patients is associated with accelerated resolution of milk allergy. (1–3) It has become common clinical practice to challenge patients with milk allergy to BM, and then add BM into the diet in increasing quantities, sometimes with repeated oral food challenges (OFCs).(4) However, while this clinical practice is widespread, long term follow up of baked milk introduction is limited with almost no information regarding the real-world introduction of baked milk outside of the research setting.
In clinical trials of baked milk introduction, successful challenge with unheated milk is often used as the measure of resolution of cow’s milk allergy.(2, 5) While this is a critical outcome, real world progression of milk ingestion is not binary, in that there are varying forms and amounts of milk tolerated in individual patients, ranging from small quantities of baked milk to baked cheese to direct milk. In prior clinical trials, adverse events in follow-up, which occurred in approximately 10% of the patient population, were most often attributed to inadequately heated milk.(2) In this study, we sought to characterize our clinical experience with the introduction of BM and other forms of milk after challenge, including the relationship of pre-challenge and in-challenge characteristics with future successful milk introduction, as well as the safety of this procedure in clinical practice.
Methods
We performed a retrospective chart review of consecutive BM OFCs performed in our clinic from 2009 – 2014. The study was approved by the Johns Hopkins Institutional Review Board. We excluded patients challenged for non-IgE mediated reactions such as food protein induced enterocolitis syndrome or eosinophilic esophagitis (EoE); 86% of the patients included had had a prior reaction to milk and all were strictly avoiding all forms of milk at the time of challenge. We included patients with at least 24 months of follow up data, whether through chart review or telephone follow up. Patients without two years of follow up data available via chart review were contacted by telephone to assess their current milk intake, as well any history of adverse reactions to milk, since the time of the last documented clinic visit. Clinical and demographic characteristics were collected, including gender, age, cow’s milk IgE at the time of challenge, details of BM OFC, and presence of other atopic diseases. In our clinic, patients typically undergo a graded challenge to a 2 gram (¼ cup) baked milk goal dose without a challenge to direct milk(6). Patient families prepare the challenge cake or muffin, using a recipe similar to those published previously(7). Occasionally patients are challenged to lower goal doses at the discretion of the clinician. We defined passing the challenge as consuming the entire goal dose without need for medications. Specific symptoms and use of medications are recorded. Of note, some patients who fail their challenge are sent home with instructions to consume an individually prescribed starting dose of BM, determined to be safe based on the details of the OFC. For example, a patient who experiences mild symptoms at the full 2 gram dose might be sent home to start BM introduction at a 0.5 gram dose.
Patients are instructed to consume the recommended starting dose a minimum of 3–5 days per week. After tolerating this dose without symptoms for 2–3 months, patients are instructed to double their dose, and continue in this fashion until tolerating at least 2 grams of baked milk at least 3–5 times per week for 2–3 months. At this point, patients are permitted to advance their milk ingestion to less-heated forms of milk such as pancakes and waffles, then progress to oven baked cheese, and eventually uncooked dairy products if there are no symptoms in prior steps. Advancements are carried out at home, except some select patients who advanced to direct milk under observation in the clinic. Patients are instructed to notify the clinic in the event of any adverse reactions. In evaluating these reactions reported during and between clinic visits, reactions were first analyzed for organ system involvement, then categorized as mild, moderate, or severe based on the number and type of organ systems involved.(8) The inciting ingestion and treatment of adverse reactions were also recorded when available.
Analysis
Data were analyzed using STATA 14. Logistic regressions were used to characterize predictors of progression to less heated and/or more concentrated forms of milk, which were categorized as less heated (e.g. pancakes or waffles), baked cheese, and direct milk. Logistic regressions were also used to characterize predictors of milk avoidance at time of follow-up. IgE values were log-transformed to account for right skewing. Challenge outcomes were categorized as tolerating <0.5 g of baked milk, 0.5–2 g of baked milk, or the full 2 g of baked milk, as well as the need for treatment and the specific treatment administered.
Results
Two hundred and six individuals were analyzed, and an additional 49 were lost to follow-up (Table 1). Of the 206, 140 were male, with a median age of 6.8 years (range 4 months-20 years) and a median milk-IgE of 7.3 kU/L (range 0.15–424) at the time of challenge. The median duration of follow-up was 49 months (range 24 – 93 months). The group lost-to follow-up was significantly older with a median age of 8.8 years (range 2–20.7 years), but otherwise was similar in composition. Ninety-nine patients passed and 107 failed their challenge. The median milk IgE of those who failed the BM OFC was 12.0 kU/L (IQR 5.4–26.0), compared to a median of 4.8 kU/L (IQR 2.5–10.2) in those who passed (P <0.001). (Figure 1) All who passed their challenge were sent home on BM and of those who failed, 19 (median ingested amount 500 mg; range 20 mg- 1 g) were recommended strict milk avoidance due to the severity or threshold of their reaction, and 88 (median ingested amount 1g; range 100 mg-2 g) were sent home with instructions to include some amount of BM in their diet. Among the 107 patients who failed the challenge, 58 required some form of treatment, including 24 who required epinephrine. Eighty-five of the 206 patients (42%) consumed the full 2 g dose, 97 (47%) consumed between 0.5–2 g, and 24 (11%) consumed less than 0.5 g of BM.
Table 1.
| 206 Individuals Analyzed | 49 Individuals without Follow-up | |
|---|---|---|
| Male (N, %) | 140 (68%) | 30 (61%) |
| Age, Median (range)* | 6.8 years (4 months-20 years) | 8.8 years (2 years-20.7 years) |
| Milk IgE, Median (range) | 7.3 kU/l (0.15 – 424 kU/l) | 5.9 kU/l (0.18 – 84 kU/l) |
| Duration of follow up | 49 months (24–93 months) | NA |
| Passed Challenge | 99 (48%) | 31 (63%) |
| Failed Challenge | 107 (52%) | 18 (37%) |
| Sent home with some milk | 88 (81%) | 16 (89%) |
| Ingested amount among those who sent home with milk | 1 gram (100 mg – 2g) | |
| Ingested amount among those who sent home on avoidance | 500 mg (20 mg – 2g) | |
| Required treatment in OFC | 58 (54%) | 7 (39%) |
| Required epinephrine in OFC | 24 (22%) | 4 (22%) |
Statistically significant difference with p<0.05
Figure 1. Relationship of Milk-IgE to BM Challenge Outcome.
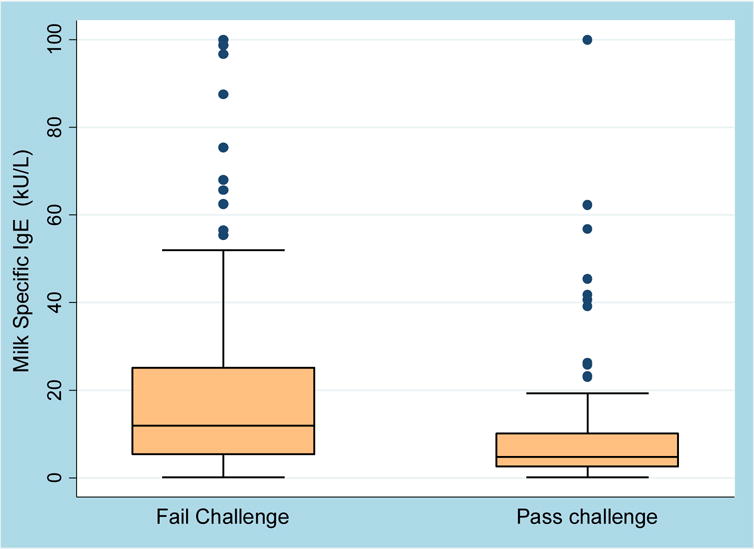
Of those who failed the BM OFC, the median IgE was 12.0 (IQR 5.4–26.0) and of those who passed, the median IgE was 4.8 (IQR 2.5–10.2, P <0.001). Values>100 are represented as 100.
At time of last follow up, 82 out of the total population (40%) had advanced to direct milk, 26 (12.6%) were eating baked cheese, 9 (4.4%) were eating less heated milk, 21 (10%) were eating BM, and 68 (33%) were avoiding all milk (Figure 2). When examining only those patients who passed their BM challenge, 54 (54%) were tolerating direct milk, 26 (26%) were ingesting baked cheese or BM, and 19 (19%) were strictly avoiding milk. When examining the 88 patients who failed their challenge and were sent home to include some amount of BM in their diets, only 26 (29%), had progressed to direct milk, 29 (33%) were consuming some baked cheese or BM, and 33 (38%) were avoiding all milk products.
Figure 2. Challenge Outcome and Milk Intake at Last Follow-up.
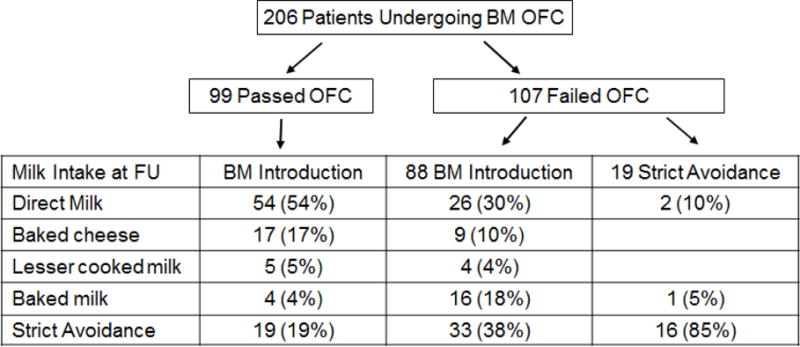
Flow chart describing challenge outcome and long term follow-up of milk intake.
The length of follow-up did not significantly alter the proportion progressing with milk ingestion. Among those with the shortest follow-up period (24–36 months), 31% had advanced to direct milk, while 42% were practicing strict avoidance (Figure 3). Among patients with 36–60 months of follow-up, 43% were consuming direct milk, and the proportion of patients practicing strict avoidance was 28%. These numbers were similar to the >60 month follow-up group, with 42% consuming direct milk and 34% practicing strict avoidance (p=0.71).
Figure 3. Relationship of Milk Advancement to Length of Follow-up.
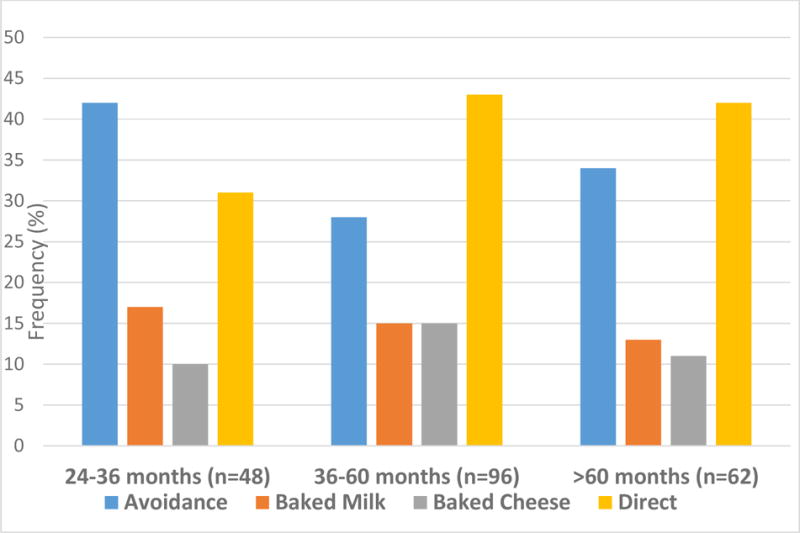
Among those with the shortest follow-up period, 31% advanced to direct milk and 42% were practicing strict avoidance at last follow-up. Among patients with 36–60 months of follow-up, 43% were consuming direct milk, and 28% practicing strict avoidance. In the longest follow-up group, 42% were consuming direct milk and 34% were practicing strict avoidance (P=0.71)
In a model accounting for length of follow up, other allergic diseases, and whether the patient/subject passed the challenge, higher log transformed milk-IgE at time of challenge was associated with decreased odds of progressing to more allergenic forms of milk during the follow-up period (OR 0.73, 95% CI 0.56–0.95), as was age at challenge (OR 0.91 95% CI 0.83–0.99 per year of age). Passing the challenge was associated with greater odds of advancing to more allergenic forms of milk (OR 4.9, 95% CI 2.5–9.6). (Table 2) Presence of asthma, eczema, other food allergy, or allergic rhinitis was not associated with odds of successfully advancing to higher levels of milk. The dose tolerated did not affect the outcome, beyond its association with passing the challenge.
Table 2.
Relationship between Predictors and Likelihood of Advancement in Milk Ingestion During Follow-up
| Crude Odds Ratios (95% confidence interval) | Adjusted Odds Ratio (95% confidence interval) | |
|---|---|---|
| Log-transformed Cow’s Milk IgE | 0.60 (0.47–0.76) | 0.73 (0.56–0.96) |
| Pass challenge | 5.32 (2.90–9.77) | 4.56 (2.46–9.56) |
| Age at challenge (years) | 0.90 (0.86–0.99) | 0.91 (0.83–0.99) |
| History of Eczema | 0.95 (0.53–1.72) | 0.94 (0.45–1.95) |
| History of Asthma | 0.72 (0.41–1.25) | 0.97 (0.49–1.92) |
| History of Rhinitis | 1.14 (0.66–1.98) | 1.61 (0.83–3.12) |
| Other food allergy | 0.73 (0.33–1.62) | 0.88 (0.34–2.23) |
| Length of Follow up (years) | 1.21 (0.98–1.49) | 0.91 (0.79–1.29) |
Those who received treatment in challenge were less likely to advance to higher levels of milk ingestion, with 71% of patients who required epinephrine and 58% of those who required any treatment in challenge practicing strict avoidance at follow up. Of the 58 patients requiring any treatment during challenge, 39 were sent home to introduce some quantity of baked milk, and of those 18 (46%) eventually returned to strict avoidance of milk. Patients who required any treatment during challenge had 2.5 times greater odds (OR 2.5, 95% CI 1.1- 5.8) of strictly avoiding milk at the time of last follow-up than those who did not require treatment during challenge, even when controlling for milk-IgE level and other atopic disease.
With regard to adverse reactions, of the 187 patients instructed to begin some level of BM introduction, there were 78 reported milk reactions involving 66 patients (35%) during follow up. Of these, the majority (59 events, 77%) were classified as mild, 11 (14%) were classified as severe, 6 (7.7%) were EOE, and 2 (2%) were unclassified (Table 3). Of the 11 severe IgE-mediated reactions, 4 were accidental exposures, 3 occurred with planned escalations (1 with pizza and 2 with increased doses of baked milk), and 4 occurred with previously tolerated doses (3 with baked milk and 1 with baked cheese). Eight reactions were treated with epinephrine, including 2 accidental exposures, 2 with planned escalations, and 4 occurring with previously tolerated doses. Three of the reactions requiring epinephrine involved post-ingestion exercise or activity, including 2 at previously tolerated doses and 1 with a planned escalation. None of the patients with severe reactions received epinephrine during their in-office challenge, 3 received an antihistamine, 2 received albuterol, and 1 received corticosteroids. There was no statistically significant difference in adverse events by challenge dose, with 46% of those consuming <0.5 g in challenge having adverse events in follow up, compared to 32% of those consuming 0.5–2 grams and 31% of those consuming 2 grams. However, of the non-accidental adverse events requiring epinephrine, none of the patients had consumed the whole 2 gram dose during in office baked milk challenge.
Table 3.
Adverse events with home milk introduction
| Occurred with | |||||
|---|---|---|---|---|---|
| 78 total reactions | Treated with epinephrine | Previously tolerated Dose | Planned escalation | Accidental exposure | |
| Severe | 11 | 2 | 4 | 3 | 4 |
| Mild | 59 | 3 | 16 | 15 | 9 |
| EOE | 6 | ||||
Among the patients sent home to initiate some level of BM, 52 (28%) were avoiding milk completely at time of follow-up. While many patients gave no single reason for stopping the BM introduction, 8 patients reported reactions to more concentrated forms of milk, often by accident, which led to discontinuation of all milk ingestion. Six stopped after the new diagnosis of EoE and 13 patients reported stopping due to bothersome symptoms with dosing, predominantly itchy mouth, GI symptoms (including the 6 with new onset EoE), and eczema, and 2 patients stopped after having a severe reaction.
Discussion
Introduction of baked milk into the diet of patients with milk allergy can substantially increase food choices and enhance the development of tolerance to other forms of milk.(2) In this study of BM introduction in a clinical setting, we found that the majority of patients who are sent home with instructions to ingest BM are able to progress to direct milk (43%), baked cheese (14%), or less heated forms of milk (5%). A small proportion (10%) remain on baked milk only, while a significant number (28%) are practicing complete milk avoidance 2 to 7 years after their initial OFC. Overall, our results are similar to those reported in other trials of BM introduction. For example, in one study at the end of the follow up period 47% could tolerate unheated-milk, 24% could tolerate some form of baked-milk/baked-cheese in their diet, and 30% were avoiding all forms of milk(3).
In assessing the clinical factors that may help predict the likelihood of success with baked milk introduction, not surprisingly we found that the results of the initial BM challenge most strongly predicted future outcomes. In addition, we found that milk-specific IgE and age were inversely associated with success of milk introduction (Table 2). However, it is also important to recognize that there is tremendous individual variability within these overall associations. For example, many patients with completely successful BM challenges were never able to successfully introduce BM into their diets, while others requiring epinephrine in their challenge are now tolerating direct milk. With regard to milk-IgE levels, while a prior study found no successful BM challenges in patients with a milk-IgE >20.4 kU/l(1, 7), we had patients with IgE levels as high as 424 kU/l successfully advance to direct milk, while others with IgE levels as low as 2.5 kU/L were unable to tolerate even small amounts of BM. Indeed, two studies of in-office baked milk challenges found similar rates of epinephrine use in challenge despite one group using a cut off of cow’s milk sIgE of 15 KU/L and the other group having no firm cut off.(7, 9) These outcomes highlight the need for individualized careful, cautious clinical judgement in the management of patients.
As noted, a large subset (28%) of patients who were sent home on BM are practicing strict avoidance 2 to 7 years later. In our population, this long term outcome was far more common in patients who required treatment during their BM challenge, with 18 of 39 (46%) patients who required treatment but were sent home with a plan for milk practicing strict avoidance at the time of last follow-up. While we have not abandoned the practice of recommending a BM introduction for some of these patients, for example a patient receiving an antihistamine for mild symptoms after the full challenge dose, these data have made us more inclined to continue milk avoidance in most of these patients.
Our experience also demonstrates that even a successful BM challenge does not preclude the possibility of future reactions, even with ingestion of a previously tolerated dose, similar to the experience of other groups who have only introduced baked milk only to those who passed baked milk challenge(10). Given that reactions with home dosing were relatively common, one could argue that advances in baked milk dose or to other forms of milk should only be done in observed challenges. However, our follow-up data suggest that similar numbers of reactions occurred to previously tolerated doses and planned escalations, and thus observed challenges may provide less security than expected. This is certainly the case with oral immunotherapy where reactions commonly occur with home dosing after successful observed dose escalations.(11) Although exercise data were not collected on all adverse events, several events involved exercise, which has also been shown to be a risk factor for adverse reaction in studies of OIT with milk and other foods.(12)
We also identified 6 patients who developed EoE coincident with baked milk introduction, and it is possible that other patients who discontinued milk intake due to gastrointestinal symptoms also had EoE. In fact, our 3.2% incidence of EoE is similar to the 2.7% reported in a meta-analysis of oral immunotherapy to milk, egg, and peanut. (13) While it has been shown that a subset of patients with milk-induced EoE are able to tolerate BM(14), it is important to recognize this potential risk of BM introduction, even in patients with no prior history of EoE.
The strengths of this study include the large sample size, minimum two year follow up, and assessment of this practice in a clinical rather than a research setting. There are, however, a number of limitations that should be recognized. First, it was conducted retrospectively, without OFCs outside of the initial challenge to confirm tolerance to other forms of milk. Second, patients were not challenged with direct milk to confirm their diagnosis of cow’s milk allergy, so the study may have included a small number who were no longer milk allergic at the time of their BM OFC. Third, the basic decision to perform a BM challenge was based on the clinicians’ judgement, with many patients with milk allergy not challenged because they were deemed to have little chance of success based on their prior reaction history. Fourth, given that some patients were unwilling to advance to more allergenic forms of milk due to taste or preference, our data may not represent the maximal advancement of milk introduction. Finally, data were limited as to the exact quantities of milk being ingested, so the results would not differentiate the patient who can tolerate unlimited amounts of baked cheese compared to the patient who can tolerate only a few bites of pizza.
Conclusions
Introduction and advancement of baked milk in the diets of milk allergic patients is frequently used in the management of cow’s milk allergy(15). Other studies have demonstrated that a majority of milk allergic patients tolerates milk in the baked form and our data demonstrate that a majority of carefully selected patients are able to advance their level of milk ingestion over time. However, it is equally important to recognize that this practice is not universally successful and that it not without risk, including the development of EoE. Patients should be counseled regarding these risks as well as ongoing availability of emergency medications and prompt treatment of reactions, even while eating forms and doses of milk that have previously been tolerated.
Figure 4. Association of Milk-IgE with Milk Advancement.
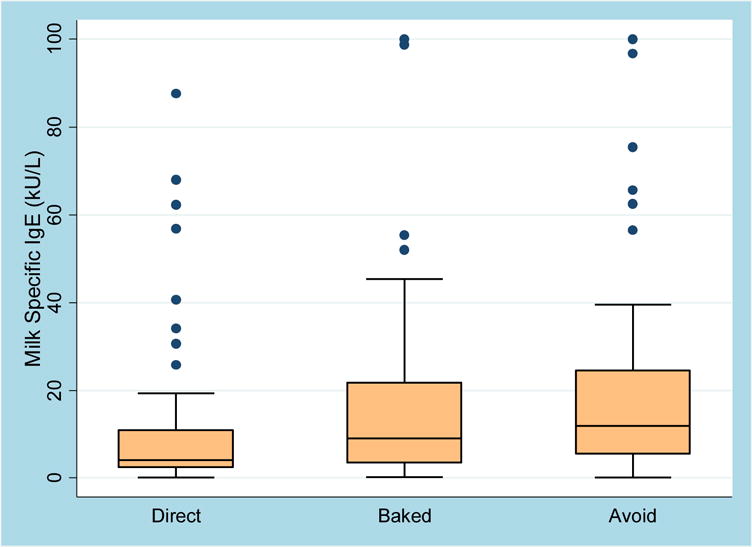
Of those who advanced to direct milk, median IgE at the time of challenge was 4.1 kU/L (IQR 2.4–10.9), compared to 9.1 kU/L (IQR 3.5–21.8) in the group that advanced to baked milk and cheese and 12.1 kU/L (IQR 5.5–24.6) in those practicing avoidance at follow-up. The difference between these three groups was statistically significant with a p-value for trend of <0.001.
Highlights box.
What is already known about this topic? Introduction of baked milk (BM) to select milk allergic patients is a common clinical practice that may help to accelerate resolution of milk allergy.
What does this article add to our knowledge? Long term follow up of baked milk introduction is limited with almost no information regarding the real-world introduction of baked milk. This study provides minimum two-year follow-up of these patients.
How does this study impact current management guidelines? While confirming that a majority of carefully selected milk-allergic patients are able to advance their level of milk ingestion over time, this study also demonstrates that this practice is not universally successful nor without risk, including the development of EoE.
Acknowledgments
Sources of support: This work was supported by NIAID T32Ai007007 and the Eudowood Foundation. No funder had any role in the design and conduct of the study; collection, management, analysis and interpretation of data, or preparation, review or approval of the manuscript.
Dr. Keet has received research support from the National Institutes of Health, and holds a patent on an immunotherapy delivery method. Dr. Wood has received research support from the National Institutes of Health, DBV, Aimmune Therapeutics, Astellas, and HAL-Allergy.
Abbreviations
- BM
Baked milk
- OFC
Oral food challenge
- EoE
Eosinophilic Esophagitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Dunlop and Ms. Mudd have no conflicts of interest to declare.
References
- 1.Bartnikas LM, Sheehan WJ, Hoffman EB, Permaul P, Dioun AF, Friedlander J, et al. Predicting food challenge outcomes for baked milk: role of specific IgE and skin prick testing. Annals of Allergy, Asthma & Immunology : Official Publication of the American College of Allergy, Asthma, & Immunology. 2012;109(5):309–13.e1. doi: 10.1016/j.anai.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. The Journal of allergy and clinical immunology. 2011;128(1):125–31.e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. The Journal of allergy and clinical immunology. 2008;122(2):342–7. 7.e1–2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Leonard SA, Caubet JC, Kim JS, Groetch M, Nowak-Wegrzyn A. Baked milk- and egg-containing diet in the management of milk and egg allergy. The journal of allergy and clinical immunologyIn practice. 2015;3(1):13–23. doi: 10.1016/j.jaip.2014.10.001. quiz 4. [DOI] [PubMed] [Google Scholar]
- 5.Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children–EuroPrevall birth cohort. Allergy. 2015;70(8):963–72. doi: 10.1111/all.12630. [DOI] [PubMed] [Google Scholar]
- 6.Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol. 2013;131(1):180–6. e1–3. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agyemang A, Feuille E, Tang J, Steinwandtner I, Sampson H, Nowak-Węgrzyn A. Outcomes of 84 consecutive open food challenges to extensively heated (baked) milk in the allergy office. The Journal of Allergy and Clinical Immunology. doi: 10.1016/j.jaip.2017.05.016. In Practice. [DOI] [PubMed] [Google Scholar]
- 8.Neuman-Sunshine DL, Eckman JA, Keet CA, Matsui EC, Peng RD, Lenehan PJ, et al. The natural history of persistent peanut allergy. Annals of Allergy, Asthma & Immunology : Official Publication of the American College of Allergy, Asthma, & Immunology. 2012;108(5):326–31.e3. doi: 10.1016/j.anai.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Al Enezi M, Lack G, Fox AT, Anagnostou K. Safety and allergic reaction profiles of children undergoing baked milk and egg challenges: a 6-year experience from a pediatric tertiary referral center. The journal of allergy and clinical immunology In practice. 2017 doi: 10.1016/j.jaip.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Weinbrand-Goichberg J, Benor S, Rottem M, Shacham N, Mandelberg A, Levine A, et al. Long-term outcomes following baked milk-containing diet for IgE-mediated milk allergy. The journal of allergy and clinical immunology In practice. 2017;5(6):1776–8.e1. doi: 10.1016/j.jaip.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Wood RA. Food allergen immunotherapy: Current status and prospects for the future. Journal of Allergy and Clinical Immunology. 2016;137(4):973–82. doi: 10.1016/j.jaci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. The Journal of allergy and clinical immunology. 2007;120(5):1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Annals of Allergy, Asthma & Immunology : Official Publication of the American College of Allergy, Asthma, & Immunology. 2014;113(6):624–9. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Leung J, Hundal NV, Katz AJ, Shreffler WG, Yuan Q, Butterworth CA, et al. Tolerance of baked milk in patients with cow’s milk-mediated eosinophilic esophagitis. J Allergy Clin Immunol. 2013;132(5):1215–6.e1. doi: 10.1016/j.jaci.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44(5):642–72. doi: 10.1111/cea.12302. [DOI] [PubMed] [Google Scholar]


