Summary
Over the last 150 years, the U.S. Food and Drug Administration (FDA) has evolved from a small division of the U.S. Patent Office to 1 of the largest consumer protection agencies in the world. Its mission includes ensuring that new medical treatments reach the public as quickly as possible while simultaneously ensuring that new treatments are both safe and effective. In the face of urgent consumer need, the FDA has faced criticism that its processes are too lengthy and costly and that the time to new drug release is significantly longer in the United States than in other Western countries. Calls from the public to loosen FDA regulations to facilitate more rapid approval of drugs and devices have been countered by the occurrence of patient harm and deaths after some approved drugs have reached the marketplace. New drug and device approval in the United States take an average of 12 and 7 years, respectively, from pre-clinical testing to approval. Costs for development of medical devices run into millions of dollars, and a recent study suggests that the entire cost for a new drug is in excess of $1 billion. For investigators seeking approval for new drugs and devices, FDA processes can be formidable. This 2-part series is intended to provide an overview of the steps involved in bringing new drugs and devices through the FDA process. Part 1 concerns the process of new drug approvals. Part 2 continues with approval of medical devices.
Key Words: drug approval, FDA, investigational new drug
Abbreviations and Acronyms: CDER, Center for Drug Evaluation and Research; EIND, emergency investigational new drug; FDA, U.S. Food and Drug Administration; IND, investigational new drug; NDA, new drug application
Regulation of the development, production, marketing, and sales of medical pharmaceuticals and devices entails paradoxical goals. It must ensure that new and effective medical treatments reach the public rapidly while simultaneously providing protection from ineffective or even unsafe therapies and from predatory marketing practices that tout unproven remedies to vulnerable patients. In the United States, these regulatory functions fall to the U.S. Food and Drug Administration (FDA).
The FDA is the oldest consumer protection agency in the United States, originating in the U.S. Patent Office in 1848, and later inherited by the Department of Agriculture in 1862 (1). The modern function of the agency in oversight of drug and medical device marketing was ultimately codified in the Pure Food and Drug Act of 1906 2, 3, which was passed in response to a pressing need to curb interstate markets for adulterated and mishandled food and pharmaceuticals. The Federal Food, Drug, and Cosmetics Act of 1938 required all drugs to be approved for safety by the FDA (1). This mission was expanded in 1962 by the Kefauver-Harris amendments that added the requirement that drugs be proven “effective” as well as safe, and placed strict controls on the use of investigational drugs (2). Regulations regarding drug safety oversight were expanded in 1976 to include medical devices 1, 2.
Over the course of the 20th century, the role of the FDA has undergone a significant metamorphosis due to expanding federal regulations, increasing complexity of drugs and devices, and the growth of the pharmaceutical industry into a major economic force in the United States. Today, the United States has among the most stringent regulations regarding medical drug and device development and marketing, and the FDA has grown from that small division in the patent office to 1 of the largest consumer safety agencies in the world. Its core mission remains the same: to provide consumers with assurance that medical drugs and devices that reach the marketplace have proven safety and efficacy in the roles for which they have been tested and approved. But, this mission has faced criticism and calls from an increasingly demanding consumer base to provide more rapid development, approval, and release of new products.
Strict regulation may have served the public with enhanced assurance of therapeutic safety, but progressive concerns of a so-called “drug lag” have resulted from an increasingly complex regulatory environment and the expense associated with drug development. Delay in the development and marketing of new pharmaceuticals was evidenced by a decline in the number of drugs approved by the FDA from an average of 50 drugs annually in the late 1950s to approximately 17 per year after 1965 2, 4. It is unclear whether FDA regulations were entirely responsible for the deceleration, because foreign countries also experienced a lag 2, 5, 6, but it was nevertheless obvious that new drugs and devices were often reaching the market in other countries months to years before achieving FDA approval in the United States (2). Modern regulations allowing for expanded access and accelerated approval for drugs to treat life-threatening conditions have their origins in the public outcry over delays in access to acquired immune deficiency syndrome treatments in the 1980s (7). But, movements to “deregulate” drug development by loosening FDA regulations have been weakened by the occurrence of major safety incidents, such as with benoxaprofen in 1982 (2). The nonsteroidal anti-inflammatory agent, marketed under the brand name Oraflex, was released to the public but then withdrawn when patient deaths were reported in the United Kingdom 8, 9. Thus the drug/device development environment in the United States involves a constant balance between accelerating pressures to expedite effective therapies to the public, and the mission to minimize major adverse events (10).
Today, the path from initial demonstration that a molecule may have therapeutic potential to the production of an approved drug involves pre-clinical testing, complex clinical trials in humans, and post-trial regulatory approval by the FDA. For drugs, this process can take 10 to 15 years and cost millions of dollars (11). A recent analysis suggests that the actual cost of taking a new drug from concept to market as of 2014 is now above $1.3 billion (12). Approximately 1 in 1,000 potential drugs is graduated to human clinical trials after pre-clinical testing in the United States, and almost 9 of every 10 new drugs then fails in the human testing phase. In 1 study, 50% of all drugs reaching the final stage (Phase III) of clinical testing did not make it to market (13). The problem is not unique to the United States; a recent analysis concluded in 2011 by the Centre for Medicine Research in the United Kingdom found that in the prior 3 years Phase II and III clinical trials had experienced rising failure rates, with only 18% of drugs making it out of Phase II to Phase III testing 14, 15.
The pathways for approval of medical devices are shorter and generally less costly when compared with the regulatory process for drugs. Although the drug development takes on average 12 years from concept to market, the same process for medical devices averages 3 to 7 years (16).
For researchers involved in the clinical development and testing of putative drugs and devices, the process of FDA approval can be daunting and difficult to navigate. This first part of a 2-part series is intended to provide an overview of the steps in bringing a drug through the process of clinical trials and FDA approval. The second part of this series will discuss the process of obtaining approval to study devices, which have their own unique pathway.
Part 1: FDA Approval of New Drugs
What is a drug?
Not every substance taken by patients “for their health” is considered a drug by the FDA (Table 1). The FDA defines herbal products, vitamins, and other complementary medical therapies as “dietary supplements” (17). As such, they are regulated by the Center for Drug Evaluation and Research (CDER) of the FDA and are subject to guidelines by the Dietary Supplement Health and Education Act of 1994 (18), but they are not subject to the rigorous tests required of substances that are defined as “drugs.”
Table 1.
What Is a Drug: the FDA Definition
| A substance recognized by an official pharmacopoeia or formulary |
| A substance intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease |
| A substance (other than food) intended to affect the structure or any function of the body |
| A substance intended for use as a component of a medicine but not a device or a component, part, or accessory of a device |
FDA = U.S. Food and Drug Administration.
Prior to ever reaching a clinical researcher's hands, all new drug development follows a common pathway. Basic research leads to conceptualization of a drug, followed by pre-clinical development involving in vitro and in vivo studies and drug prototype design (Table 2). When a substance is ready for clinical study, but prior to any testing in human subjects, the drug developer must involve the FDA. This process begins when the drug's sponsor (usually the drug manufacturer or distributor) files an investigational new drug (IND) application with the agency. Federal law requires that a drug be the subject of an approved marketing application for it to be legally shipped across state lines. An approved IND application provides the developer with a technical exemption to this federal regulation, so that clinical investigators can distribute a drug to different study centers across the United States (19).
Table 2.
Pre-Clinical Steps in New Drug Development
| Understand the disease process |
| Identify potential targets for pharmaceutical action |
| Identify chemicals that modify the targets |
| Conduct pre-clinical studies: in vivo and in vitro studies to determine the efficacy and safety of proposed drugs in animal models, including carcinogenicity, mutagenicity, and teratogenicity |
| On the basis of results from pre-clinical studies, begin to design proposed clinical trials in humans to study safety and efficacy |
| Begin initial work to determine pharmaceutical formulation and outline potential manufacturing processes |
| Evaluate the formulated drug's purity and stability through the manufacturing process |
The IND application: 3 basic pathways to approval
There are 2 categories of INDs (“commercial” and “research”) and 3 types of IND applications: investigator IND, emergency use investigational new drug (EIND), and treatment IND (19).
All drugs will go through review by a committee, or “new drug division,” specializing in the class of drug in question on the basis of the anticipated purpose of the drug. The FDA encourages investigators to seek early consultation with the appropriate new drug review division through the Pre-Investigational New Drug Application Consultation Program (20) prior to submitting a formal IND application. Early collaboration prior to IND applications can avoid issuance of suggestions, mandatory changes, or clinical holds on the application and are well worth the time and effort. Collaborative conversations can be initiated by contacting the appropriate review division (20). The review division can provide valuable guidance about information necessary for an IND submission. The FDA provides specific names and contact numbers at the FDA web site, arranged according to therapeutic class of drug (21).
In addition to the pre-investigational consultation, the FDA provides a number of guidance documents that can be useful in assembling the necessary data and materials for the IND application (22), as well as information and contact numbers for submitting an EIND application (detailed in the following text).
All IND applications require the investigator to supply 3 basic categories of information: 1) data regarding pre-clinical animal and toxicological studies and any previous human experience with the drug (e.g., foreign experience); 2) manufacturing information, including the composition, manufacturer, stability, and controls; and 3) the clinical protocols of the study, information about the investigators proving that they are qualified to conduct the trial, as well as commitments to obtain informed consent from subjects, obtain institutional review board (IRB) approval, and adhere to any regulations regarding INDs (23).
Pathway 1: the investigator IND
An investigator IND is submitted by a physician, sometimes on behalf of an institution or “sponsor” such as a pharmaceutical company (Table 3). The investigator will both initiate and conduct the investigation and direct the dispensing and administration of the drug.
Table 3.
Summary of Steps and Timeline in the Investigator IND Application
| Step 1 | Contact the appropriate division of the FDA and set up a Pre-IND Consultation Program; check FDA guidance documents to be sure the new drug does not qualify for an exemption from IND application (uncommon, but can occur with some generic drugs and radiological products) |
| Step 2 | Submit the application (original and 2 copies) to:
|
| Step 3 | If the FDA does not raise an objection within 30 days of submission of the application, the investigator may proceed |
| Step 4 | If the FDA issues a “clinical hold,” or responds with suggestions or mandatory changes, address these issues and resubmit the application |
FDA = U.S. Food and Drug Administration; IND = investigational new drug.
The investigator must wait at least 30 days after submitting an IND application to begin any clinical trials. If the FDA does not object within that time, clinical Phase I testing can begin (24). However, the FDA may respond to the application with suggestions for the study or with mandatory change requirements. Although “suggestions” are changes that are not required for the IND study to proceed, they should nevertheless be taken very seriously. The FDA review panel consists of clinicians and researchers who may have considerable experience with related drugs or drugs in a similar class, and such suggestions are not made lightly. Furthermore, it is vital for the approval process that the investigator maintains a collaborative relationship with the FDA reviewers to avoid issues further down the road. Mandatory changes are just that: failure to make the required changes will result in FDA issuance of a “clinical hold” (24), preventing the study from legally proceeding until the FDA is assured of the safety of the study. Although the FDA must respond within 30 days to challenges of a clinical hold by the investigator, technically there is no deadline for resolution of issues causing clinical holds. Typically, a clinical hold will result in a study delay of a year or more (25).
Pathway 2: the EIND
An EIND asks the FDA to approve use of an experimental drug in an emergency situation that does not allow time for a standard IND process or IRB approval (26). This type of application may also be submitted to authorize use in a patient or patients who do not meet study criteria or if no approved study protocol exists. EINDs are initiated by direct contact with the appropriate division of the FDA. Special contact numbers for EIND applications, including an emergency contact number for night and weekend contacts, are listed in Table 4. This contact information is current as of March 4, 2016, but may be updated and can be found at the FDA web site (26). In emergency cases, the FDA will often authorize use of the agent in advance of a full IND, which must then be completed in a timely fashion. The process and timeline for EIND applications are summarized in Table 5.
Table 4.
FDA Contact Numbers and Resources for Emergency IND (or Individual Patient IND) Application∗
| Purpose | Resource |
|---|---|
| FDA URL for phone and fax numbers for specific drug review divisions | http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm107434.htm |
| General info for emergency INDs during normal business hours (8:00 am to 4:30 pm EST) | Contact CDER's DDI:
|
| For emergency use of a specific investigational drug | Contact the review division for the drug if known, or the DDI if not known |
| If the DDI or review division are unknown or unavailable | Contact the CDER Emergency Coordinator of the Counterterrorism and Emergency Coordination Staff
|
| After hours on weekdays, or all day on weekends | Office of Crisis Management and Emergency Operations Center
|
CDER = Center for Drug Evaluation and Research; DDI = Division of Drug Information; other abbreviations as in Table 3.
Contact numbers are current as of March 4, 2016. Available at: http://www.fda.gov/RegulatoryInformation/Guidances/ucm126491.htm.
Table 5.
EIND Timeline and Investigator Required Actions
| Time | Action |
|---|---|
| Day 0 to 1 | Contact supplier of the investigational drug to obtain their agreement to provide the drug for emergency use. Obtain a letter of authorization from the supplier granting the right of reference to information contained in the supplier's existing IND application. This must be sent to the FDA at the time of EIND application, by day 15. |
| Day 1 | Call the FDA and request to open an EIND application and obtain FDA authorization for investigational treatment. Fax or e-mail the information on the Physician's Checklist for Emergency IND Application. The checklist can be found at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/UCM343041.pdf |
| Once the EIND is in effect, the manufacturer may ship the investigational drug directly to the physician | |
| Obtain informed consent from the patient or legally authorized representative prior to treatment and treat the patient. Send informed consent form to the FDA at the time of EIND application by day 15. | |
| Post-treatment, no later than day 5 | Notify the institutional review board of the emergency treatment. Submit documentation as required to the local institutional review board. |
| No later than day 5 | Submit full EIND application to the Center for Drug Evaluation and Research at the FDA. The application includes: IND application cover letter; completed FDA forms; letter of authorization from the supplier for the right of reference to information in the existing IND application; clinical protocol for the emergency treatment, including rationale, description of the patient's condition, method of administration, description of clinical monitoring, laboratory testing, and procedures to minimize risk and evaluate effects of treatment; copy of the informed consent; and (optional) copy of the investigator's brochure. |
| As soon as possible, but no later than 7 days after occurrence | Mandatory report of life-threatening or fatal occurrences. |
| As soon as possible, but no later than 15 days after occurrence | Report serious or unexpected suspected adverse reactions. |
| Any time during the IND application life | Submit amendments to the EIND applications if there are any changes to information sent with the initial EIND application. |
| Following completion of EIND treatment | Send FDA written summary of the results of the investigational treatment. |
| After 1 year (if EIND application is still active) and within 60 days of the anniversary of the FDA's authorization date | Send EIND application annual report to the appropriate review division of Center for Drug Evaluation and Research. |
EIND = emergency investigational new drug; other abbreviations as in Table 3.
Pathway 3: the treatment IND
Treatment IND applications ask for approval to use an experimental drug that is showing promise in clinical studies before completion of the studies, FDA review, and final approval. These are also called “expanded use INDs” (27). Treatment IND regulations went into effect in 1987, largely as a response to public activism surrounding the limited availability of azidothymidine during the drug's development (7). Additional accelerated approval measures were instituted by The Food and Drug Administration Modernization Act of 1997 (28), which allows the FDA to “fast track” certain products that meet 2 criteria: 1) the product must concern a life-threatening or serious condition; and 2) it must have the potential to address an unmet medical need (29). Four requirements must be met before issuance of a treatment IND: 1) the drug is intended for treatment of a serious or immediately life-threatening disease; 2) there is no satisfactory alternative treatment; 3) the drug is already under investigation or trials are complete; and 4) the drug sponsor is actively pursuing approval. Prospective IRB review and informed consent are mandatory. The process and timelines for treatment IND applications follow a similar pathway to those of regular INDs, but requirements for clinical evidence differ.
The clinical trials
Clinical trials establish the safety, efficacy, and effectiveness (Table 6) of new drugs and are divided into Phase 0, I, II, and III trials. Post-approval surveillance trials are generally termed Phase IV trials. Characteristics of the different clinical trials phases can be found in Table 7, and major steps in the clinical trials phase and IND review are summarized in Table 8. The FDA encourages investigators and sponsors to communicate directly with the appropriate FDA review section during each phase of testing.
Table 6.
Safety, Efficacy, and Effectiveness
| Safety determines the highest tolerable dose or optimal dose needed to achieve the desired clinical benefit and potential adverse effects in that exposure range. |
| Efficacy determines whether a drug has a positive clinical benefit over placebo or other intervention. Efficacy tests involve “ideal,” that is, strictly controlled conditions. |
| Effectiveness describes a drug's clinical benefits in a “real world” situation, for example, where patients may have comorbid conditions or other medications that interact with the drug, and where drug administration may not following strict study guidelines. |
Table 7.
Characteristics of Clinical Trial Phases
| Phase 0 “Exploratory” |
Phase I | Phase II | Phase III | Phase IV | |
|---|---|---|---|---|---|
| Description | First-in-man early trial to determine if drug engages its expected target | Initial safety evaluations, determine safe dosage range, identify common side effects, study toxicity profile of the drug | Begin to explore efficacy while maintaining safety | Final confirmation of safety and efficacy | Any trials conducted after FDA approval of the drug |
| Number of subjects | 10–15 healthy volunteers | 20–80 healthy volunteers | 100–300 volunteers with the targeted medical condition | 1,000–3,000 subjects with the targeted medical condition | Number of subjects depends on trial endpoints |
| Dose | Single, low dose (<1% of dose calculated to produce a clinical effect) |
|
Multiple dose trials, often conducted against placebo | Multiple dose trials, ascending doses | Variable |
| Endpoints | Not expected to show clinical effect or significant adverse effects. Helps to choose between competing chemical analogs for further study. | Escalation of dose ends when unacceptable side effects occur; the previous dose is considered the maximum tolerated dose. | Explores clinical effects against the targeted condition, and reveals the less-common side effects | Confirms clinical efficacy of the drug against the targeted condition and evaluates safety and side effects | Confirms clinical efficacy and safety and explores other possible drug uses; may be required as a condition of drug approval |
| Timing | Can be conducted with prior approval while final IND review is pending | Together with Phase 0 trials, first clinical trials conducted in an IND process | Conducted after report to FDA of results of Phase I trials | Conducted after report to FDA of results of Phase II trials | Conducted after release of the drug by the FDA for marketing |
Abbreviations as in Table 3.
Table 8.
Major Steps in the Clinical Trials Phase and Review for INDs
| Review information from the FDA regarding clinical trials and guidance documents relevant to the drug being investigated | |
| Investigator contacts the review section at the FDA | http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/Overview/UCM166356.pdf |
| IND application submitted | |
| 30-day review |
|
| Phase 0 clinical trial | Proceed during finalization of full IND |
| Phase I clinical trial | Small study to determine toxicity and safety |
| Report submitted to FDA after Phase I | FDA and sponsors discuss how Phase II studies will be conducted |
| Phase II clinical trial | Moderate-size study to explore efficacy and less-common side effects |
| Report submitted to FDA | FDA and sponsors discuss how large-scale Phase III studies will be conducted |
| Phase III clinical trial | Large prospective studies of clinical efficacy |
| Pre-NDA period | Sponsor meets with the FDA |
| Submission of NDA | NDA asks the FDA for marketing approval of the drug |
| 60-day waiting period | FDA has 60 days to determine if they will file the application and start the review process |
| FDA review team assigned to the drug |
|
| FDA approval | Drug is approved for marketing OR response letter from FDA outlining further actions |
NDA = new drug application; other abbreviations as in Table 3.
Phase 0 clinical trials
Only about 10% of IND applications ever result in clinically approved drugs 15, 30. Drug development is lengthy and expensive, and many drugs fail very late in the process after expenditure of significant time and resources. As new agents increasingly are molecularly targeted, it seems rational to perform early testing to assess such agents early in the pharmacodynamic assay. Such trials, termed “Phase 0,” or “exploratory” trials, were first allowed in 2006 as a means of facilitating the drug approval (and elimination) process (30). Phase 0 clinical trials require submission of an exploratory IND followed by a full IND.
Phase 0 trials represent the earliest, first-in-man use of a proposed drug therapy (31). They are carried out in very small cohorts (10 to 15 patients), with dose levels <1% of the dose calculated to produce a clinical effect 32, 33 and administration schedules not expected to produce any clinical toxicity. Duration of dosing in any patient is anticipated to be <1 week (34).
A Phase 0 trial can help determine whether a drug engages its expected target and is likely to have the anticipated clinical effect in human subjects (33). It can also illuminate the pharmacokinetic and pharmacodynamic characteristics of the drug. These trials may weed out ineffective therapies early in the FDA process and help the researcher choose between competing analogue agents for further clinical development. Approval for a Phase 0 trial generally requires less toxicity testing than for full Phase I trials. Phase 0 trials can also be conducted while awaiting FDA review of a standard IND application, thus providing valuable information regarding human effects while avoiding delays in the full FDA approval process.
Phase I trials
The exploratory (Phase 0) INDs are actually a subset of Phase I human subject trials of all new drugs. Exploratory INDs progress to “full” Phase I clinical trials if early results are promising. The purposes of a Phase I trial are to provide initial safety evaluation, determine safe dosing ranges, and identify common side effects and the toxicity profile of the drug. The number of subjects is still low, usually between 20 and 80 (35), and subjects are generally healthy because clinical effectiveness is not an endpoint of the trial.
Single-dose studies are the usual starting place of Phase I trials: the subject is given a single dose of drug no greater than one-tenth the highest dose associated with no adverse effects in the most sensitive animal safety studies (34). Many researchers now believe that single-dose toxicity trials should not be carried out simultaneously in multiple subjects—meaning that the drug should be tested in a single subject and enough time be allowed between subjects such that a severe reaction in any subject will lead to termination of the study before other subjects are exposed 25, 36. A recent, disastrous clinical trial serves to underscore this concern; in the initial Phase I trial of TGN1412, an immunomodulatory drug to treat leukemia and autoimmune diseases, all 6 subjects were dosed on the same day successively. Every single subject experienced unexpected, agonizing, and immediate life-threatening complications. Had dosing of participants been spread out over several days, reaction of the first subject likely would have led to termination of the trial prior to other participant exposures (37).
Single-dose trials are followed by single and multiple ascending-dose trials (Phase Ia and Ib trials, respectively). In single ascending-dose (Phase Ia) trials, a small group of subjects (typically 3) are all given a single, higher dose. If no adverse effects are noted, another small set of subjects receives a further escalated dose, and this process continues until either pre-calculated pharmacokinetic safety levels are reached or until adverse effects begin appearing. If at a given dosing level any subject experiences an unacceptable side effect, then additional subjects (e.g., 3 more) are dosed to confirm. When unacceptable side effects appear, the drug is determined to have reached its maximum tolerated dose, generally described as the dose preceding the one with the intolerable adverse effects.
Multiple ascending-dose (Phase Ib) studies evaluate the pharmacokinetics and pharmacodynamics of multiple doses of the drug. Groups of patients receive multiple low doses of the drug, and biological samples (blood, fluids, urine) are collected and analyzed. The dose is then escalated in further groups, to a pre-determined level.
Phase II clinical trials
Following favorable initial safety testing, the investigator or sponsor submits the safety information regarding the drug to the FDA, incorporating any new information that has been gained in Phase I testing. The submission includes any changes in drug manufacture or preparation anticipated for Phase II studies.
The goals of Phase II trials are to explore the efficacy of the drug while continuing to establish safety. These trials are larger (100 to 300 subjects), so that less-common side effects can be seen, and the trials involve patients who have a condition that is a therapeutic target of the drug (35). Tests are often conducted in comparison to placebo. Escalating dosing may be incorporated, exploring the therapeutic range of the drug.
Phase III clinical trials
Prior to initiating Phase III trials, the investigator or sponsor must again submit updated information to the FDA regarding continuing safety for subjects, incorporating any safety and toxicity information gained in Phase II testing. Phase III trials are the final confirmation of safety and efficacy, and are carried out in large cohorts (1,000 to 3,000 subjects). The trials evaluate effectiveness, monitor side effects, and compare the drug with commonly used alternative treatments.
Following successful completion of Phase III clinical trials, the drug sponsor can file a New Drug Application (NDA) with the CDER of the FDA. This application constitutes a request by the sponsor to manufacture and sell the drug in the United States.
The NDA
The NDA (38) includes all data concerning the drug; all information about the manufacturing process and facilities, quality control, and assurance; a complete product description (chemical formula, specifications, pharmacodynamics, and pharmacokinetics); indications; labeling; and proposed risk evaluation and mitigation processes if applicable. A typical NDA can run 100,000 pages, and according to the Office of the Federal Register, the application fee in 2016 for an NDA that requires clinical data is $2,374,200 39, 40. The FDA has 60 days to determine if they will file the application once it is received (41).
FDA reviewers will evaluate clinical data, analyze drug samples, inspect the production facilities, and check proposed labeling. The Federal Food, Drug, and Cosmetics Act requires that there be “substantial evidence” of drug safety and efficacy (42). The FDA interprets this as needing at least 2 adequate and well-controlled Phase III trials with convincing evidence of effectiveness (29), although this is not a guarantee of approval. The FDA often convenes advisory panels of experts to review the data, and usually follows panel recommendations. Approval may include specific conditions, such as requirements for post-approval (Phase IV) clinical studies, distribution restrictions, changes to labeling, or other requirements.
FDA review occurs within 180 days of receipt of a complete application (38). An accelerated process is available for generic drugs, products that provide “meaningful therapeutic benefit” over existing drugs, those that concern serious or life-threatening conditions, or those that address a previously unmet medical need. If the application is found to have deficiencies, the clock stops on review while the manufacturer is given an opportunity to respond to the deficiencies or withdraw the application. If approval of the NDA is denied, the FDA sends a complete response letter describing specific deficiencies and recommending ways for the applicant to make the application viable. Unsuccessful applicants may request a hearing.
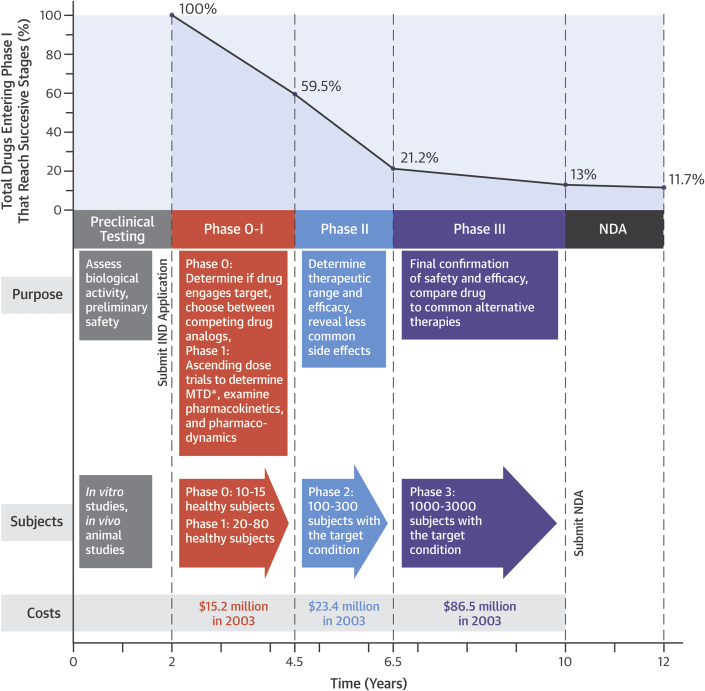
Upon review and approval of the NDA, the manufacturer is free to manufacture and market the drug. A summary of the timeline, costs, and overall probability of success for the drug development process can be found in Figure 1 (12,41).
Figure 1.
Time, Money, and Success: Stages in Drug Development
The highest failure rates occurs in Phase II testing, which is the first stage in which doses of drug in humans are escalated to reach levels expected to be clinically active (i.e., the first doses at which efficacy may fail and less common side effects appear). Cumulative probability of a drug reaching U.S. Food and Drug Administration approval declines with each stage. The overall probability of a drug passing all stages is approximately 11% as of 2014 (12).
Summary
The United States has arguably the most stringent regulations regarding approval of medical drugs and devices in the world. The average time from FDA application to approval of drugs is 12 years, and the estimated average cost of taking a new drug from concept to market exceeds $1 billion. The FDA faces constant, often contradictory pressure to shorten the approval process, while still preserving or enhancing the safety and efficacy of drugs and devices. Thus, regulatory processes are under constant scrutiny to identify means of streamlining approval processes while not compromising the primary mission of the agency.
After approval of an IND application, the FDA allows human Phase 0, I, II, and III studies, provided safety and efficacy are demonstrated at the appropriate clinical testing phase. For NDA, the FDA requires “substantial evidence” of drug safety and efficacy, and interprets this as needing at least 2 adequate and well-controlled Phase III trials with convincing evidence of effectiveness (Table 9). Some approvals may require more studies.
Table 9.
Levels of Evidence for a Clinical Therapeutic Study
| Level I |
|---|
|
| Level II |
|---|
|
CI = confidence interval; RCT = randomized controlled trial.
Data from DeVries JG, Berlet GC. Understanding levels of evidence for scientific communication. Foot Ankle Special 2010;3:205–10.
The FDA encourages early and regular communication from investigators and sponsors seeking drug approval, with a purpose of avoiding application failures or required modifications or clinical holds that may waste valuable time, effort, and finances in the process of bringing a drug to market.
Footnotes
Dr. Van Norman has received financial support from the American College of Cardiology.
References
- 1.U.S. Food and Drug Administration. History. Available at: http://www.fda.gov/AboutFDA/WhatWeDo/History/default.htm. Accessed March 4, 2016.
- 2.Gieringer D.H. The safety and efficacy of new drug approval. Cato J. 1985;5:177–201. [PubMed] [Google Scholar]
- 3.The Wiley Act. Public Law Number 59-384, 34 Stat 768. 59th Cong (1906).
- 4.Grabowski H.G., Vernon J.M. American Enterprise Institute; Washington DC: 1983. The Regulation of Pharmaceuticals; the 1962 Amendments; pp. 29–30. [Google Scholar]
- 5.Kennedy D. A calm look at the drug lag. JAMA. 1987;230:423–426. [PubMed] [Google Scholar]
- 6.Daemmrich A. Invisible monuments and the costs of pharmaceutical regulation: twenty-five years of drug lag debate. Pharm Hist. 2003;45:3–17. [PubMed] [Google Scholar]
- 7.Vogel D. AIDs and the politics of drug lag. Public Int. 1989;96:73–85. [PubMed] [Google Scholar]
- 8.Taggart H.M., Alderdice J.M. Fatal cholestatic jaundice in elderly patients taking benoxaprofen. Br Med J. 1982;284:1372. doi: 10.1136/bmj.284.6326.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lueck T.J. At Lilly, the side-effects of Oraflex. The New York Times. Aug 15, 1982 Available at: www.nytimes.com/1982/08/15/business/at-lilly-the-side-effects-of-oraflex.html. Accessed March 4, 2016. [Google Scholar]
- 10.Eichler H.G., Pgnatti F., Flamion B., Leufkens H., Breckenridge A. Balancing early market access to new drugs with the need for benefit; risk data: a mounting dilemma. Nature Rev Drug Discov. 2008;7:818–826. doi: 10.1038/nrd2664. [DOI] [PubMed] [Google Scholar]
- 11.Morgan S., Grootendorst P., Lexchin J., Cunningham C., Greyson D. The cost of drug development: a systematic review. Health Policy. 2011;100:4–17. doi: 10.1016/j.healthpol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Dimasi J.A., Grabowski G.H., Hansen R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Arrowsmith J. Trial watch: Phase II and submission failures 2007–2010. Nature Rev Drug Discov. 2011;10:87. doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- 14.Arrowsmith J. Trial watch: Phase II failures 2008–2010. Nature Rev Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 15.Arrowsmith H., Miller P. Trial watch: Phase II and Phase III attrition rates 2011–2012. Nature Rev Drug Discov. 2013;12:569. doi: 10.1038/nrd4090. [DOI] [PubMed] [Google Scholar]
- 16.Fargen K.M., Frei D., Fiorella D. The FDA approval process for medical devices. J Neurointervent Surg. 2013;5:269–275. doi: 10.1136/neurintsurg-2012-010400. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. Dietary supplements. Available at: http://www.fda.gov/Food/DietarySupplements/default.htm. Accessed March 4, 2016.
- 18.The Dietary Health and Education Act of 1994. S.784. 103rd Cong (1992–1994).
- 19.U.S. Food and Drug Administration. Investigational new drug (IND) application. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/default.htm#Introduction. Accessed March 4, 2016.
- 20.U.S. Food and Drug Administration. Pre-IND consultation program. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/Overview/default.htm. Accessed March 4, 2016.
- 21.U.S. Food and Drug Administration. Center for Drug Evaluation and Research Pre-IND consultation contacts. Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/Overview/UCM166356.pdf. Accessed March 4, 2016.
- 22.U.S. Food and Drug Administration. Guidance documents for drug applications. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm090361.htm. Accessed March 4, 2016.
- 23.U.S. Food and Drug Administration. Guidance for industry: content and format of investigational new drug applications (INDs) for Phase 1 studies of drugs, including well-characterized, therapeutic, biotechnology-derived products. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM074980.pdf. Accessed March 4, 2016.
- 24.U.S. Food and Drug Administration. Manual of policies and procedures; Center for Drug Evaluation and Research: review management. IND process and review procedures (including clinical holds). Available at: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ManualofPoliciesProcedures/UCM082022.pdf. Accessed March 4, 2016.
- 25.Friedhoff L.T. New Drugs: An Insider's Guide to the FDA's New Drug Approval Process for Scientists, Investors and Patients. Pharmaceutical Special Projects Group LLC (PSPG) Publishing; New York NY: 2009. Initial human testing: the IND application and Phase I testing; pp. 41–51. [Google Scholar]
- 26.U.S. Food and Drug Administration. Emergency use of an investigational drug or biologic—information sheet. Available at: http://www.fda.gov/RegulatoryInformation/Guidances/ucm126491.htm. Accessed March 4, 2016.
- 27.U.S. Food and Drug Administration. Final rules for expanded access to investigational drugs for treatment use and charging for investigational drugs. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm172492.htm. Accessed March 4, 2016.
- 28.The Food and Drug Administration Modernization Act of 1997. H.R. 1411. 105th Congress (April 23, 1997).
- 29.Thaul S. How FDA approves drugs and regulates their safety and effectiveness. CRS report for congress. Congressional Research Service. July 25, 2012. Available at: http://fas.org/sgp/crs/misc/R41983.pdf. Accessed March 4, 2016.
- 30.U.S. Food and Drug Administration. Guidance for industry, investigators, and reviewers. Exploratory IND studies. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078933.pdf. Accessed March 4, 2016.
- 31.Rubenstein L.V., Steinberg S.M., Kummar S. The statistics of phase 0 trials. Statistics Med. 2010;29:1072–1076. doi: 10.1002/sim.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsen-Kram J. Improving the quality of cancer clinical trials. Office of New Drugs, Center for Drug Evaluation and Research, Food and Drug Administration. Available at: http://iom.nationalacademies.org/∼/media/Files/Activity%20Files/Disease/NCPF/OverviewoftheExploratoryINDDifferencesfromtheTraditionalINDJacobsonKram.pdf. Accessed March 4, 2016.
- 33.Kummar S., Rubinstein L., Kinders R. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 2008;14:133–137. doi: 10.1097/PPO.0b013e318172d6f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guidance for industry, investigators, and reviewers; exploratory IND studies US DHHS, Division of Drug Information, Center for Drug evaluation and Research, Food and Drug Administration. January 2006. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm078933.pdf. Accessed March 15, 2016.
- 35.U.S. Food and Drug Administration. CFR—Code of Federal Regulations Title 21. Chapter I, Sec 312.21, phases of an investigation. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.21. Accessed March 4, 2016.
- 36.Goodyear M. Learning from the TGN1412 trial. BMJ. 2006;332:677. doi: 10.1136/bmj.38797.635012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attarwala H. TGN1412: from discovery to disaster. J Young Pharm. 2010;2:332–336. doi: 10.4103/0975-1483.66810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration. New Drug Application (NDA). Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/default.htm. Accessed March 4, 2016.
- 39.U.S. Food and Drug Administration. Prescription Drug User Fee Act (PDUFA). Available at: http://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/default.htm. Accessed March 4, 2016.
- 40.Department of Health and Human Services, Federal Food and Drug Administration. prescription drug user fee rates for fiscal year 2016: a notice by the Food and Drug Administration on 08/03/2015. Available at: https://www.federalregister.gov/articles/2015/08/03/2015-18914/prescription-drug-user-fee-rates-for-fiscal-year-2016#h-11. Accessed March 4, 2016.
- 41.U.S. Food and Drug Administration. FDA’s drug review process: continued. Drug review steps simplified. Available at: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm289601.htm. Accessed March 4, 2016.
- 42.Federal Food Drug and Cosmetics Act. P.L. 75-717, §505(c) and (d). 75th Cong (1938).