Abstract
Some DNA or RNA sequences rich in guanine (G) nucleotides can adopt non-canonical conformations known as G-quadruplexes (G4). In the nuclear genome, G4 motifs have been associated with genome instability and gene expression defects, but they are increasingly recognized to be regulatory structures. Recent studies have revealed that G4 structures can form in the mitochondrial genome (mtDNA) and G4 forming potential sequences are associated with the origin of mtDNA deletions. However, little is known about the regulatory role of G4 structures in mitochondria. In this short review, we will explore the potential for G4 structures to regulate mitochondrial function, based on evidence from the nucleus.
Keywords: G-quadruplexes, mtDNA, mitochondrial gene expression, mitochondrial genome instability, mtDNA deletions, mtDNA depletion, G4 ligand
Introduction
DNA or RNA sequences particularly abundant in adjacent guanines (G) can fold into non-canonical four-stranded nucleic structures known as G-quadruplexes (G4; Fig. 1)1,2. G4 structures have been widely studied in vitro, and under physiological conditions they display high thermodynamic stability2,3. In brief, the G4 core is formed by the assembly of four guanines bound through Hoogsteen-hydrogen bonds into a planar conformation called a G-tetrad4 (Fig. 1A), and two or more G-tetrads stack together to form a stable quadruplex (Fig. 1B). The source of guanines can be intra- or inter-strand, DNA or RNA, that may be connected by loops of variable length1. The relative orientation of the strands can form parallel, antiparallel or mixed orientation G4 structures5. G4 stability depends on π-π interactions between the stacked G-tetrads and metal cations, particularly K+ and to a lesser extent Na+, which fit into the central anionic cavity within or between the G-tetrads to coordinate the carbonyl oxygens of the guanines3. Additional details regarding the structural dynamics and stability of G4 structures have been reviewed elsewhere6,7.
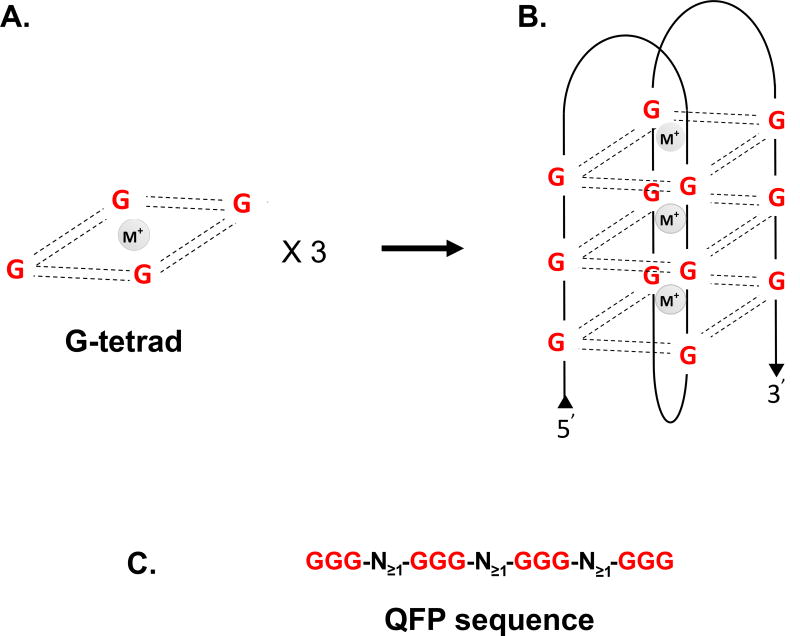
Figure 1. G-quadruplex structure.
(A) Four guanines (G) form planar cyclic conformations through eight Hoogsteen-hydrogen bonds (dashed lines) and stabilized by the presence of a central metal cation (M+). (B) G4 structure in antiparallel conformation. Numerous topologies are possible. (C) From the requirement of four runs of guanine, QFP sequences can be predicted within the same strand. Traditional prediction requires at least two G per run and one nucleotide to form a loop. Shown is a prediction scheme typically used for identifying three guanine stack G4-forming potential (QFP) sequences. Limits to size of window for search usually range from 30–50 bp.
Computational approaches have been used to predict sequences with quadruplex forming potential (QFP)8–10, usually based on the G3+-N≥1-G3+-N≥1-G3+-N≥1-G3+ consensus motif (Fig. 1C). Some of these early tools predicted more than 370,000 QFP sequences in the human genome8,11. Many QFP sequences from plants12, protozoa13, bacteria14, yeast15 and mammalian cells16,17 have been confirmed in vitro to form G4 structures. Unfortunately, these computational approaches suffer from significant false negative and positive predictions compared with in vitro data, and the type of G4 topological fold is also not readily predicted. In a recent study, Bedrat A. et al. developed a more sophisticated algorithm (G4Hunter) that takes into account both the G-richness and G-skewness of sequences, and which they validated using over 200 G-quadruplexes formed in vitro9. Using G4Hunter in comparative studies, genomes with high guanine and cytosine (GC) content and GC strand asymmetry showed higher incidence of QFP sequences, and the authors estimated that the QFP regions present in the human genome could be from 2 to 10-fold more abundant than previously predicted9. Despite these advances, it is important to consider that the rules dictating G4 levels in vivo, where G4 stability could be modulated by many factors (including molecular crowding, dehydration, DNA supercoiling, helicase activity, and binding by RNA and proteins), are still only poorly understood.
The mitochondrial DNA (mtDNA) contains hundreds of QFP and is a classic example of a genome with high potential to form G4. Recently, evidence of potential roles for G4s in mtDNA replication18,19 and their association with human mtDNA deletions20–22 have been described; however, the contribution of these structures to mitochondrial function may be broader. The purpose of this review is to provide a perspective on potential functions of G4 in the mitochondria that draw from lessons learned in the study of nuclear nucleic acids and to discuss the current data on G4 structures in mtDNA.
A BRIEF REVIEW OF G-QUADRUPLEXES IN THE NUCLEAR GENOME
Evidence for G4 in the nucleus
Before reviewing the evidence for G4s in the nucleus, we emphasize that proving definitively that G4s form and function in vivo is challenging. Demonstrating that a G4 ligand (e.g. a small molecule or antibody) binds to a subcellular target is only weak evidence of physiologic G4 formation, because such ligands may also have substantial affinity for non-G4 targets, and also because they may artifactually induce or stabilize G4s to yield levels that do not exist naturally. Similarly, there is no genetic manipulation (e.g. the deletion or overexpression of gene encoding a G4-unwinding helicase) that is known to fully and selectively impact G4 levels. Nonetheless, strong indications of G4 function have been obtained by intersecting independent lines of evidence.
Attempts to directly identify genomic regions that can form G4s have to date primarily used anti-G4 antibodies or the capacity of G4s in a DNA template to block DNA polymerase synthesis23. Immunoprecipitation of purified genomic DNA using a single chain antibody fragment (scFv), HF2, revealed enrichment for sequences with QFP24. Similarly, enhanced blocking of DNA polymerase under conditions of G4 stabilization (using K+ or the G4 small molecule ligand PDS) on a purified genomic DNA template occurred preferentially at QFP sequences, which were termed “observed quadruplexes” (OQs)25. Because these techniques use isolated DNA, they do not reveal direct evidence that these sequences form G4s in live cells.
However, more recent genome-wide chromatin immunoprecipitation (ChIP-seq) experiments using different scFvs, BG4 or D1, and chromatin isolated from cultured human cells showed a preferential enrichment for QFP sequences26,27. Such BG4 peaks occurred at only about one percent of the OQs. This may indicate that only some OQs form G4 in vivo, or that BG4 does not bind all G4s. We suspect both are true, and indeed it would be surprising if any antibody (or small molecule ligand) could bind all possible G4 folds, given their structural diversity. Isolation of G4s within chromatin may also be possible using G4 small molecule ligands, although it is not yet clear if any have sufficient affinity and specificity. To test the fidelity of the above approaches, it will be important to determine whether apparent G4 levels in chromatin change as expected under conditions expected to alter G4 stability, for example loss of expression of a G4 helicase.
Telomeres
Early interest in G4 biology focused primarily on telomeres, the structures at the ends of linear chromosomes. Telomeres are critical for chromosome stability, but they tend to shorten with cell division, which can lead ultimately to loss of telomere function, called telomere “uncapping”, and thus cell death or permanent cell cycle arrest. The enzyme telomerase can lengthen telomeres, and most cancers (80–90%) overexpress telomerase to support their replicative immortality28. Therefore, to block the perpetual division of cancer cells, one experimental strategy has been to inhibit telomerase activity. Telomeres typically comprise tandem repeats of DNA sequences possessing a run of consecutive guanines which endow them with high QFP. For example, vertebrate telomeres contain thousands of repeats of the sequence 5’-TTAGGG-3’. Indeed, telomere repeats have long been known to form stable G4s in vitro2,29, and several lines of evidence support potential roles for telomeric G4s in vivo30, but the extent to which telomeric G4s play beneficial or deleterious roles under natural conditions remains poorly understood.
Evidence that G4s and G4 small ligands can inhibit telomerase stimulated the development of such molecules as potential anti-cancer drugs31 and more in depth summary of G4 ligands with anti-cancer activity has been reviewed elsewhere32. The predominant mechanism of in vitro cancer cell death is associated with telomerase inhibition and/ or telomeres uncapping along with DNA damage and consequent activation of senescence and apoptotic pathways33. Several G4 binding ligands have been shown to induce selective cancer cell senescence34–36. Additionally, exogenous G4 DNAs (both telomeric and non-telomeric sequence G4s) transfected into cancer cells can induce apoptosis37, suggesting that the presence of stabilized G4s can induce apoptosis in cancer cells. On the other hand, there is evidence in both O. nova and human cells that telomeres in G4 conformations can be good substrates for telomerase38,39, arguing that stabilization of G4s might enhance cancer cell survival by improving the healing of uncapped telomeres. In summary, to what extent G4 ligands inhibit cancer cells via effects on telomerase and telomeres is generally not established. The anti-cancer activity of G4 ligands or stabilized G4 structures might be through effects on mitochondria, on DNA integrity, or on gene expression.
Gene Expression
A large number of studies indicate that G4s are involved in the regulation of transcription. More than 40% of promoter regions of human genes contain sequences that have the potential to fold into G4 structures40, and QFP is also enriched in the promoters of other species including yeast41 and bacteria14. Functional evidence for regulation of transcription by G4 come from genome-wide analyses of cells treated with G4 small molecule ligands41,42, expressing anti-G4 scFvs26, or lacking the activity of DNA helicases with G4-unwinding activity, for example yeast Sgs1 and human Werner’s syndrome protein (WRN), Bloom syndrome protein (BLM)43–45. These treatments lead to preferential alteration of mRNA levels from genes with high QFP, indicating G4-based mechanisms. Although QFP on the sense strand of such genes could reflect G4-DNA or G4-RNA based effects, QFP in these studies were found upstream of transcription start sites and without strand bias, suggesting at least some role for G4-DNA. Numerous studies have revealed the high propensity of proto-oncogene promoters to form G4 structures40,46, such as c-MYC47,48, VEGF49, c-KIT50, KRAS51, hTERT52, and BCL253. There are G-quadruplexes located upstream of the transcription start site of these genes and at least some can negatively modulate gene expression when treated with small molecule G4 ligands48,54–57. G4s are also associated with upregulation of gene expression, and one underlying mechanism may involve their tendency to destabilize nucleosomes, since QFP is associated with reduced histone occupancy at promoters, with DNase I hypersensitive sites, and BG4 ChIP revealed enrichment for nucleosome poor chromatin25,41,58.
Other mechanisms by which G4s may affect gene expression include regulation of RNA splicing, polyadenylation, stability, targeting, and translation into protein. These have been well reviewed elsewhere59. We note that although it has long been assumed that G4-RNA might be more prevalent than G4-DNA, because G4-RNA formation can be unimpeded by competitive base pairing by complementary strand as in duplex DNA, a recent study concluded that in eukaryotes potential G4-RNA is primarily in an unfolded state60. More work will be essential to fully understand to what extent transient formation of G4-RNA occurs and plays an important role in RNA biology.
DNA replication and genome stability
Several lines of evidence indicate that G4 structures can impact DNA replication and genome stability61–63. For example, ~80 % of apparent replication origins overlap with QFP elements in mouse and human cells61,64. Functional dissection of two model replication origins in chicken DT40 cells demonstrated that their QFP elements are required, though not sufficient, for origin firing65. At the same time, particularly under conditions of replicative stress, G4 structures may imperil DNA replication and genome stability. Insertion of the human minisatellite CEB1, which has high QFP, into the S. cerevisiae genome generates a replication fork stalling and genome instability when the cells lack the Pif1 G4 DNA helicase or are treated to the G4 ligand Phen-DC366,67. Similarly, Pif1 is important for replication though natural QFP elements in yeast77. Furthermore, cultured human cells treated with the G4 ligand PDS develop replication-dependent phosphorylation of H2AX near QFP elements, indicating G4 stabilization might cause damage to replication forks68. In cancer cells, a strong association between nuclear genome deletion breakpoints and QFP sites has been identified, suggesting G4-induced genome instability may contribute to carcinogenesis69. Furthermore, cancer cells deficient in homologous recombination (HR) secondary to BRCA1 or BRCA2 deficiency were found to be highly susceptible to PDS induced DNA double strand break generation and toxicity70. The HR dependent repair is further corroborated by recent evidence showing cells deficient in BLM helicase show increased sister chromatid exchange events at QFP sequences throughout the human and mouse genome71. These findings suggest that HR-dependent mechanisms are important for the repair of G4-related genome damage, and for maintaining genome stability.
MTDNA: A FAVORABLE ENVIRONMENT FOR G4 FORMATION
The mitochondria
Mitochondria have a double membrane structure and play numerous crucial roles in cell biology (Fig. 2). They are signaling organelles, involved in bioenergetics signaling, apoptosis, calcium signaling, and immune signaling. As the hub of cellular metabolism, mitochondria are the home of heme biosynthesis, iron-sulfur cluster biosynthesis, branched-chain amino acid biosynthesis, fatty acid biosynthesis and catabolism, the Kreb’s cycle, and high efficiency ATP production through oxidative phosphorylation (OXPHOS). During OXPHOS, electron transport coupled proton (H+) translocation across the inner membrane into the intermembrane space generates an electrochemical gradient harnessed by Complex V to catalyze the ATP synthesis (Fig. 2A). It is important to note that OXPHOS Complexes I, III, IV, and V are derived from the protein products of mitochondrial and nuclear genomes, and are thus bigenomic in origin.
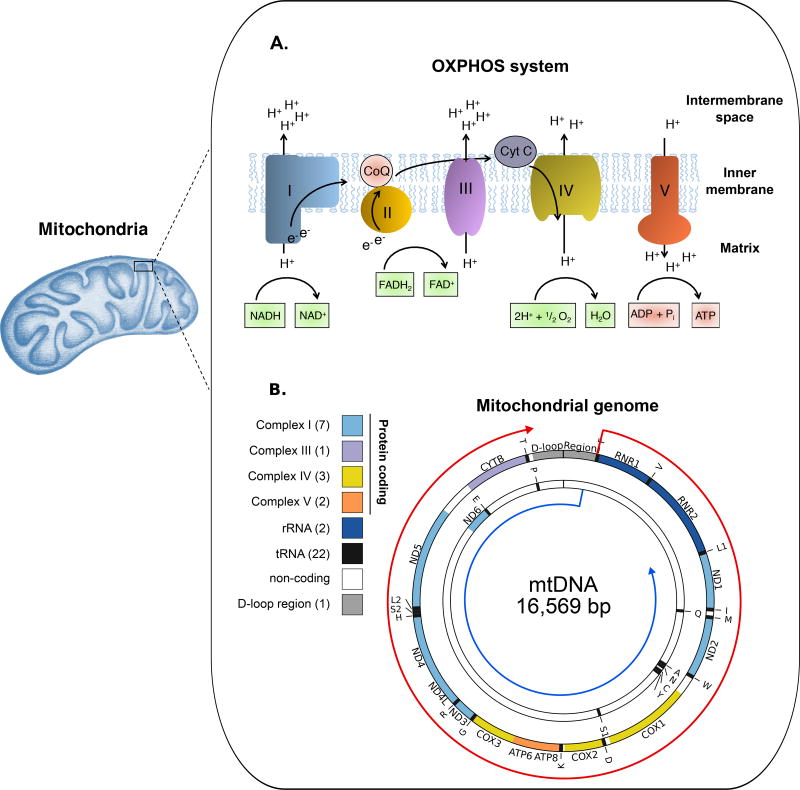
Figure 2. Schematic representation of the oxidative phosphorylation (OXPHOS) system and mitochondrial DNA (mtDNA).
(A) Main components of the mitochondrial OXPHOS system: Complex I (blue), Coenzyme Q (CoQ; pink), Complex II (orange), Complex III (purple), Cytochrome C (CytC; grey), Complex IV (yellow) and Complex V (red). The redox metabolites are shown in green boxes and the final OXPHOS reaction is represented in red boxes. (B) The mitochondrial genome is a 16,569 bp circular double-stranded DNA molecule that contains 37 genes encoding for 13 OXPHOS proteins (colors matched panel A) and the tRNAs (black) and rRNAs (dark blue) required for their expression. The number of genes per group are indicated in parenthesis. The genes are encoded on either strand, shown with the two strands separated to indicate the relevant coding sequences on each polycistronic pre-mRNA transcript (indicated by red and blue arrows).
The mitochondrial DNA
The human mitochondrial genome is a double-stranded circular molecule of 16,569 nucleotides that encodes 13 proteins essential for OXPHOS function, as well as the 22 tRNAs and two rRNAs required for their translation72 (Fig. 2B). The other OXPHOS proteins are nuclear encoded, cytoplasmically translated, and imported into the mitochondria. Mitochondria contain multiple copies of their genome mtDNA with copy numbers ranging from ~102 to ~105 copies in somatic cells and to even higher levels (~4 × 106) in oocytes73. Somatic cells with a high metabolic demand, such as neurons and muscle cells, require more ATP and have a higher number of mtDNA compared to those with low energy demands, such as fibroblasts.
The mitochondrial genome displays several unique features relative to the nuclear genome. In brief, the mtDNA is packaged into small structures termed nucleoids, with estimates of 1–2 genome copies per nucleoid74. Notably, the mtDNA lacks histones and the diverse DNA repair mechanisms of the nucleus, with only base excision repair (BER) activities having been detected75. The limited DNA repair pathways available in the mitochondria, coupled with its close proximity to the OXPHOS complexes, which are a major source of reactive oxygen species (ROS), render the mtDNA more vulnerable to damage when compared to nuclear DNA76. The primary defense against damage may be the high mtDNA copy number. The constant damage to mtDNA from ROS is thought to contribute to progressive age-related decline in mitochondrial function77. Whether aging lowers copy number, oxidative damage contributes to mtDNA mutations, and mtDNA mutations contribute to aging, are subjects of continued debate78–80.
Introduction to mtDNA replication
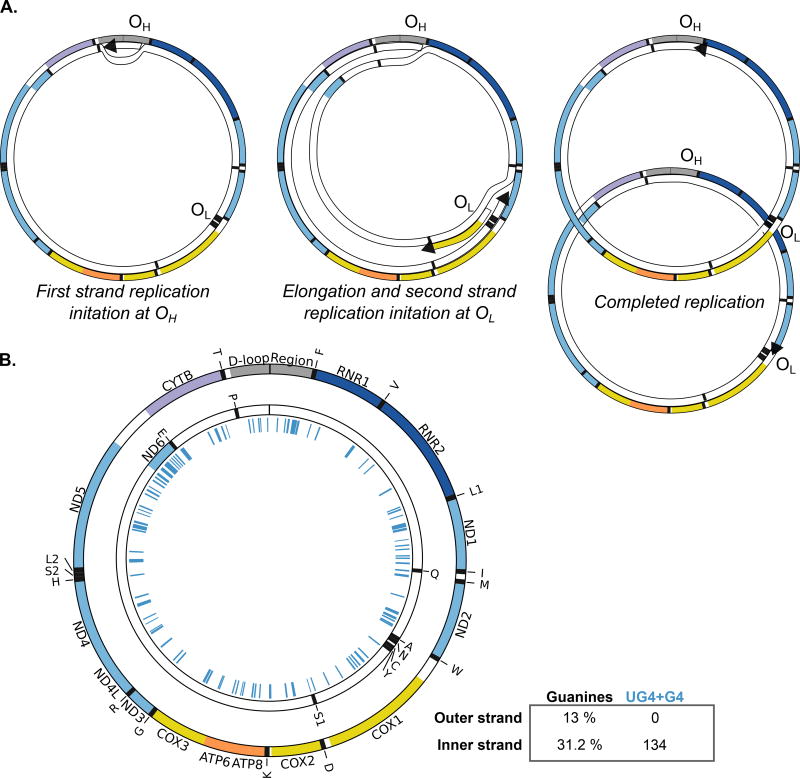
The mammalian mitochondrial genome replicates in a manner distinct from nuclear DNA. Although there is a clear leading (first) and lagging (second) strand, Okazaki fragments are not apparent in mtDNA replication81. The lack of Okazaki fragments is due to asymmetric replication of mtDNA, which is the predominant mechanism of DNA synthesis and generates primarily continuous nascent strands. There is significant debate about the specifics of mtDNA replication, so a simplified view of the common features of the mitochondrial genome replication in cultured cells is provided in Fig. 3. The major start site of first strand synthesis is within the control region, at ori-H (OH; Fig. 3A left). Truncated mtDNA replication molecules, which form a D-loop structure, are common in vivo82, so elongation beyond the D-loop region is thought to indicate productive replication. First strand replication produces a nascent heavy strand, displacing the parental heavy strand. The single-stranded region can be bound by single-stranded DNA binding protein83 or hybridized with mitochondrial RNA84. Although second-strand replication initiation can occur anywhere on the displaced strand, replication primed by hybridized RNA initiates predominantly at ori-L83 (OL; Fig. 3A middle). Continued elongation of both strands yields two DNA molecules replicated in a semiconservative fashion (Fig. 3A right).
Figure 3. Mitochondrial genome replication and predicted G4 forming potential sequences.
(A) Simplified diagram of mtDNA asynchronous replication. Left: first strand replication initiation at ori-H (OH). DNA starts as duplex, with synthesis of the heavy strand initiated in the heavy strand origin (OH). Middle: elongation of the first strand and initiation of the second strand replication at ori-L (OL). Continuous elongation of the first strand displaces the parental heavy strand of mtDNA with nascent DNA. Once replication crosses the primary second strand origin (OL), replication in the other direction begins. Right: Elongation of both strands continues until two semi-conservative molecules are generated. Note that only the heavy strand is single stranded in this process. (B) Representation of the G4-forming sequences in the heavy strand (inner strand; 31.2% guanines) of the human mtDNA. The figure represents validated unstable G4 (UG4; n=63) and G4 (n=71) out of 209 sequences experimentally tested9.
THE MITOCHONDRIAL VIEWPOINT OF G-QUADRUPLEXES
Mitochondrial G4 forming sequences
The mode of mtDNA replication may contribute directly to G4 structures formation and genome instability, thereby causing disease. The mammalian mitochondrial genome shows significant asymmetry in strand composition with a two-fold enrichment of guanines on one strand (inner strand; Fig. 3B). The reason for this highly conserved strand asymmetry arises from selective pressure to limit cytosine content in the displaced strand, as cytosine can be deaminated to uracil to cause cytosine to thymine transversions85. This enrichment of guanines contributes to the higher QFP density of the mitochondrial vs. nuclear genome. In a recent in silico study, QFP per kb in the human mtDNA is estimated to be 2.4 to 3.6-fold higher than in the nuclear DNA9. During replication and transcription, the DNA strand rich in QFP sequences is temporarily single stranded, suggesting an increased opportunity to form G4 structures. Additionally, the mitochondrial environment would be permissive to G4 formation, as the potassium concentration in the matrix is estimated to be 150 mM86.
Mitochondrial G4 formation and transcription/replication switching
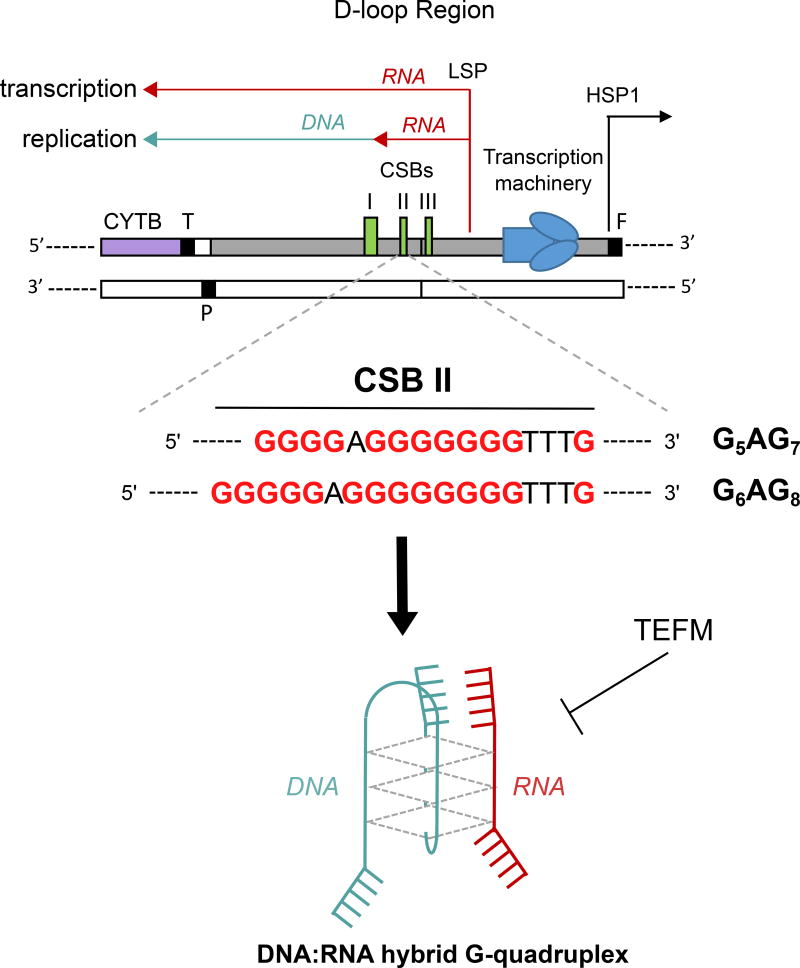
An intriguing feature of mtDNA is the mechanism of replication initiation at OH (Fig. 4). Transcription from the light strand promoter (LSP) generates all RNAs that contain heavy strand sequence. Immediately downstream of the LSP are three conserved sequence boxes (CSBs), which are conserved from yeast to human mtDNA87. Although the specific transition point reported varies, CSBs are the sites where transcript termination can occur, forming the primer for replication. In in vitro transcription assays, multiple groups have shown that mitochondrial RNA polymerase (POLRMT) terminates at CSB II18,88. This termination depends of the formation RNA-DNA G4 hybrid structures18,88,89. Termination is more efficient when CSB II contains the typical sequence G6AG8, rather than the rare variant G5AG7, consistent with a higher stability G4 promoting termination. Furthermore, the RNA helicase TEFM, which can be efficiently photo-crosslinked to the G4 forming sequence of CSB II, inhibits termination19. Therefore, these in vitro studies support a contribution of G4 structure formation and resolution to the switch between mtDNA replication and transcription.
Figure 4. Mitochondrial control region and transcription termination induced by G4 structure.
The light strand promoter (LSP; red) transcription can be arrested by a hybrid DNA:RNA G4 structure formed at the conserved block sequence II (CSB II). The arrested RNA is thought to serve as a primer to initiate the leading-strand DNA (green) replication. Two alternative regions at the CSB II could lead to the G4 formation, the G5AG7 (rare polymorphism) and the G6AG8 (most genomes) sequences. The mitochondrial transcription elongation factor (TFEM) prevents the CSB II transcription termination.
QFP sequences and mtDNA instability
Several groups have queried whether predicted secondary structures in mtDNA might associate with mtDNA deletion breakpoints in human diseases20–22. As described above, G4 stabilization can lead to genome instability, and QFP sequences are common sequence elements associated with deletion breakpoints in nuclear genomes of cancer cells, suggesting that G4s could affect the stability of both nuclear and mitochondrial DNA. Among the various sequences that form non-B form secondary structures tested by the different groups20–22, QFP sequences showed the strongest association with the ends of deletions in the mitochondrial genome. When tested, the QFP sequences form G4 structures in vitro20,21. Given their apparently negative impact on mtDNA stability, the persistence over evolutionary time of QFP sequences in mitochondrial genomes raises the possibility that their negative effects are more than counterbalanced by beneficial regulatory functions.
A potential role for G4 structures to alter mitochondrial replication, transcription, and translation
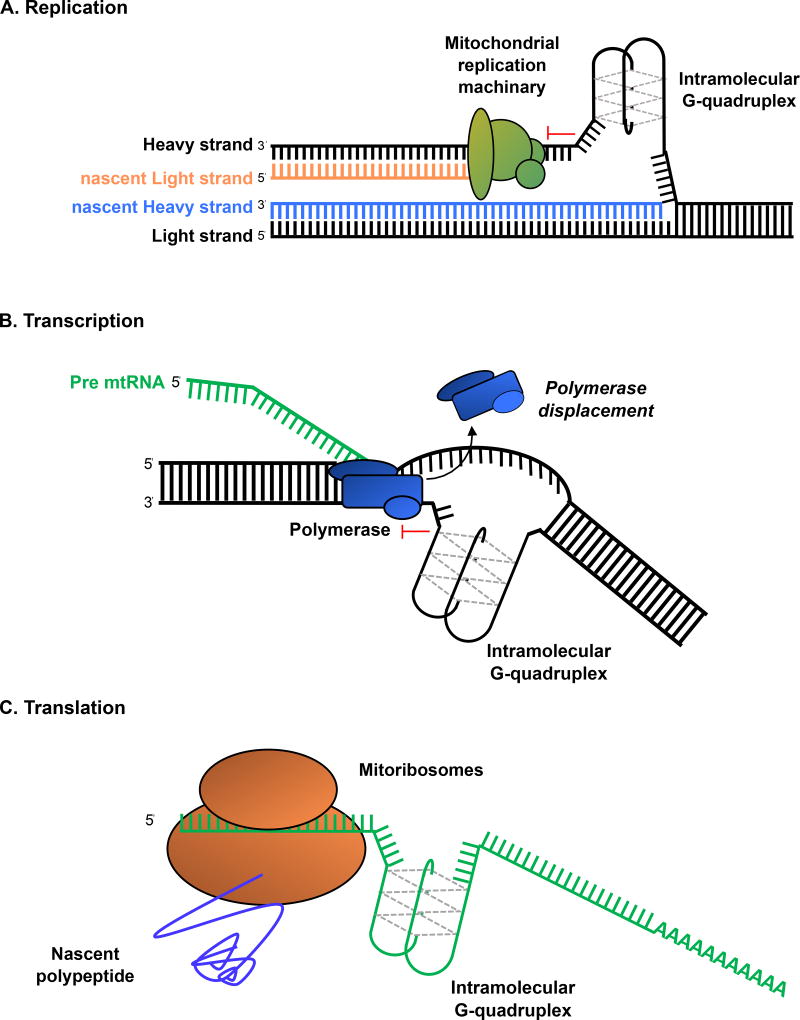
Evidence continues to build for G4 structures altering the function of mitochondrial sequences18,21,90,91. Alteration of G4 stability at CSB II would be expected to alter the balance between transcription and replication from LSP18. This switch likely specifies a gene expression or mtDNA copy number maintenance role to each mitochondrial genome (Fig. 4). More broadly, the enrichment of QFP on the displaced strand, which is the template for second strand synthesis, promotes the speculation that G4 stabilization would interfere with second-strand synthesis to cause mtDNA depletion20,21 (Fig. 5A). These studies point toward a regulatory role of G4 structures in both DNA replication initiation and elongation. Because QFP are found in both RNA sequences and transcription templates, the formation of G4 structures may also regulate mitochondrial transcription elongation (Fig. 5B), perhaps through hybrid formation as observed in CSB II or template interference. Similarly, RNA G4 may have a regulatory role in translation (Fig. 5C). As was the case in the nucleus, development of tools specific to the mitochondria will be instrumental in testing this hypothesis.
Figure 5. Potential G4 formation during mtDNA replication, transcription and translation.
G4 structures can form in single-stranded nucleic acids. (A) During mtDNA replication, the displaced G-rich heavy strand is a potential hotspot for the G4 formation, which would arrest the mitochondrial replication machinery (green) during second strand synthesis. (B) A model of transcription inhibition by G4 structures. The unresolved G4 structures would occur in the mtDNA template strand preventing the polymerase (blue) activity. (C) A model of translation inhibition by G4 structures forming in the mRNA.
The potential for G4 helicases to regulate mitochondrial function
The Pif1 helicase shows strong G4 resolving activity and is dual localized to both telomeres and mitochondria92–95. Pif1 helicases are an evolutionary conserved class of enzymes present in bacteria, yeast and mammals96. Depletion of the nuclear Pif1 isoform in human cell lines induces replication fork pausing and DNA deletions96. In yeast, mutations in the PIF1 gene affect mtDNA stability and its susceptibility to oxidative stress97. Moreover, it has been observed that the presence of unresolved G4 structures due to Pif1 helicase deficiency causes DNA instability and DNA breakpoint formation98,99. Interestingly, PIF1 ablation in mouse shows no effect on telomere length100, but causes a modest increase in mtDNA deletion load in skeletal muscle101.
Other potential G4-interacting proteins have been identified to have a role in mitochondria. Human primary fibroblasts knocked-down for RECQL4, which localizes in part to the mitochondria102, show an increased susceptibility to mtDNA damage and impaired maximal respiratory function. More recently, an in vitro study has identified the mtDNA binding protein TFAM as a G-quadruplex binding protein103, suggesting that G4 structures might occur in the mitochondrial genome. TFAM has also been suggested to bind recombination intermediates104, but neither G4 binding nor Holliday-junction binding has been validated in mammalian cells. Like single-stranded DNA, RNA may also be implicated in forming G4 structures. The mitochondrial RNA-binding protein G-rich binding factor1 (GRSF1) shows strong preference for non-coding mitochondrial RNA sequences that are predicted to form G4 structures105. The mitochondrial SLIRP RNA helicase that regulates mitochondrial gene expression post-transcriptionally106 has recently been identified biochemically to bind G4 structures in vitro107. The formation of G4 in mitochondrial RNA, and a role for GRSF1 and SLIRP have yet to be established. Identifying the key G4 resolving activities may be crucial to understanding the impact of G4 on mitochondrial function.
G4-ligands in mitochondria
Whether G4s play a specific role in transcription/replication switching, or more broadly in replication, transcription, and translation has yet to be established in mammalian mitochondria. Key helicases need to be identified. In addition, the development of mitochondrial G4 reagents may play an important role in probing G4 in mitochondria and understanding their potential implications in respiratory function. Recently, it has been reported that a derivative of 3,6-bis(1-methyl-4-vinylpyridinium) carbazole diiodide (BMVC) localizes to mitochondria in cancer cells and induces apoptosis108. The mechanisms of action of BMVC on mitochondrial nucleic acids and the impact on respiratory function has not been clarified. Other known G4 ligands, TMPyP4 and DoDC, do not localize preferentially to the mitochondria, but do cause mitochondrial dysfunction concomitant with cytochrome C release and apoptosis109,110. Further investigation is necessary to identify and characterize mitochondrial targeted G4 ligands to test their impact on respiratory function.
Future perspectives
Our current knowledge of G4 structures role in the mitochondrial has been limited due the lack of efficient experimental tools. Using the information that have been provided by the studies of G4 structures in the nucleus, we speculate that the mitochondrial G4 formation may affect mitochondrial replication, transcription and translation. To study G4 structures in mitochondrial biology, ligands and antibodies would need to be developed to selectively detect or isolate mitochondrial G4 structures in mtDNA or RNA. As such, scFv antibodies currently expressed in the nucleus might be applied to mitochondrial G4 detection, although they will need to be modified so they can be imported efficiently into the mitochondria. Additionally, scFv molecules may be used to capture and characterize by sequencing the changes in G4 forming sequences in response to helicase or ligand alterations. For application to mitochondria, known G4 ligands need to be tested, or new molecules need to be generated, that specifically localize to mitochondria and then assessed for function in mitochondrial nucleic acid biology. Furthermore, the identification of the mitochondrial-localized helicases that resolve G4 structures, the development of mitochondrial-specific G4 antibodies, and the identification of mitochondrial-localized G4 ligands may represent a powerful combinatorial approach for investigating G4 function in mitochondrial biology.
In summary, the mitochondrial genome is highly skewed for guanine content and QFP sequences. The high copy number and gene density of mtDNA has the potential to amplify the effects of G4 dysregulation on mitochondrial respiratory function and cell viability. The potential impact of G4 structures on DNA replication and deletion formation is shared between the nuclear and mitochondrial DNAs, as QFP sequences are a common feature near the junctions of deletions in both genomes. To extend our understanding further, however, requires testing if the G4-functions described in the nucleus apply to the mitochondria. Ultimately the mitochondrial G4 reagents development will lead to an improved understanding of the major G4s, how they are resolved, and their normal and pathophysiological functions in the mitochondria.
Acknowledgments
BAK, MF, and FBJ were all supported by the National Institutes of Health under award number R01GM110424 (to BAK).
References
- 1.Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–15. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334:364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 3.Sen D, Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29; doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- 4.Hazel P, Parkinson GN, Neidle S. Topology variation and loop structural homology in crystal and simulated structures of a bimolecular DNA quadruplex. J. Am. Chem. Soc. 2006;128:5480–7. doi: 10.1021/ja058577+. [DOI] [PubMed] [Google Scholar]
- 5.Karsisiotis AI, Hessari NM, Novellino E, Spada GP, Randazzo A, Webba da Silva M. Topological characterization of nucleic acid G-quadruplexes by UV absorption and circular dichroism. Angew. Chem. Int. Ed. Engl. 2011;50:10645–8. doi: 10.1002/anie.201105193. [DOI] [PubMed] [Google Scholar]
- 6.Lane AN, Chaires JB, Gray RD, Trent JO. Stability and kinetics of G-quadruplex structures. Nucleic Acids Res. 2008;36:5482–515. doi: 10.1093/nar/gkn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harkness RW, Mittermaier AK. G-quadruplex dynamics. Biochim. Biophys. Acta. 2017 doi: 10.1016/j.bbapap.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Huppert JL, Balasubramanian S. Prevalence of quadruplexes in the human genome. Nucleic Acids Res. 2005;33:2908–16. doi: 10.1093/nar/gki609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedrat A, Lacroix L, Mergny JL. Re-evaluation of G-quadruplex propensity with G4Hunter. Nucleic Acids Res. 2016;44:1746–1759. doi: 10.1093/nar/gkw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok CK, Merrick CJ. G-Quadruplexes: Prediction, Characterization, and Biological Application. Trends Biotechnol. 2017 doi: 10.1016/j.tibtech.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Todd AK, Johnston M, Neidle S. Highly prevalent putative quadruplex sequence motifs in human DNA. Nucleic Acids Res. 2005;33:2901–7. doi: 10.1093/nar/gki553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg R, Aggarwal J, Thakkar B. Genome-wide discovery of G-quadruplex forming sequences and their functional relevance in plants. Sci. Rep. 2016;6:28211. doi: 10.1038/srep28211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paeschke K, Simonsson T, Postberg J, Rhodes D, Lipps HJ. Telomere end-binding proteins control the formation of G-quadruplex DNA structures in vivo. Nat. Struct. Mol. Biol. 2005;12:847–854. doi: 10.1038/nsmb982. [DOI] [PubMed] [Google Scholar]
- 14.Rawal P, Kummarasetti VBR, Ravindran J, Kumar N, Halder K, Sharma R, Mukerji M, Das SK, Chowdhury S. Genome-wide prediction of G4 DNA as regulatory motifs: Role in Escherichia coli global regulation. Genome Res. 2006;16:644–655. doi: 10.1101/gr.4508806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capra JA, Paeschke K, Singh M, Zakian VA. G-quadruplex DNA sequences are evolutionarily conserved and associated with distinct genomic features in Saccharomyces cerevisiae. In: Stormo GD, editor. PLoS Comput. Biol. Vol. 6. 2010. p. e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson A, Wu Y, Huang YC, Chavez EA, Platt J, Johnson FB, Brosh RM, Sen D, Lansdorp PM. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014;42:860–869. doi: 10.1093/nar/gkt957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanrooij PH, Uhler JP, Simonsson T, Falkenberg M, Gustafsson CM. G-quadruplex structures in RNA stimulate mitochondrial transcription termination and primer formation. Proc. Natl. Acad. Sci. 2010;107:16072–16077. doi: 10.1073/pnas.1006026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agaronyan K, Morozov YI, Anikin M, Temiakov D. Replication-transcription switch in human mitochondria. Science (80-.) 2015;347:548–551. doi: 10.1126/science.aaa0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong DW, Pereira F, Barrett SP, Kolesar JE, Cao K, Damas J, Yatsunyk LA, Johnson F, Kaufman BA. Association of G-quadruplex forming sequences with human mtDNA deletion breakpoints. BMC Genomics. 2014;15:677. doi: 10.1186/1471-2164-15-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharti SK, Sommers JA, Zhou J, Kaplan DL, Spelbrink JN, Mergny J-L, Brosh RM., Jr DNA sequences proximal to human mitochondrial DNA deletion breakpoints prevalent in human disease form G-quadruplexes, a class of DNA structures inefficiently unwound by the mitochondrial replicative Twinkle helicase. J. Biol. Chem. 2014;289:29975–93. doi: 10.1074/jbc.M114.567073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira PH, da Silva CL, Cabral JMS. An appraisal of human mitochondrial DNA instability: new insights into the role of non-canonical DNA structures and sequence motifs. In: Bielas JH, editor. PLoS One. Vol. 8. 2013. p. e59907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han H, Hurley LH, Salazar M. A DNA polymerase stop assay for G-quadruplex-interactive compounds. Nucleic Acids Res. 1999;27:537–542. doi: 10.1093/nar/27.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernando H, Rodriguez R, Balasubramanian S. Europe PMC Funders Group Selective Recognition of a DNA G-Quadruplex by an Engineered Antibody †. 2009;47:9365–9371. doi: 10.1021/bi800983u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers VS, Marsico G, Boutell JM, Di Antonio M, Smith GP, Balasubramanian S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015;33:877–881. doi: 10.1038/nbt.3295. [DOI] [PubMed] [Google Scholar]
- 26.Hänsel-Hertsch R, Beraldi D, Lensing SV, Marsico G, Zyner K, Parry A, Di Antonio M, Pike J, Kimura H, Narita M, Tannahill D, Balasubramanian S. G-quadruplex structures mark human regulatory chromatin. Nat. Genet. 2016;48:1267–1272. doi: 10.1038/ng.3662. [DOI] [PubMed] [Google Scholar]
- 27.Conformation Selective Antibody Enables Genome Profiling and Leads to Discovery of Parallel G-Quadruplex in Human Telomeres. Cell Chem. Biol. 2016;23:1261–1270. doi: 10.1016/j.chembiol.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 29.Henderson E, Hardin CC, Walk SK, Tinoco I, Blackburn EH. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987;51:899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, Mavrakis KJ, Jiang M, Roderick JE, Meulen J, Van Der, Schatz JH, Rodrigo CM, Zhao C, Rondou P, Stanchina E, De, Teruya-feldstein J, Kelliher MA, Speleman F, Wendel H, Amrane S, Adrian M, Heddi B, Serero A, Nicolas A, Mergny J-LL, Tua A, Arora A, Nair DR, Maiti S, Biffi G, Di Antonio M, Tannahill D, Balasubramanian S, John M, Balasubramanian S, Bourdoncle a, Torres aE, Gosse C, Lacroix L, Vekhoff P, Le Saux T, Jullien L, Mergny J-LL, Brooks Ta, Hurley LH, Bryan TM, Baumann P, Bugaut A, Balasubramanian S, Cahoon La, Seifert HS, Chung WJ, Heddi B, Schmitt E, Lim KW, Mechulam Y, Phan ATT, Guédin A, Alberti P, Mergny J-LL, De Cian A, Gros J, Lacroix L, Mergny J-LL, Henderson A, Wu Y, Huang YC, Chavez Ea, Platt J, Johnson FB, Brosh RM, Sen D, Lansdorp PM, Jixun Dai, Megan Carver 1, D Y, Johnson JE, Cao K, Ryvkin P, Wang LS, Johnson FB, König SLB, Huppert JL, Sigel RKO, Evans AC, Lane AN, Maizels N, Martadinata H, Phan ATT, Melko M, Bardoni B, Palacký J, Vorlíčková M, Kejnovská I, Mojzeš P, Phan ATT, Kuryavyi V, Burge S, Neidle S, Patel DJ, Renciuk D, Kejnovská I, Skoláková P, Bednárová K, Motlová J, Vorlícková M, Schaffitzel C, Berger I, Postberg J, Hanes J, Lipps HJ, Plückthun a, Smith JS, Chen Q, Yatsunyk LA, Nicoludis JM, Garcia MS, Kranaster R, Balasubramanian S, Monchaud D, Teulade-Fichou M-P, Abramowitz L, Schultz DC, Johnson FB, Wanrooij PH, Uhler JP, Simonsson T, Falkenberg M, Gustafsson CM, Weisi Lu1, Yi Zhang2, Dan Liu2, Zhou Songyang1,2,3, M W, Yang D, Okamoto K, Tippana R, Xiao W, Myong S, Smith JS, Chen Q, Yatsunyk LA, Nicoludis JM, Garcia MS, Kranaster R, Balasubramanian S, Monchaud D. Rudimentary G-quadruplex-based telomere capping in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;18:764–7. [Google Scholar]
- 31.Paulo A, Francisco AP. Oncogene Expression Modulation in Cancer Cell Lines by DNA G-Quadruplex-Interactive Small Molecules. Curr. Med. Chem. 2016 doi: 10.2174/0929867323666160829145055. [DOI] [PubMed] [Google Scholar]
- 32.Vy Thi Le T, Han S, Chae J, Park H-J. G-quadruplex binding ligands: from naturally occurring to rationally designed molecules. Curr. Pharm. Des. 2012;18:1948–72. doi: 10.2174/138161212799958431. [DOI] [PubMed] [Google Scholar]
- 33.Neidle S. Human telomeric G-quadruplex: The current status of telomeric G-quadruplexes as therapeutic targets in human cancer. FEBS J. 2010 doi: 10.1111/j.1742-4658.2009.07463.x. [DOI] [PubMed] [Google Scholar]
- 34.Riou JF, Guittat L, Mailliet P, Laoui a, Renou E, Petitgenet O, Mégnin-Chanet F, Hélène C, Mergny JL. Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2672–2677. doi: 10.1073/pnas.052698099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burger AM, Dai F, Schultes CM, Reszka AP, Moore MJ, Double JA, Neidle S. The G-quadruplex-interactive molecule BRACO-19 inhibits tumor growth, consistent with telomere targeting and interference with telomerase function. Cancer Res. 2005;65:1489–96. doi: 10.1158/0008-5472.CAN-04-2910. [DOI] [PubMed] [Google Scholar]
- 36.Müller S, Sanders DA, Di Antonio M, Matsis S, Riou J-F, Rodriguez R, Balasubramanian S. Pyridostatin analogues promote telomere dysfunction and long-term growth inhibition in human cancer cells. Org. Biomol. Chem. 2012;10:6537. doi: 10.1039/c2ob25830g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi H, Lin C-P, Fu X, Wood LM, Liu Aa, Tsai Y-C, Chen Y, Barbieri CM, Pilch DS, Liu LF. G-quadruplexes induce apoptosis in tumor cells. Cancer Res. 2006;66:11808–11816. doi: 10.1158/0008-5472.CAN-06-1225. [DOI] [PubMed] [Google Scholar]
- 38.Moye AL, Porter KC, Cohen SB, Phan T, Zyner KG, Sasaki N, Lovrecz GO, Beck JL, Bryan TM. Telomeric G-quadruplexes are a substrate and site of localization for human telomerase. Nat. Commun. 2015;6:7643. doi: 10.1038/ncomms8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oganesian L, Moon IK, Bryan TM, Jarstfer MB. Extension of G-quadruplex DNA by ciliate telomerase. EMBO J. 2006;25:1148–1159. doi: 10.1038/sj.emboj.7601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huppert JL, Balasubramanian S. G-quadruplexes in promoters throughout the human genome. Nucleic Acids Res. 2007;35:406–413. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershman SG, Chen Q, Lee JY, Kozak ML, Yue P, Wang L-S, Johnson FB. Genomic distribution and functional analyses of potential G-quadruplex-forming sequences in Saccharomyces cerevisiae. Nucleic Acids Res. 2008;36:144–56. doi: 10.1093/nar/gkm986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halder R, Riou J-F, Teulade-Fichou M-P, Frickey T, Hartig JS. Bisquinolinium compounds induce quadruplex-specific transcriptome changes in HeLa S3 cell lines. BMC Res. Notes. 2012;5:138. doi: 10.1186/1756-0500-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang W, Robles AI, Beyer RP, Gray LT, Nguyen GH, Oshima J, Maizels N, Harris CC, Monnat RJ. The Werner syndrome RECQ helicase targets G4 DNA in human cells to modulate transcription. Hum. Mol. Genet. 2016;25:2060–2069. doi: 10.1093/hmg/ddw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen GH, Tang W, Robles AI, Beyer RP, Gray LT, Welsh JA, Schetter AJ, Kumamoto K, Wang XW, Hickson ID, Maizels N, Monnat RJ, Harris CC. Regulation of gene expression by the BLM helicase correlates with the presence of G-quadruplex DNA motifs. Proc. Natl. Acad. Sci. U. S. A. 2014;111:9905–10. doi: 10.1073/pnas.1404807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JE, Cao K, Ryvkin P, Wang L-S, Johnson FB. Altered gene expression in the Werner and Bloom syndromes is associated with sequences having G-quadruplex forming potential. Nucleic Acids Res. 2010;38:1114–22. doi: 10.1093/nar/gkp1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddy J, Maizels N. Gene function correlates with potential for G4 DNA formation in the human genome. Nucleic Acids Res. 2006;34:3887–3896. doi: 10.1093/nar/gkl529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan AT, Modi YS, Patel DJ. Propeller-Type Parallel-Stranded G-Quadruplexes in the Human c-myc Promoter. J. Am. Chem. Soc. 2004;126:8710–8716. doi: 10.1021/ja048805k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11593–8. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun D, Liu W-J, Guo K, Rusche JJ, Ebbinghaus S, Gokhale V, Hurley LH. The proximal promoter region of the human vascular endothelial growth factor gene has a G-quadruplex structure that can be targeted by G-quadruplex-interactive agents. Mol. Cancer Ther. 2008;7:880–9. doi: 10.1158/1535-7163.MCT-07-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rankin S, Reszka AP, Huppert J, Zloh M, Parkinson GN, Todd AK, Ladame S, Balasubramanian S, Neidle S. Putative DNA Quadruplex Formation within the Human c-kit Oncogene. J. Am. Chem. Soc. 2005;127:10584–10589. doi: 10.1021/ja050823u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cogoi S, Xodo LE. G-quadruplex formation within the promoter of the KRAS proto-oncogene and its effect on transcription. Nucleic Acids Res. 2006;34:2536–2549. doi: 10.1093/nar/gkl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palumbo SML, Ebbinghaus SW, Hurley LH. Formation of a unique end-to-end stacked pair of G-quadruplexes in the hTERT core promoter with implications for inhibition of telomerase by G-quadruplex-interactive ligands. J. Am. Chem. Soc. 2009;131:10878–10891. doi: 10.1021/ja902281d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dexheimer TS, Sun D, Hurley LH. Deconvoluting the structural and drug-recognition complexity of the G-quadruplex-forming region upstream of the bcl-2 P1 promoter. J. Am. Chem. Soc. 2006;128:5404–15. doi: 10.1021/ja0563861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cogoi S, Rapozzi V, Cauci S, Xodo LE. Critical role of hnRNP A1 in activating KRAS transcription in pancreatic cancer cells: A molecular mechanism involving G4 DNA. Biochim. Biophys. Acta - Gen. Subj. 2017;1861:1389–1398. doi: 10.1016/j.bbagen.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 55.Lopergolo A, Perrone R, Tortoreto M, Doria F, Beretta GL, Zuco V, Freccero M, Grazia Borrello M, Lanzi C, Ricther SN, Zaffaroni N, Folini M. Targeting of <i>RET</i> oncogene by naphthalene diimide-mediated gene promoter G-quadruplex stabilization exerts anti-tumor activity in oncogene-addicted human medullary thyroid cancer. Oncotarget. 2014 doi: 10.18632/oncotarget.10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu P, Ma DL, Leung CH, Yan SC, Zhu N, Abagyan R, Che CM. Stabilization of G-quadruplex DNA with platinum(II) Schiff base complexes: Luminescent probe and down-regulation of c-myc oncogene expression. Chem. - A Eur. J. 2009;15:13008–13021. doi: 10.1002/chem.200901943. [DOI] [PubMed] [Google Scholar]
- 57.Murat P, Gormally MV, Sanders D, Di Antonio M, Balasubramanian S. Light-mediated in cell downregulation of G-quadruplex-containing genes using a photo-caged ligand (ESI) Chem. Commun. (Camb) 2013;49:8453–5. doi: 10.1039/c3cc44737e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halder K, Halder R, Chowdhury S. Genome-wide analysis predicts DNA structural motifs as nucleosome exclusion signals. Mol. Biosyst. 2009;5:1703–12. doi: 10.1039/b905132e. [DOI] [PubMed] [Google Scholar]
- 59.Song J, Perreault J-P, Topisirovic I, Richard S. RNA G-quadruplexes and their potential regulatory roles in translation. 2016;4:e1244031. doi: 10.1080/21690731.2016.1244031. doi.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo JU, Bartel DP. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science (80-.) 2016;353:aaf5371–aaf5371. doi: 10.1126/science.aaf5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Besnard E, Babled A, Lapasset L, Milhavet O, Parrinello H, Dantec C, Marin J-M, Lemaitre J-M. Unraveling cell type-specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat. Struct. Mol. Biol. 2012;19:837–44. doi: 10.1038/nsmb.2339. [DOI] [PubMed] [Google Scholar]
- 62.Bochman ML, Paeschke K, Zakian VA. DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012;13:770–780. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langley AR, Gräf S, Smith JC, Krude T. Genome-wide identification and characterisation of human DNA replication origins by initiation site sequencing (ini-seq) Nucleic Acids Res. 2016;44:gkw760. doi: 10.1093/nar/gkw760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cayrou C, Coulombe P, Vigneron A, Stanojcic S, Ganier O, Peiffer I, Rivals E, Puy A, Laurent-Chabalier S, Desprat R, Méchali M. Genome-scale analysis of metazoan replication origins reveals their organization in specific but flexible sites defined by conserved features. Genome Res. 2011;21:1438–1449. doi: 10.1101/gr.121830.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valton AL, Hassan-Zadeh V, Lema I, Boggetto N, Alberti P, Saintomé C, Riou JF, Prioleau MN. G4 motifs affect origin positioning and efficiency in two vertebrate replicators. EMBO J. 2014;33:732–746. doi: 10.1002/embj.201387506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopes J, Piazza A, Bermejo R, Kriegsman B, Colosio A, Teulade-Fichou M-P, Foiani M, Nicolas A. G-quadruplex-induced instability during leading-strand replication. EMBO J. 2011;30:4033–46. doi: 10.1038/emboj.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piazza A, Boulé J-B, Lopes J, Mingo K, Largy E, Teulade-Fichou M-P, Nicolas A. Genetic instability triggered by G-quadruplex interacting Phen-DC compounds in Saccharomyces cerevisiae. Nucleic Acids Res. 2010;38:4337–48. doi: 10.1093/nar/gkq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez R, Miller KM, Forment JV, Bradshaw CR, Nikan M, Britton S, Oelschlaegel T, Xhemalce B, Balasubramanian S, Jackson SP. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat. Chem. Biol. 2012;8:301–10. doi: 10.1038/nchembio.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De S, Michor F. DNA secondary structures and epigenetic determinants of cancer genome evolution. Nat. Struct. Mol. Biol. 2011;18:950–955. doi: 10.1038/nsmb.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmer J, Tacconi EMC, Folio C, Badie S, Porru M, Klare K, Tumiati M, Markkanen E, Halder S, Ryan A, Jackson SP, Ramadan K, Kuznetsov SG, Biroccio A, Sale JE, Tarsounas M. Targeting BRCA1 and BRCA2 Deficiencies with G-Quadruplex-Interacting Compounds. Mol. Cell. 2016;61:449–460. doi: 10.1016/j.molcel.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Wietmarschen N, Merzouk S, Halsema N, Spierings DCJ, Guryev V, Lansdorp PM. BLM helicase suppresses recombination at G-quadruplex motifs in transcribed genes. Nat. Commun. 2018;9:271. doi: 10.1038/s41467-017-02760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 73.Kang E, Wu J, Gutierrez NM, Koski A, Tippner-Hedges R, Agaronyan K, Platero-Luengo A, Martinez-Redondo P, Ma H, Lee Y, Hayama T, Van Dyken C, Wang X, Luo S, Ahmed R, Li Y, Ji D, Kayali R, Cinnioglu C, Olson S, Jensen J, Battaglia D, Lee D, Wu D, Huang T, Wolf DP, Temiakov D, Belmonte JCI, Amato P, Mitalipov S. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- 74.Kukat C, Wurm CA, Spåhr H, Falkenberg M, Larsson N-G, Jakobs S. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13534–9. doi: 10.1073/pnas.1109263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexeyev M, Shokolenko I, Wilson G, LeDoux S. The maintenance of mitochondrial DNA integrity--critical analysis and update. Cold Spring Harb. Perspect. Biol. 2013;5:a012641. doi: 10.1101/cshperspect.a012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1997;94:514–9. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safdar A, Annis S, Kraytsberg Y, Laverack C, Saleem A, Popadin K, Woods DC, Tilly JL, Khrapko K. Amelioration of premature aging in mtDNA mutator mouse by exercise: the interplay of oxidative stress, PGC-1α, p53, and DNA damage. A hypothesis. Curr. Opin. Genet. Dev. 2016;38:127–132. doi: 10.1016/j.gde.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valente WJ, Ericson NG, Long AS, White PA, Marchetti F, Bielas JH. Mitochondrial DNA exhibits resistance to induced point and deletion mutations. Nucleic Acids Res. 2016;44:8513–8524. doi: 10.1093/nar/gkw716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szczepanowska K, Trifunovic A. Origins of mtDNA mutations in ageing. Essays Biochem. 2017;61:325–337. doi: 10.1042/EBC20160090. [DOI] [PubMed] [Google Scholar]
- 80.Kauppila TES, Kauppila JHK, Larsson N-G. Mammalian Mitochondria and Aging: An Update. Cell Metab. 2017;25:57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 81.McKinney EA, Oliveira MT. Replicating animal mitochondrial DNA. Genet. Mol. Biol. 2013;36:308–15. doi: 10.1590/S1415-47572013000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shadel GS, Clayton DA. MITOCHONDRIAL DNA MAINTENANCE IN VERTEBRATES. Annu. Rev. Biochem. 1997;66:409–435. doi: 10.1146/annurev.biochem.66.1.409. [DOI] [PubMed] [Google Scholar]
- 83.Eoff RL, Raney KD. A catch and release program for single-stranded DNA. J. Biol. Chem. 2017;292:13085–13086. doi: 10.1074/jbc.H117.791392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamada T, Akiyama H, McGeer PL. Complement-activated oligodendroglia: a new pathogenic entity identified by immunostaining with antibodies to human complement proteins C3d and C4d. Neurosci. Lett. 1990;112:161–6. doi: 10.1016/0304-3940(90)90196-g. [DOI] [PubMed] [Google Scholar]
- 85.Reyes A, Gissi C, Pesole G, Saccone C. Asymmetrical directional mutation pressure in the mitochondrial genome of mammals. Mol. Biol. Evol. 1998;15:957–66. doi: 10.1093/oxfordjournals.molbev.a026011. [DOI] [PubMed] [Google Scholar]
- 86.Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am. J. Physiol. Cell Physiol. 2007;292:C157–63. doi: 10.1152/ajpcell.00272.2006. [DOI] [PubMed] [Google Scholar]
- 87.Clayton DA. Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 1992;141:217–32. doi: 10.1016/s0074-7696(08)62067-7. [DOI] [PubMed] [Google Scholar]
- 88.Wanrooij PH, Uhler JP, Shi Y, Westerlund F, Falkenberg M, Gustafsson CM. A hybrid G-quadruplex structure formed between RNA and DNA explains the extraordinary stability of the mitochondrial R-loop. Nucleic Acids Res. 2012;40:10334–10344. doi: 10.1093/nar/gks802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng K-w, Wu R-y, He Y-d, Xiao S, Zhang J-y, Liu J-q, Hao Y-h, Tan Z. A competitive formation of DNA:RNA hybrid G-quadruplex is responsible to the mitochondrial transcription termination at the DNA replication priming site. Nucleic Acids Res. 2014;42:10832–10844. doi: 10.1093/nar/gku764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyonnais S, Tarrés-Soler A, Rubio-Cosials A, Cuppari A, Brito R, Jaumot J, Gargallo R, Vilaseca M, Silva C, Granzhan A, Teulade-Fichou M-P, Eritja R, Solà M. The human mitochondrial transcription factor A is a versatile G-quadruplex binding protein. Sci. Rep. 2017;7:43992. doi: 10.1038/srep43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agaronyan K, Morozov YI, Anikin M, Temiakov D. Replication-transcription switch in human mitochondria. Science (80-.) 2015;347:548–551. doi: 10.1126/science.aaa0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boulé J-B, Zakian VA. Roles of Pif1-like helicases in the maintenance of genomic stability. Nucleic Acids Res. 2006;34:4147–4153. doi: 10.1093/nar/gkl561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Futami K, Shimamoto A, Furuichi Y. Mitochondrial and nuclear localization of human Pif1 helicase. Biol. Pharm. Bull. 2007;30:1685–92. doi: 10.1248/bpb.30.1685. [DOI] [PubMed] [Google Scholar]
- 94.Mendoza O, Bourdoncle A, Boulé JB, Brosh RM, Mergny JL. G-quadruplexes and helicases. Nucleic Acids Res. 2016;44:1989–2006. doi: 10.1093/nar/gkw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paeschke K, Bochman ML, Garcia PD, Cejka P, Friedman KL, Kowalczykowski SC, Zakian VA. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature. 2013;497:458–62. doi: 10.1038/nature12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sabouri N. The functions of the multi-tasking Pfh1Pif1 helicase. Curr. Genet. 2017;63:621–626. doi: 10.1007/s00294-016-0675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Rourke TW, Doudican NA, Mackereth MD, Doetsch PW, Shadel GS. Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol. Cell. Biol. 2002;22:4086–93. doi: 10.1128/MCB.22.12.4086-4093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribeyre C, Lopes J, Boulé J-B, Piazza A, Guédin A, Zakian VA, Mergny J-L, Nicolas A. The yeast Pif1 helicase prevents genomic instability caused by G-quadruplex-forming CEB1 sequences in vivo. In: Cohen-Fix O, editor. PLoS Genet. Vol. 5. 2009. p. e1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paeschke K, Capra JA, Zakian VA. DNA Replication through G-Quadruplex Motifs Is Promoted by the Saccharomyces cerevisiae Pif1 DNA Helicase. Cell. 2011;145:678–691. doi: 10.1016/j.cell.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Snow BE, Mateyak M, Paderova J, Wakeham A, Iorio C, Zakian V, Squire J, Harrington L. Murine Pif1 Interacts with Telomerase and Is Dispensable for Telomere Function In Vivo. Mol. Cell. Biol. 2007;27:1017–1026. doi: 10.1128/MCB.01866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bannwarth S, Berg-Alonso L, Augé G, Fragaki K, Kolesar JE, Lespinasse F, Lacas-Gervais S, Burel-Vandenbos F, Villa E, Belmonte F, Michiels J-F, Ricci J-E, Gherardi R, Harrington L, Kaufman BA, Paquis-Flucklinger V. Inactivation of Pif1 helicase causes a mitochondrial myopathy in mice. Mitochondrion. 2016;30:126–37. doi: 10.1016/j.mito.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Croteau DL, Rossi ML, Canugovi C, Tian J, Sykora P, Ramamoorthy M, Wang Z, Singh DK, Akbari M, Kasiviswanathan R, Copeland WC, Bohr VA. RECQL4 localizes to mitochondria and preserves mitochondrial DNA integrity. Aging Cell. 2012;11:456–466. doi: 10.1111/j.1474-9726.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyonnais S, Tarrés-Solé A, Rubio-Cosials A, Cuppari A, Brito R, Jaumot J, Gargallo R, Vilaseca M, Silva C, Granzhan A, Teulade-Fichou M-P, Eritja R, Solà M. The human mitochondrial transcription factor A is a versatile G-quadruplex binding protein. Sci. Rep. 2017;7:43992. doi: 10.1038/srep43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohno T, Umeda S, Hamasaki N, Kang D. Binding of human mitochondrial transcription factor A, an HMG box protein, to a four-way DNA junction. Biochem. Biophys. Res. Commun. 2000;271:492–8. doi: 10.1006/bbrc.2000.2656. [DOI] [PubMed] [Google Scholar]
- 105.Antonicka H, Sasarman F, Nishimura T, Paupe V, Shoubridge EA. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 2013;17:386–398. doi: 10.1016/j.cmet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Emerman AB, Zhang Z-R, Chakrabarti O, Hegde RS. LRPPRC and SLIRP Interact in a Ribonucleoprotein Complex That Regulates Posttranscriptional Gene Expression in Mitochondria. Mol. Biol. Cell. 2010;21:4325–4337. doi: 10.1091/mbc.E10-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams P, Li L, Dong X, Wang Y. Identification of SLIRP as a G Quadruplex-Binding Protein. J. Am. Chem. Soc. 2017;139:12426–12429. doi: 10.1021/jacs.7b07563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang W-C, Tseng T-Y, Chen Y-T, Chang C-C, Wang Z-F, Wang C-L, Hsu T-N, Li P-T, Chen C-T, Lin J-J, Lou P-J, Chang T-C. Direct evidence of mitochondrial G-quadruplex DNA by using fluorescent anti-cancer agents. Nucleic Acids Res. 2015;43:gkv1061. doi: 10.1093/nar/gkv1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li C-P, Huang J-H, Chang A-C, Hung Y-M, Lin C-H, Chao Y, Lee S-D, Whang-Peng J, Huang T-S. A G-quadruplex ligand 3,3’-diethyloxadicarbocyanine iodide induces mitochondrion-mediated apoptosis but not decrease of telomerase activity in nasopharyngeal carcinoma NPC-TW01 cells. Pharm. Res. 2004;21:93–100. doi: 10.1023/b:pham.0000012166.44521.1f. [DOI] [PubMed] [Google Scholar]
- 110.Zhuang X-Y, Yao Y-G. Mitochondrial dysfunction and nuclear-mitochondrial shuttling of TERT are involved in cell proliferation arrest induced by G-quadruplex ligands. FEBS Lett. 2013;587:1656–1662. doi: 10.1016/j.febslet.2013.04.010. [DOI] [PubMed] [Google Scholar]