Abstract
Screening for hereditary cardiomyopathies and arrhythmias (HCA) may enable early detection, treatment, targeted surveillance, and result in effective prevention of debilitating complications and sudden cardiac death. Screening at-risk family members for HCA is conducted through cascade screening. Only half of at-risk family members are screened for HCA. To participate in screening, at-risk family members must be aware of their risk. This often relies on communication from diagnosed individuals to their relatives. However, family communication is not well understood and is ripe for developing interventions to improve screening rates. Until very recently, family communication of genetic risk has been mostly studied in non-cardiac disease. Using this non-cardiac literature, we developed the family communication of genetic risk (FCGR) conceptual framework. The FCGR has four main elements of the communication process: influential factors, communication strategies, communication occurrence, and reaction to communication. Using the FCGR, we conducted an integrated review of the available literature on genetic risk communication in HCA families. Descriptive analysis of 12 articles resulted in the development of categories describing details of the FCGR elements in the context of HCA. This review synthesizes what is known about influential factors, communication strategies, communication occurrence, and outcomes of communication in the context of HCA.
Keywords: hypertrophic cardiomyopathy, long QT syndrome, cascade screening, family communication, genetic risk, disclosure, inherited arrhythmia, inherited cardiomyopathies, sudden cardiac death
Introduction
Hereditary cardiomyopathies and arrhythmias (HCA) account for most sudden cardiac death (SCD) in young people (Vetter et al., 2008). The risk for SCD and other complications of HCAs (e.g. heart failure, stroke, syncope) are reduced through interventions including medication, implanted defibrillators, and lifestyle modifications. HCAs including hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C), familial dilated cardiomyopathy (DCM), long QT syndrome (LQTS), and Brugada Syndrome (BS) are generally inherited in an autosomal dominant (AD) pattern. This means that all first-degree relatives (FDR; parents, siblings, and children) of a diagnosed individual have a 50% chance of having the same disease-causing genetic variant. When an individual is diagnosed with an HCA it is critical that relatives are notified of their risk, tested, and treated prior to development of life-threatening complications. Screening of at-risk family members is conducted through cascade screening, a process of sequentially testing at-risk family members for HCA through genetic testing and clinical exam, and is recommended by the American College of Cardiology Foundation/American Heart Association Task Force (Gersh et al., 2011) and the European Society of Cardiology (Ponikowski et al., 2016). However, to optimize cascade screening, the risk of disease must be communicated timely and accurately to all at-risk relatives.
In several nations worldwide, family communication is the principal mechanism through which family members are notified of increased risk for HCA. Recently, the American Heart Association (AHA)/American College of Cardiology (ACC) described a new quality measure recommending documentation that all FDRs of adult survivors of sudden cardiac arrest from an HCA have been notified of their need to be screened (Al-Khatib et al., 2017). However, the process and extent to which family communication about genetic risk for HCA occurs is poorly understood. Historically, research on family communication of genetic risk focused on non-cardiac diseases; more recently, we find research articles specific to HCAs.
The purpose of this review is to develop a conceptual framework for family communication about genetic risk for HCA in the pre-test phase. Genetic information disclosed in the pre-test phase leads to decisions about testing in at-risk relatives. This is distinct from the post-test state where genetic information is shared between individuals who have been informed about a positive or negative test result and discussions focus on adapting to the HCA diagnosis. This integrative review synthesizes the current literature on family communication of genetic risk for HCA. The outcome of this review is a conceptual framework that can guide clinical practice, research, and policy. First, we reviewed family communication about genetic risk in non-cardiac disease and identified major elements of family communication of genetic risk. This review culminated in the development of the Family Communication of Genetic Risk (FCGR) conceptual framework. We then applied the FCGR conceptual framework to guide an integrative review about family communication of genetic risk for HCA.
Family Communication of Genetic Risk (FCGR) Conceptual Framework
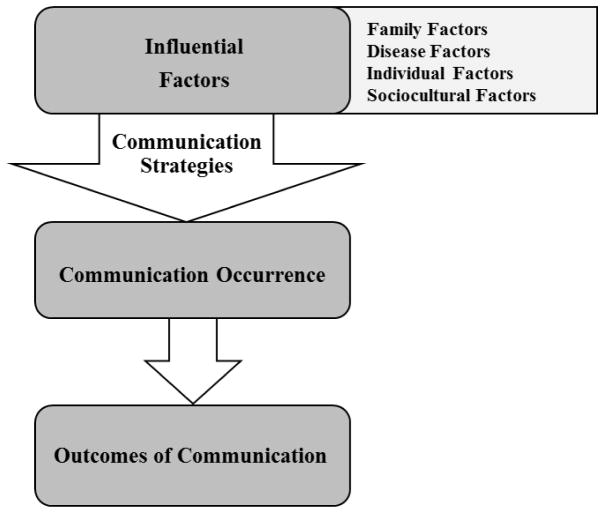
Multiple reviews of the literature on family communication of genetic risk include Wilson et al. (2004), Gaff et al. (2007), Wiseman, Dancyger, & Michie (2010), and Weins et al. (2013). None of these reviews included articles pertaining to families with HCAs. Major findings from each review are summarized in Table I and synthesized into four major elements of family communication of genetic risk: influential factors, communication strategies, communication occurrence, and outcomes of communication. Influential factors motivate communication decisions and include disease, individual, family, and sociocultural factors that influence the extent of communication (e.g. what will be communicated, when, and to whom).
Table I.
Elements of Communicating Genetic Risk Identified by Reviews
| Element | Description of Element |
|---|---|
| Wilson et al. (2004) | |
|
| |
| Disease factors | Characteristics of the disease that impact the likelihood of communication about genetic risk (e.g., inheritance pattern, certainty and comprehensibility of genetic testing information, and availability of risk management strategies or interventions). |
| Individual factors | Characteristics of probands that may influence the likelihood that individuals share genetic risk information and with whom they share it (e.g., individual’s coping mechanisms, emotional barriers to communication, psychological defenses, and risk perception). |
| Family factors | Characteristics of the relationships in families and the family system (e.g., communication patterns, family representatives, control of information, and family myths). |
| Sociocultural factors | Characteristics of the culture or society in which individuals and families live (e.g., gender roles, taboos, concern about discrimination, and notions of kinship and inheritance). |
|
| |
| Gaff et al. (2007) | |
|
| |
| Deliberation before communication | Probands’ considerations of what information to share, when to share it, and the possible effects of communicating inherited disease risk information to family members. |
| Communication strategies | Individual approaches to communicating risk information (e.g., complete openness with family members, total secrecy, passing on responsibility to others in the family to communicate). |
| Outcomes of communication | Effects of the disease risk communication on probands, relatives, and families measured as uptake of genetic testing; family members’ knowledge of disease risk; or the impact of the communication on probands, relatives, and their relationships. |
|
| |
| Wiseman et al. (2010) | |
|
| |
| Functions & influences of communication | Reasons for communicating about disease to relatives as well as the factors that either motivate or inhibit disease risk communication. |
| Processes of communication | Who is told, how they are told, and what they are told. |
| Outcomes of communication | The impact of the disease risk communication on probands, relatives, and family relationships. |
|
| |
| Weins et al. (2013) | |
|
| |
| Attitude | The desire to protect; perceptions of relevance, responsibility, and the right to know; and the usefulness of communicating which determine intention to communicate. |
| Subjective Norm | Pressure from family members, health professionals, and society which determine intention to communicate. |
| Perceived Behavioral Control | Family dynamics and relationships, communication skills, ability to understand, and coping skills which determine intention to communicate. |
| Intention | The determination of an individual to engage in communication of genetic risk to another family member as a function of attitude, subjective norms, and perceived behavioral control. |
| Behavior | Communication of genetic risk to another family member as a function of attitude, subjective norms, and perceived behavioral control. |
Communication strategies are the methods used to carry out communication in families. Communication occurrence is defined as how many family members were told about the genetic risk. Outcomes of communication are the emotions, behaviors, and actions resulting from communication experienced by probands, relatives, or families. Figure 1 organizes these four major elements into the family communication of genetic risk conceptual framework (FCGR).
Figure 1.
Family Communication of Genetic Risk Conceptual Framework (FCGR) Based on Non-Cardiac Conditions
Limitations of reviewed studies included small samples sizes, measurement of attitudes and not actual behavior, data collection soon after undergoing genetic testing which may not have given people enough time to communicate the risk to their relatives, over reporting of communication extremes, absence of studies describing the content of communication, lack of cultural diversity, no reports on the impact of communication on individuals or families, underdevelopment of the unique and sensitive issues related to communication genetic risk to young children, and study focus on general disease communication rather than risk communication (Gaff et al., 2007; Weins et al., 2013; Wilson et al., 2004; Wiseman et al., 2010).
Methods
The FCGR conceptual framework (Figure 1) was used to guide the analysis of this subsequent integrative review on family communication about genetic risk for HCAs. We applied methodology described by Whittemore & Knafl (2005) to conduct the integrative review in five stages: 1. Problem Identification, 2. Literature Search, 3. Data Evaluation, 4. Data Analysis, and 5. Presentation of Results.
Stage 1: Problem Identification
About half of at-risk family members are not screened for HCAs, placing large numbers of individuals at high risk for life-threatening complications of a disease amendable to treatment (Christiaans et al., 2008; Hanninen et al., 2015; Miller et al., 2013). One critical step in cascade screening for HCAs is ensuring that at-risk family members are aware of their increased risk and the need for screening. This generally requires accurate and timely communication from diagnosed individuals to their at-risk family members. However, family communication about genetic risk for HCA is not well understood. This integrative review addresses the problem that family communication about genetic risk, a critical step in cascade screening, is poorly understood in the context of HCA. Improved understanding of how families communicate about genetic risk for HCA may then help address the problem of low screening rates for HCA.
Stage 2: Literature Search
Identification of all relevant literature on family communication about genetic risk for HCA was conducted through electronic database searching and ancestry searching. Gray literature was not included. Six electronic databases were searched (PubMed, CINAHL, PsychINFO, Scopus, EMBASE, and Family Studies Abstracts) on March 14, 2016 without limits or filters. Search terms were based on terms used in the non-cardiac literature reviews (Wilson et al., 2004; Gaff, et al., 2007; Wiseman, et al., 2010) with additional terms used to focus the search on genetic cardiac diseases, specifically HCM and LQTS. Search terms included long QT syndrome [MeSH], hypertrophic cardiomyopathy [MeSH], inherited heart conditions, inherited heart disease, sudden cardiac death, genetic risk communication, disclosure, family communication, and cascade screening. Endnote X7 was used to manage citations from the multiple searches.
Articles were excluded if they were not in English; reported family screening behaviors only without mention of communication; focused on provider to patient communication, clinical management of individuals, disease pathophysiology or pharmacology, population screening or community programs, registries, or multidisciplinary care programs; published conference abstracts; did not include an inherited cardiomyopathy or channelopathy that can result in SCD; or focused on bereavement or end of life care.
The electronic database search generated 506 articles. Duplicate articles were removed resulting in 317 articles. LS reviewed the title and abstract of these 317 articles using the inclusion/exclusion criteria and excluded 235 articles, leaving 82 articles requiring a full text review. Articles with no abstract were automatically included for a full text review. LS then reviewed the full text of all 82 articles using the inclusion/exclusion criteria and excluded 72 articles. Ancestry search methods identified eight additional articles for review from the full-text review of 82 articles and their bibliographies. The full texts of these eight articles were reviewed, applying the inclusion/exclusion criteria and excluded seven articles.
Eleven articles were used for initial data analysis. However, to ensure that all relevant literature was included in analysis, a final search of the electronic databases was repeated prior to publication using limits to identify literature published between March 15, 2016 and June 6, 2017. This search yielded 93 articles which were reviewed using the same process described previously. This final search resulted in the addition of one article and a final sample of 12 articles included in the analysis. Figure 2 illustrates the process of article identification, screening, selection, and inclusion in an adapted PRISMA flow diagram.
Figure 2.
Adapted PRISMA Flow Diagram Illustrating the Steps of the Literature Search Process
Stage 3: Data Evaluation of HCA Literature
The final sample of 12 articles included qualitative, quantitative, mixed methods, ethical case study, and personal narrative designs. Kmet, Lee, & Cook (2004) quality assessment criteria were used to evaluate qualitative, quantitative, and mixed methods studies. Fourteen criteria were used to evaluate quantitative studies and 10 criteria were used for qualitative studies. Criteria were evaluated and scored (2 = Yes, 1= Partial, 0 = No, N/A= Not Applicable) based on specific definitions and instructions for each criterion, summed, and adjusted to provide a summary score (Kmet et al., 2004). The mixed method study (Haukkala et al., 2013) was evaluated using both quantitative and qualitative criteria. Ethical case studies and personal narratives were not evaluated for quality because validated instruments for these types of studies have not been developed. However, both were judged to be acceptable quality based on being in peer-reviewed journals. Tables II and III show the quality assessment criteria and evaluation of the articles included in the review. A minimum quality score was set at .75 out of 1.00 (Kmet et al., 2004). All articles exceeded the minimum standard for quality.
Table II.
Quality Evaluation of Qualitative Studies
| Criteria for Qualitative Studies | Articles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Smart et al. (2010) | Geelen et al. (2011a) | Geelen et al. (2011b) | Mangset el al. (2014) | Ormondroyd et al. (2014) | Vavolizza et al. (2015) | Haukkala et al. (2013) | Whyte et al. (2016) | ||
| 1 | Question/objective sufficiently described? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 2 | Study design evident and appropriate? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 3 | Context for the study clear? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 4 | Connection to a theoretical framework/wider body of knowledge? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 5 | Sampling strategy described, relevant and justified? | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 |
| 6 | Data collection methods clearly described and systematic? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 7 | Data analysis clearly described and systematic? | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 |
| 8 | Use of verification procedure(s) to establish credibility? | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| 9 | Conclusions supported by the results? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 10 | Reflexivity of the account? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total Sum | 18 | 19 | 19 | 19 | 19 | 17 | 19 | 19 | |
| Total Possible Sum (20) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Summary Score (total sum/total possible sum) | .90 | .95 | .95 | .95 | .95 | .85 | .95 | .95 | |
2= Yes, 1=Partial, 0=No, N/A=Not Applicable
Table III.
Quality Evaluation of Quantitative Studies
| Criteria for Quantitative Studies | Articles | |||
|---|---|---|---|---|
| Batte et al. (2015) | Burns et al. (2016) | Haukkala et al. (2013) | ||
| 1 | Question/objective sufficiently described? | 2 | 2 | 2 |
| 2 | Study design evident and appropriate? | 2 | 2 | 2 |
| 3 | Method of subject/comparison group selection or source of information/input variables described and appropriate? | 2 | 2 | 2 |
| 4 | Subject (and comparison group, if applicable) characteristics sufficiently described? | 2 | 2 | 2 |
| 5 | If interventional and random allocation was possible, was it described? | N/A | N/A | N/A |
| 6 | If interventional and blinding of investigators was possible, was it reported? | N/A | N/A | N/A |
| 7 | If interventional and blinding of subjects was possible, was it reported? | N/A | N/A | N/A |
| 8 | Outcome and (if applicable) exposure measure(s) well defined and robust to measurement/misclassification bias? means of assessment reported? | 2 | 2 | 1 |
| 9 | Sample size appropriate? | 2 | 2 | 2 |
| 10 | Analytic methods described/justified and appropriate? | 2 | 2 | 2 |
| 11 | Some estimate of variance is reported for the main results? | 2 | 2 | 1 |
| 12 | Controlled for confounding? | 1 | 1 | N/A |
| 13 | Results reported in sufficient detail? | 2 | 2 | 2 |
| 14 | Conclusions supported by the results? | 2 | 2 | 2 |
| Total Sum | 21 | 21 | 18 | |
| Total Possible Sum (28 number of “N/A” *2) | 22 | 22 | 20 | |
| Summary Score (total sum/total possible sum) | .95 | .95 | .90 | |
2= Yes, 1=Partial, 0=No, N/A=Not Applicable
Stage 4: Data Analysis of HCA Literature
Data analysis began by extracting the year published, authors, country where the research took place, study design, sample characteristics, sample recruitment, and genetic testing status of participants from the 12 articles. The full text of each article was uploaded into NVivo 10 to facilitate analysis (NVivo Qualitative Data Analysis Software). LS read each article in its entirety and coded study findings into the four major elements of the FCGR (influential factors, communication strategies, communication occurrence, outcomes of communication). Data included in analysis were generally found in the ‘results’ sections of the articles, however data were also found in the ‘discussion’ section because some studies present original findings in both sections of an article (Sandelowski & Leeman, 2012). A second reviewer (SDH) then examined coded text for each element and discrepancies were discussed until agreement was reached. The next round of coding consisted of LS reviewing the coded text for each of the four elements and developing categories to capture the details within each element. SDH reviewed the categories and again discrepancies were discussed until agreement was reached.
Results
Stage 5: Presentation of Results of HCA Literature Integrative Review
Table IV summarizes the articles included in this review. Studies were conducted in the United Kingdom (UK; n = 3), United States of America (USA; n = 3), Netherlands (n = 2), Australia (n = 1), Finland (n = 1), Norway (n = 1), and Ireland (n = 1). Studies were generally small with the largest sample size being 383 (Batte et al., 2015). Studies varied in design, including qualitative (n = 7), quantitative (n = 2), and mixed-methods (n = 1). Two non-research articles, an ethical case study (n = 1) and a personal narrative (n = 1), were also included in the review. While all studies included individuals with HCM or LQTS, only three studies included individuals with both HCM and LQTS. Nine studies included only participants that had undergone genetic testing, two studies included participants both with and without genetic testing, and one study did not specify genetic testing status. In studies with participants that had genetic testing, two articles included only those with positive genetic test results and seven included participants with positive, negative, or inconclusive results. Recruitment of participants for the research studies was through clinical settings, disease support groups, national disease registries, and research biobanks.
Table IV.
Summary of Articles Included in Review
| Year | Author | Country | Design | Sample | Sample Recruitment | Genetic Testing Status of Participants | Quality Score |
|---|---|---|---|---|---|---|---|
| 2010 | Ladd | UK | Personal narrative | N=1 | N/A | PGT | Not rated |
| 2010 | Smart | UK | Qualitative | N=27 HCM and LQTS | Clinical setting | All participants had genetic testing: 17 PGT, 7 NGT, 3 IGT | .90 |
| 2011a | Geelen et al. | Netherlands | Qualitative | N=57 with HCM (from 6 families) | Clinical setting | All families had an identified genetic mutation. Precise results of individuals not given. | .95 |
| 2011b | Geelen et al. | Netherlands | Qualitative | N=57 with HCM (from 6 families) | Clinical setting | All families had an identified genetic mutation. Precise results of individuals not given. | .95 |
| 2012 | Cohen et al. | USA | Ethical case study | 3 case studies | N/A | 1 PGT, 2 in process of parental decision about genetic testing | Not rated |
| 2013 | Haukkala et al. | Finland | Mixed Methods | N=17 LQTS found incidentally | Research biobank | All PGT | .90/.95* |
| 2014 | Mangset et al. | Norway | Qualitative | N=13 parents of children with LQTS (9 nuclear families) | Disease support group | All participants had genetic testing. 54% PGT | .95 |
| 2014 | Ormondroyd et al. | UK | Qualitative | N=22 with HCM or LQTS | Clinical setting | All participants had genetic test. 77% PGT | .95 |
| 2015 | Batte et al. | USA | Quantitative | N=383 with HCM | Disease support group | 64% had genetic testing. 43% PGT | .95 |
| 2015 | Vavolizza et al. | USA | Qualitative | N=50 with personal or family history of genetic arrhythmia (32 families) | Clinical setting and disease support groups | Gene test status not specified | .85 |
| 2016 | Burns et al. | Australia | Quantitative | N=75 probands and relatives (38 families) with LQTS | National disease registry | 15 probands & 27 relatives PGT. 17 probands IGT. 6 probands and 1 relative did not have genetic testing. | .95 |
| 2016 | Whyte et al. | Ireland | Qualitative | N=9 family members taking part in cascade screening for HCM/LQTS | Clinical setting | All participants had genetic testing. 4 PGT, 5 NGT | .95 |
HCM = hypertrophic cardiomyopathy; LQTS = long QT syndrome; BS = Brugada syndrome; ARVC = arrythmogenic right ventricular cardiomyopathy; DCM = dilated cardiomyopathy; CPVT = catecholaminergic polymorphic ventricular tachycardia; PGT = Positive genetic test; NGG = Negative genetic test; IGT = Indeterminate genetic test.
Haukkala et al. (2013), a mixed methods study, had a quality score of .90 for the quantitative evaluation and .95 for the qualitative evaluation.
Results of the qualitative synthesis are presented in Table V by the four major elements of the FCGR (influential factors, communication strategies, communication occurrence, and outcomes of communication). The table contains conceptual definitions for each developed category along with supporting data from the reviewed articles.
Table V.
Qualitative Synthesis of Literature on Family Communication of Genetic Risk for HCA
| FCGR Element | Category: Conceptual Definition |
|
|---|---|---|
| Sub-Category: Conceptual Definition | ||
| Citation | Supporting Literature | |
| Influential Factors (Family Factors) | Established Family Dynamics Persist: Patterns and styles already present in a family in terms of communication, conduct towards each other, and relationships. |
|
| Whyte et al. (2016) |
|
|
| Haukkala et al. (2013) |
|
|
| Geelen et al. (2011b) |
|
|
| Burns et al. (2016) |
|
|
| Family Contact & Closeness: The influence that the family structure, quality of relationships within the family, amount of contact between family members, geographical proximity, and emotional closeness have on the communication of HCA risk. | ||
| Burns et al. (2016) |
|
|
| Smart (2010) |
|
|
| Batte et al. (2015), Ormondroyd et al. (2014), Smart (2010) |
|
|
| Whyte et al. (2016) |
|
|
| Batte et al. (2015) |
|
|
| Geelen et al. (2011b) |
|
|
| Ladd (2010) |
|
|
| Ormondroyd et al. (2014), Smart (2010) |
|
|
| Cohen et al. (2012) |
|
|
| Milestones: Normal life happenings such as marriage, pregnancy, or childbirth that influence HCA risk communication. | ||
| Geelen et al. (2011b) |
|
|
| Haukkala et al. (2013) |
|
|
| Influential Factors (Disease Factors) | Understanding of Disease: An individual’s measured or perceived understanding or confidence in their knowledge of various aspects of their disease and the disease risk for others (e.g. inheritance, genetic tests, perception of risk). |
|
| Batte et al. (2015) |
|
|
| Burns et al. (2016) |
|
|
| Smart (2010), Vavolizza et al. (2015) |
|
|
| Batte et al. (2015) |
|
|
| Haukkala et al. (2013) |
|
|
| Whyte et al. (2016) |
|
|
| Batte et al. (2015) |
|
|
| Smart (2010) |
|
|
| Burns et al. (2016) |
|
|
| Mangset et al. (2014) |
|
|
| Ormondroyd et al. (2014) |
|
|
| Disease Experience: An individual’s experience with the disease, including how they came to be diagnosed or aware of their risk, symptoms, coping with the disease, and test results. | ||
| Geelen et al. (2011b), Ormondroyd et al. (2014) |
|
|
| Geelen et al. (2011b) |
|
|
| Haukkala et al. (2013) |
|
|
| Geelen et al. (2011b) |
|
|
| Ladd (2010) |
|
|
| Haukkala et al. (2013) |
|
|
| Whyte et al. (2016) |
|
|
| Batte et al. (2015) |
|
|
| Geelen et al. (2011a) |
|
|
| Haukkala et al. (2013) |
|
|
|
||
| Geelen et al. (2011b) |
|
|
| Geelen et al. (2011a) |
|
|
|
||
| Ormondroyd et al. (2014) |
|
|
| Smart (2010) |
|
|
| Batte et al. (2015) |
|
|
| Batte et al. (2015), Burns et al. (2016) |
|
|
| Influential Factors (Individual Factors) | Reasons to Communicate Risk: An individual’s conscious reasons to communicate risk and included three distinct subcategories; moral and ethical conviction; desire to inform, encourage, and help; and reciprocal communication. |
|
| Moral & Ethical Conviction: Feelings of responsibility, duty, or obligation to communicate risk to various relatives. | ||
| Batte et al. (2015), Burns et al. (2016), Geelen et al. (2011b), Mangset et al. (2014), Ormondroyd et al. (2014), Vavolizza et al. (2015), Whyte et al. (2016) |
|
|
| Geelen et al. (2011b) |
|
|
| Geelen et al. (2011b), Mangset et al. (2014) |
|
|
| Vavolizza et al. (2015) |
|
|
| Ormondroyd et al. (2014), Vavolizza et al. (2015) |
|
|
| Ladd (2010) |
|
|
| (Mangset et al. (2014) |
|
|
| Altruism: Desire to inform, encourage, and help as reasons to communicate. | ||
| Batte et al. (2015), Burns et al. (2016), Haukkala et al. (2013), Mangset et al. (2014), Ormondroyd et al. (2014), Vavolizza et al. (2015) |
|
|
| Burns et al. (2016), Haukkala et al. (2013), Mangset et al, (2014), Vavolizza et al. (2015) |
|
|
| Batte et al. (2015), Burns et al. (2016) |
|
|
| Burns et al. (2016) |
|
|
| Ormondroyd et al. (2014), Vavolizza et al. (2015) |
|
|
| Reciprocal Communication: The expectation that communication of risk to relatives would have some benefit to the proband. | ||
| Batte et al. (2015) |
|
|
| Ladd (2010) |
|
|
| Psychological Functioning: General or disease related anxiety, depression, or stress that affects or is associated with communication about genetic risk. | ||
| Haukkala et al. (2013) |
|
|
| Burns et al. (2016) |
|
|
| Smart (2010) |
|
|
| Haukkala et al. (2013) |
|
|
| Mangset et al. (2014) |
|
|
| Mangset et al. (2014), Smart (2010) |
|
|
| Smart (2010), Vavolizza et al. (2015) |
|
|
| Doubts, Ambivalence, & Reluctance: The view that negative consequences of risk communication may outweigh the positive, leading to hesitation or blocking communication about genetic risk to relatives. | ||
| Geelen et al. (2011b), Ormondroyd et al. (2014), Smart (2010) |
|
|
| Burns et al. (2016), Smart (2010), Vavolizza et al. (2015) |
|
|
| Burns et al. (2016) |
|
|
| Cohen et al. (2012 |
|
|
| Smart (2010) |
|
|
| Mangset et al. (2014), Ormondroyd et al. (2014) |
|
|
| Burns et al. (2016) |
|
|
| Batte et al. (2015) |
|
|
| Whyte et al. (2016) |
|
|
| Difficulty with the Conversation: Not knowing what to say or how to start a conversation about risk for HCA. | ||
| Smart (2010) |
|
|
| Batte et al. (2015) |
|
|
| Gender Influence: Differences in communication styles that are thought to be related to gender. | ||
| Batte et al. (2015) |
|
|
| Conflicting Interests for Children: Conflict between the need to protect children from physical, psychological, or social burdens related to HCA and HCA risk, now and in the future. | ||
| Smart (2010) |
|
|
| Cohen et al. (2012), Mangset et al. (2014), Vavolizza et al. (2015) |
|
|
| Cohen et al. (2012) |
|
|
| Mangset et al. (2014) |
|
|
| Vavolizza et al. (2015). |
|
|
| Geelen et al. (2011a) |
|
|
| Haukkala et al. (2013), Mangset et al. (2014) |
|
|
| Desire to Protect Elderly: The need to protect elderly relatives, generally parents, from psychological burdens related to HCA (e.g. guilt of passing on bad gene, worry, stigma). | ||
| Ormondroyd et al. (2014), Smart (2010) |
|
|
| Smart (2010) |
|
|
| Whyte et al. (2016) |
|
|
| Communication | Delivery: Various modalities, styles, tones, or approaches used to communicate HCA risk to relatives. |
|
| Burns et al. (2016) Strategies |
|
|
| Haukkala et al. (2013), Ormondroyd et al. (2014) |
|
|
| Burns et al. (2016) Geelen et al. (2011b) |
|
|
| Vavolizza et al. (2015). |
|
|
| Whyte et al. (2016) |
|
|
| Geelen et al. (2011b) |
|
|
| Ladd (2010) |
|
|
| Smart (2010) |
|
|
| Mangset et al. (2014) |
|
|
| Ormondroyd et al. (2014) |
|
|
| Cohen et al. (2012) |
|
|
| Content: Specific content of communications about HCA risk. | ||
| Vavolizza et al. (2015) |
|
|
| Smart (2010) |
|
|
| Geelen et al. (2011b) |
|
|
| Ormondroyd et al. (2014) |
|
|
| Vavolizza et al., 2015, p. 612). |
|
|
| Ladd (2010) |
|
|
| Whyte et al. (2016) |
|
|
| Communication Occurence | Batte et al. (2015) |
|
| Burns (2016) |
|
|
| Haukkala et al. (2013) |
|
|
| Geelen et al. (2011b) |
|
|
| Ormondroyd et al. (2014), p. 92 |
|
|
| Burns et al. (2016) |
|
|
| Ladd (2010) |
|
|
| Outcomes of Communication | Clinical Screening & Genetic Testing: Reported uptake of clinical care including screening, and genetic testing because of communication of HCA risk. |
|
| Burns et al. (2016) |
|
|
| Cohen et al. (2012) |
|
|
| Ladd (2010) |
|
|
| Haukkala et al. (2013) |
|
|
| Geelen et al. (2011a) |
|
|
| Family Functioning: Describes positive and negative changes in family dynamics as a result of communication of HCA risk. | ||
| Vavolizza et al. (2015) |
|
|
| Whyte et al. (2016) |
|
|
| Geelen et al. (2011b) |
|
|
| Ladd (2010) |
|
|
| Vavolizza et al. (2015) |
|
|
| Ormondroyd et al. (2014) |
|
|
| Geelen et al. (2011b) |
|
|
| Responsibility Completed: Discharging of responsibility for disease risk after communication is done. | ||
| Geelen et al. (2011b), p. 1755 |
|
|
| Mangset et al. (2014) |
|
|
| Ladd (2010) |
|
|
| Relative’s Lack of Interest or Denial: Describes relatives whose response to learning their risk was apparent or real denial or lack of interest. | ||
| Whyte et al. (2016) |
|
|
| Ormondroyd et al. (2014) |
|
|
| Geelen et al. (2011b) |
|
|
| Haukkala et al. (2013) p. 249 |
|
|
| Vavolizza et al., 2015 |
|
|
| Ladd (2010) |
|
|
| Parental Concern: Concerns or worry related to a non-adult child’s health, coping, or response to being aware of their disease or disease risk. | ||
| Geelen et al. (2011b) |
|
|
| Haukkala et al. (2013) |
|
|
| Cohen et al. (2012) |
|
|
| Ormondroyd et al. (2014) |
|
|
| Emotional Reaction: Emotions of the relative that occurred in response to the communication of HCA risk. | ||
| Haukkala et al. (2013) |
|
|
| Vavolizza et al. (2015) | One relative expressed anger and fear about learning about the HCA risk and wished they had never known the information. | |
| Ormondroyd et al. (2014) |
|
|
| Geelen et al. (2011b) |
|
|
| Ladd (2010) |
|
|
| Discontinuation of the Cascade: The breakdown in communication that occurs when relatives who learn about HCA risk fail to pass on risk information to subsequent relatives. | ||
| Ormondroyd et al. (2014) |
|
|
| Haukkala et al. (2013) |
|
|
| Geelen et al. (2011b) |
|
|
| Haukkala et al. (2013), Vavolizza et al. (2015) |
|
|
| Difficulty with Relatives’ Reactions: Frustration, disappointment, anger, or disagreement with how relatives responded to communication of HCA risk. | ||
| Geelen et al. (2011a; 2011b) |
|
|
| Haukkala et al. (2013), Vavolizza et al. (2015), Whyte et al. (2016) |
|
|
Synthesis of Evidence
The integrated review of the HCA literature resulted in a modification of the original FCGR framework (which was constructed based on non-cardiac inherited conditions) in the context of HCA. Figure 3 summarizes modifications of the FCGR based on the HCA integrated review with the original major elements of the FCGR in bold text and modifications to the FCGR in italics. All 12 articles discussed influential factors in communication (12 discussed individual factors, 11 discussed disease factors, and nine discussed family factors) while only six articles discussed communication occurrence. The number of articles describing each element, category, and sub-category of the updated FCGR are indicated in parentheses in Figure 3. This integrative review provides a detailed framework for understanding family communication of genetic risk for HCAs.
Figure 3.
Modification of the Family Communication of Genetic Risk Conceptual Framework (FCGR) Based on Integrative Review of Family Communication About Genetic Risk for HCA Number in parentheses indicates the number of articles describing each element, category, and sub-category of the updated FCGR.
Discussion
This review analyzed 12 studies related to family communication of genetic risk for HCA. Several findings in this review are congruent with the literature on non-cardiac genetic risk communication in families. Specifically, family, disease, and individual factors were influential in the extent to which families communicated about genetic risk.
Individual factors were the most extensively developed influential factors and were identified in all 12 studies. One category, conflicting interests for children, is of particular relevance for HCA. The onset of HCA often occurs in minor children, whereas in many non-cardiac diseases (e.g., Huntington Disease, Hereditary Breast and Ovarian Cancer), onset in minor children is very rare. Rowland & Metcalfe (2013) reviewed genetic risk communication between parents and their minor children and found that for diseases with young onset (Cystic Fibrosis, Familial Adenomatous Polyposis, Sickle Cell Disease, and Neurofibromatosis), parents communicated more with children who were affected and less with children who were unaffected. Similar to Rowland & Metcalfe’s (2013) findings, communication of genetic risk for HCA to minor age children is tightly intertwined with testing and diagnosis because parents take on the role of communicating disease risk and managing their child’s health and wellbeing. Unlike communication to adult children or adult relatives, communication about HCA to minor age children seemed to focus on the risks related to the disease itself (such as SCD) rather than the risk for disease and inherited nature of the disease. This creates the potential that children who are told about their disease risk at a young age may not realize the heritability element of their HCA as they transition to adulthood. This presents an opportunity to create developmentally appropriate systems that address HCA risk and inheritance with children throughout their development (Rowland & Metcalfe, 2013).
Influential family factors were not specific to families with HCA as family dynamics, closeness, and contact have been similarly described in the non-cardiac literature (Wilson et al., 2004; Gaff, et al., 2007; Wiseman, et al., 2010). However, unlike in families with non-cardiac disease, some of the milestones such as childbirth, and pregnancy seemed to be meaningful in families with HCA due to the risk for children. Family contact and closeness had varied and somewhat contradictory findings in regards to its influences on communication about HCA in families, however the majority of evidence supports that lack of contact and closeness with family members is an important barrier to communication of HCA risk.
The description of influential disease factors highlights the contributions of both the understanding and the personal experience with HCA to communication. HCAs, although inherited in a predictable AD pattern, have variable and largely unpredictable expressivity and severity within families. This variable expressivity likely contributes to the difficulties that participants in some studies had in understanding disease inheritance and identifying who was at risk.
Communication strategies includes multiple methods of delivery of communication and variation in the content of these communications, similar to the non-cardiac literature (Gaff, et al., 2007; Wiseman, et al., 2010). Participants in the included HCA studies identified multiple modes of communicating genetic risk to relatives. However, letters were the only clinician-provided mode of communication described. van der Roest et al. (2009) reported that providing family letters to probands led to higher family member screening rates, vs. families not receiving letters (57% vs. 35%, respectively, p < .01). Clinicians have the opportunity to support other modes of communication in addition to letters, such as scripts for in-person or telephone conversations, which seemed to be more common modes of communication in families than letters, or identifying a family informant to carry out communication, as is suggested for communication of familial cancer risk (Bowen et al., 2017). As with non-cardiac disease (Gaff, et al., 2007; Wiseman, et al., 2010), the content of communication to relatives was varied among those with HCA, and still very little is known about what information relatives actually received or understand.
Only six studies described communication occurrence. Overall, reports of communication occurrence were not specific to which family members were told about risk, nor which relatives had not been told about their risk. Most studies focused on immediate family members, who clearly are at highest risk and should be tested first, but did not examine communication to more distant relatives, who are also at high risk. If communication was optimal, relatives who were told of their risk would communicate risk to the next tier of relatives, following the same path as cascade screening. However, communication of risk breaks down in each successive wave and it is unknown if the cascade screening model can rely on communication in each wave or if the communication may be best coming from the original proband or informed family member. Research was also lacking in identifying which relatives are not told, and why, and did not examine communication occurrence from the perspective of the relative.
The most clinically relevant outcome of communication is uptake of clinical or genetic testing of family members because it identifies individuals who would benefit from treatments, and clarifies risk status for additional family members. Findings related to clinical or genetic testing in relatives were scarce, considering the large number of family members at risk. More research is needed to understand the complexity of why at-risk relatives do not get screened for HCA. It is known that making relatives aware of their risk is essential, but not sufficient to ensure that relative will get tested. The familial risk perception model identifies that individuals must develop salience or awareness of risk as an essential step in their development of personal feelings of vulnerability to risk that lead them take action to control their risk (Walter & Emery, 2005; Daack-Hirsch, Shah, & Cady, 2017). However, although SCD of a family member may be the salient event that brings awareness of the HCA to a family, those experiencing SCD in their family are no more likely to participate in screening than those who have not experienced SCD (Christiaans et al., 2008; Miller et al., 2013) potentially due to the emotions experienced by the sudden loss of a loved one (Christian et al., 2017).
Study Limitations
The 12 articles included in this integrative review are exclusively from western countries where healthcare systems are protective of individual privacy. Healthcare systems and cultures vary significantly in how genetic information is viewed and how families communicate, which may limit the application of these findings in other cultures or healthcare systems which have less restrictive privacy practices. Many of the studies included in this review that had larger sample sizes drew from disease support groups. Samples from support groups likely differ from clinic based samples (Wiseman et al., 2010) in a number of ways including disease experience, coping, and level of interest and knowledge about their HCA. In addition, the retrospective survey designs of the quantitative studies included in this review may be limited by recall bias and confounding, and are unable to establish temporality, a key component in detecting causal relationships between various factors and communication about HCA risk in families.
Practice Implications
The FCGR framework is clinically practical and describes several elements pertinent to the process of family communication about genetic risk for HCAs. Healthcare practitioners providing genetic counseling can directly apply this model by discussing influential factors important to probands and family specific needs and developing personalized communication strategies such as family letters (van der Roest et al. (2009) and utilization of other family members to facilitate communication (Bowen et al., 2017). Ethical-based conceptual frameworks (McConkie-Rosell & Spiridigliozzi, 2004) and multi-disciplinary team approaches (Caleshu et al., 2016) may help clinicians address the variety of issues that impede communication about HCA risk in families particularly with children. A team approach is important because while genetic counseling lays the foundation, communication of risk for HCA is an ongoing process and individuals with HCA will likely have needs related to family communication throughout their life. Probands may interact with physicians or nurses more frequently than genetic counselors for their healthcare and a team approach provides flexibility for various healthcare roles to provide continued support for individuals communicating HCA risk with family members.
Counseling sessions with individuals and families with HCA should specifically address the conflicting interests regarding communication to children and address doubts, ambivalence, and reluctance that may prevent communication to relatives. Practitioners should monitor the success of communication by assessing occurrence and outcomes of communication throughout the counseling process. Probands may benefit from counseling about the range of outcomes of their communication beyond clinical screening and genetic testing. This review provides insight into range of influential factors that may be important to the proband’s communication with family members about HCA risk.
Policy & Research Recommendations
Findings from this study support the recognition of family communication as a key step in cascade screening in the next update of the American Heart Association (AHA)/American College of Cardiology (ACC) Guidelines. Al-Khatib et al.’s (2017) recent quality measure to document communication of genetic risk to first-degree family members is a good step toward the recognition of the key role of family communication in cascade screening. This new quality measure also provides an opportunity for additional research on family communication and family screening which can be used to promote this quality measure into a performance measure, which would be subject to public reporting or pay for performance programs (Al-Khatib et al., 2017).
The updated FCGR in the context of HCA identifies several areas for further research. Future research should seek to understand the specific content of genetic risk communications to family members, as this was a particularly sparse area of research. Understanding the content of communications is key to ensure that family members can make an informed decision about screening. Future research on communication occurrence should strive to determine specifically which family members are not told about their risk in order to more accurately design interventions aimed at improving communication with those relatives who are otherwise unlikely to be told about their risk. Deliberate efforts to include participants with diverse disease experiences and backgrounds in this research is crucial because they may have very different needs and this is a current limitation to the current body of literature. Research aimed at investigating communication about genetic risk for HCA from the point of view of family members would fill an important gap in our understanding about how family members view the communication and their reaction to the communication which would lead to better understanding of how messages are received and translated into screening action. Future research should also aim to develop novel interventions to improve family communication as this may improve the rates of family members participating in screening and genetic testing (Cirino et al., 2017). In addition, utilization of prospective study designs or using control groups can advance our understanding of the relationships among various factors and communication, which may be particularly useful in intervention development.
Conclusion
This article presents an analysis of family communication of genetic risk for HCA and helps to better understand a critical step in the cascade screening process. Family communication about genetic risk for HCA fits into the major elements of the FCGR developed from the literature on family communication about genetic risk for non-cardiac disease. This review expands understanding about family communication of genetic risk for HCA by synthesizing what is known about influential factors, communication strategies, communication occurrence, and the outcomes of communication in the context of HCA. Findings can be used to inform practice and the development of future studies, interventions, and policy aimed at improving communication and cascade screening in families with HCA.
Acknowledgments
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award numbers F31NR014758 and T32NR009759 and the Midwest Nurses Research Society Dissertation Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge Janet K. Williams for thoughtful review of this work.
Footnotes
Conflict of Interest
Lisa L. Shah and Sandra Daack-Hirsch declare that they have no conflict of interest.
Informed Consent
No protected health information or human subjects were used for this article. No animal studies were carried out by the authors for this article.
References
- Al-Khatib SM, Yancy CW, Solis P, Becker L, Benjamin EJ, Carrillo RG, … Varosy PD. 2016 AHA/ACC clinical performance and quality measures for prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Journal of the American College of Cardiology. 2017;69(6):217–744. doi: 10.1016/j.jacc.2016.09.933. [DOI] [PubMed] [Google Scholar]
- Batte B, Sheldon J, Arscott P, Huismann D, Salberg L, Day S, Yashar B. Family communication in a population at risk for hypertrophic cardiomyopathy. Journal of Genetic Counseling. 2015;24(2):336–348. doi: 10.1007/s10897-014-9774-8. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Hay JL, Harris-Wai JN, Meischke H, Burke W. All in the family? Communication of cancer survivors with their families. Familial Cancer. 2017 doi: 10.1007/s10689-017-9987-8. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C, McGaughran J, Davis A, Semsarian C, Ingles J. Factors influencing uptake of familial long QT syndrome genetic testing. American Journal of Medical Genetics Part A. 2016;170(2):418–425. doi: 10.1002/ajmg.a.37455. [DOI] [PubMed] [Google Scholar]
- Caleshu C, Kasparian NA, Edwards KS, Yeates L, Semsarian C, Perez M, … Ingles J. Interdisciplinary psychosocial care for families with inherited cardiovascular diseases. Trends in Cardiovascular Medicine. 2016;26(7):647–653. doi: 10.1016/j.tcm.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Christiaans I, Birnie E, Bonsel G, Wilde AA, van Langen IM. Uptake of genetic counseling and predictive DNA testing in hypertrophic cardiomyopathy. European Journal of Human Genetics. 2008;16:1201–1207. doi: 10.1038/ejhg.2008.92. [DOI] [PubMed] [Google Scholar]
- Christian S, Atallah J, Clegg R, Guiffre M, Huculak C, Dzwiniel T, … Somerville M. Uptake of predictive genetic testing and cardiac evaluation for children at risk for an inherited arrhythmia or cardiomyopathy. Journal of Genetic Counseling. 2017 doi: 10.1007/s10897-017-0129-0. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Cirino AL, Harris S, Lakdawala NK, Michels M, Olivetto I, Day SM, … Ho CY. Role of genetic testing in inherited cardiovascular disease: A review. JAMA Cardiology. 2017 doi: 10.1001/jamacardio.2017.2352. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Cohen LL, Stolerman M, Walsh C, Wasserman D, Dolan SM. Challenges of genetic testing in adolescents with cardiac arrhythmia syndromes. Journal of Medical Ethics. 2012;38(3):163–167. doi: 10.1136/medethics-2011-100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daack-Hirsch S, Shah LL, Cady A. Mental models of cause and inheritance for type 2 diabetes among unaffected individuals who have a positive family history. Qualitative Health Research. 2017 doi: 10.1177/1049732317745052. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, … Edwards A. Process and outcome in communication of genetic information within families: A systematic review. European Journal of Human Genetics. 2007;15:999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- Geelen E, Van Hoyweghen I, Doevendans PA, Marcelis CLM, Horstman K. Constructing “best interests”: Genetic testing of children in families with hypertrophic cardiomyopathy. American Journal of Medical Genetics Part A. 2011a;155:1930–1938. doi: 10.1002/ajmg.a.34107. [DOI] [PubMed] [Google Scholar]
- Geelen E, Van Hoyweghen I, Horstman K. Making genetics not so important: Family work in dealing with familial hypertrophic cardiomyopathy. Social Science & Medicine. 2011b;72:1752–1759. doi: 10.1016/j.socscimed.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, … Yancy CW. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2011;58(25):2–47. doi: 10.1016/j.jacc.2011.10.825. [DOI] [PubMed] [Google Scholar]
- Hanninen M, Klein GJ, Laksman Z, Conacher SS, Skanes AC, Yee R, … Krahn AD. Reduced uptake of family screening in genotype-negative versus genotype-positive long QT syndrome. Journal of Genetic Counseling. 2015;24(4):558–564. doi: 10.1007/s10897-014-9776-6. [DOI] [PubMed] [Google Scholar]
- Haukkala A, Kujala E, Alha P, Salomaa V, Koskinen S, Swan H, Kaariainen H. The return of unexpected research results in a biobank study and referral to health care for heritable long QT syndrome. Public Health Genomics. 2013;16(5):241–250. doi: 10.1159/000354105. [DOI] [PubMed] [Google Scholar]
- Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Alberta Heritage Foundation for Medical Research; 2004. [Google Scholar]
- Ladd M. Maps of beauty and disease: thoughts on genetics, confidentiality, and biological family. Journal of Medical Ethics. 2010;36(8):479–482. doi: 10.1136/jme.2009.033514. [DOI] [PubMed] [Google Scholar]
- Mangset M, Hofmann B. LQTS parents’ reflections about genetic risk knowledge and their need to know or not to know their children’s carrier status. Journal of Genetic Counseling. 2014;23(6):1022–1033. doi: 10.1007/s10897-014-9727-2. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Spiridigliozzi GA. “Family matters”: A conceptual framework for genetic testing in children. Journal of Genetic Counseling. 2004;13(1):9–29. doi: 10.1023/b:jogc.0000013379.90587.ef. [DOI] [PubMed] [Google Scholar]
- Miller EM, Wang Y, Ware SM. Uptake of cardiac screening and genetic testing among hypertrophic cardiomyopathy and dilated cardiomyopathy families. Journal of Genetic Counseling. 2013;22:258–267. doi: 10.1007/s10897-012-9544-4. [DOI] [PubMed] [Google Scholar]
- Ormondroyd E, Oates S, Parker M, Blair E, Watkins H. Pre-symptomatic genetic testing for inherited cardiac conditions: A qualitative exploration of psychosocial and ethical implications. European Journal of Human Genetics. 2014;22(1):88–93. doi: 10.1038/ejhg.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, … van der Meer 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Journal of Heart Failure. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- Rowland E, Metcalfe A. Communicating inherited genetic risk between parent and child: A meta-thematic synthesis. International Journal of Nursing Studies. 2013;50:870–880. doi: 10.1016/j.ijnurstu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Sandelowski M, Leeman J. Writing usable qualitative health research findings. Qualitative Health Research. 2012;22(10):1404–1413. doi: 10.1177/1049732312450368. [DOI] [PubMed] [Google Scholar]
- Smart A. Impediments to DNA testing and cascade screening for hypertrophic cardiomyopathy and long QT syndrome: A qualitative study of patient experiences. Journal of Genetic Counseling. 2010;19:630–639. doi: 10.1007/s10897-010-9314-0. [DOI] [PubMed] [Google Scholar]
- van der Roest WP, Pennings JM, Bakker M, van den Berg MP, van Tintelen JP. Family letters are an effective way to inform relatives about inherited cardiac disease. American Journal of Medical Genetics Part A. 2009;149A:357–363. doi: 10.1002/ajmg.a.32672. [DOI] [PubMed] [Google Scholar]
- Vavolizza R, Kalia I, Aaron K, Silverstein L, Barlevy D, Wasserman D, … Dolan S. Disclosing genetic information to family members about inherited cardiac arrhythmias: An obligation or a choice? Journal of Genetic Counseling. 2015;24(4):608–615. doi: 10.1007/s10897-014-9783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter VL, Elia J, Erickson C, Berger S, Blum N, Uzark K, Webb CL. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- Walter FM, Emery J. ‘Coming down the line’-patients’ understanding of their family history of common chronic disease. Annals of Family Medicine. 2005;3:405–414. doi: 10.1370/afm.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte S, Green A, McAllister M, Shipman H. Family Communication in Inherited Cardiovascular Conditions in Ireland. Journal of Genetic Counseling. 2016;25:1317–1326. doi: 10.1007/s10897-016-9974-5. [DOI] [PubMed] [Google Scholar]
- Wiens ME, Wilson BJ, Honeywell C, Etchgary H. A family genetic risk communication framework: Guiding tool development in genetics health services. Journal of Community Genetics. 2013;4:233–242. doi: 10.1007/s12687-012-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Forrest K, van Teijlingen ER, McKee L, Haites N, Matthews E, Simpson SA. Family communication about genetic risk: The little that is known. Community Genetics. 2004;7:15–24. doi: 10.1159/000080300. [DOI] [PubMed] [Google Scholar]
- Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: A review. Familial Cancer. 2010;9:691–701. doi: 10.1007/s10689-010-9380-3. [DOI] [PubMed] [Google Scholar]