Abstract
An antibacterial peptide-encoding gene from alfalfa seeds, alfAFP, was fused to the C-terminal part of chitin-binding domain (CBD) of the rice chitinase-encoding gene (CBD-alfAFP) and introduced to tobacco by Agrobacterium-mediated transformation. Polymerase chain reaction (PCR) technique was used to confirm the integration of the recombinant CBD-alfAFP encoding gene in transgenic tobacco plants. A number of transgenic lines and a non-transgenic control plant were selected for further molecular analyses. The result of analyzing the transgenic plants by semi-quantitative RT-PCR showed that the recombinant gene is expressed in transgenic plants and there is a difference between the transgenic plants in terms of the level of CBD-alfAFP expression. The total protein was extracted from a few selected transgenic plants and used to evaluate the antibacterial/antifungal of recombinant protein activity against some important plant and human pathogens. The results of this experiment showed that the total protein extract obtained from transgenic lines significantly (P < 0.05) inhibited the growth of various bacteria and fungi compared to the non-transgenic plants. Transgenic lines showed a significant (P < 0.01) difference considering their ability to inhibit bacterial and fungal pathogens growth. The recombinant CBD-alfAFP protein significantly (P < 0.01) increased the resistance of the transgenic plants against Fusarium solani. Transgenic lines showed no significant wilting symptoms and obvious wilting symptoms were not observed even 30 days post-inoculation (dpi) with Fusarium solani spores. These results suggest that transgenic tobacco plants are resistant to Fusarium solani wilt and fusion of CBD to the alfAFP antimicrobial peptide is an efficient approach to control fungal diseases.
Keywords: Antibacterial, Chitin-binding domain, Fungi, Genetic engineering, Transformation
Introduction
More than a billion people worldwide, most of them living in developing countries, suffer from food shortage (FAO 2017). It is estimated that plant pests and diseases destroy a total of 30% of agricultural crops with fungi alone accounting for over 80% of losses (Becker-Ritt and Carlini 2012). Unfortunately, there is no reliable statistics on the extent of damage caused by fungal diseases in developing countries, but the damage to the crop plants as a result of these diseases seems to be higher than the global average.
Currently, fungal diseases are controlled using chemical fungicides that, in addition to environmental hazards, cause fungicide resistance in fungal plant pathogens (Komárek et al. 2010). Among fungal pathogens, chitin-containing pathogens such as Fusarium solani, Fusarium oxysporum, and Alternaria solani incite the greatest damage to crop plants such as potatoes, rice, soybeans, wheat, and tobacco. For instance, Fusarium solani is an important plant pathogen and causal agent of several important diseases such as root and stem rot in pea, sudden death syndrome of soybeans, foot rot of bean, and dry rot of potato tuber(s) in the storage (Aoki et al. 2003; Alastruey-Izquierdo et al. 2008; Zaccardelli et al. 2008). The fungal cell wall is a complex structure composed of chitin, β-1,3-glucans, and β-1,6 glucans, which are interconnected by a large number of proteins. Chitin, as the second most frequent polysaccharide in nature after cellulose, constitutes the main component of some pathogenic fungi cell wall (Free 2012). In addition to cell wall strength, chitin plays an essential role in fungi development such that fungal cells die if chitin biosynthesis is hampered (Khan et al. 2011). After being released from the fungal cell wall, chitin and its constituent units such as N-acetylglucosamine are sensed by the plasma membrane receptor kinases (RKs). Next, these RKs stimulate the immune system of the host plants through self-phosphorylation/and or phosphorylation of other proteins (Bhattacharyya and Jha 2012). Finally, plant chitinase enzymes degrade chitin by releasing chitin oligomers and N-acetylglucosamine, which lead to the death of fungal cells (Gow and Hube 2012). In addition to a catalytic domain, chitinase enzymes have at least one domain capable of chitin, oligosaccharides, N-acetylglucosamine binding, and chitin-binding domain (CBD) (Salas et al. 2015). The main function of CBD is similar to the carbohydrate-binding domain (CBM) in the large family of alpha-amylases such as “starch binding domain” that hydrolyzes starch granules (Shoseyov et al. 2006).
Antimicrobial peptides constitute an important part of the innate immune system in many living organisms, including plants, with antibacterial properties similar to antibiotics (Guaní-Guerra et al. 2010). Although different antimicrobial peptides have been used to produce resistant transgenic crop plants (Li et al. 2011; Gao et al. 2000; Parashina et al. 2000), it seems that their preventive role is reduced due to the low inherent affinity of most peptides to bind to the cell wall and their inadequate concentration on the plasma membrane (Kaur et al. 2011). However, some peptides, like defensins, have small motifs and specific amino acids, which play a role in chitin binding. The chitin-binding capability of defensins plays a crucial role in the antifungal activity. Therefore, if defensins’ chitin-binding property is enhanced, the activity of the antimicrobial peptide may synergistically increase (Yokoyama et al. 2009).
The alfAFP, a cationic peptide, is a member of the family of antifungal plant defensins that is expressed in alfalfa seeds. Unlike animal defensins, plant defensins exhibit their antibacterial activity specifically by the permeabilization of the plasma membrane (Sagaram et al. 2011). There are many reports that describe various transgenic plant species harboring AMPs encoding genes resistance to plant pathogens. For instance, Terras et al. (1992) showed that a cysteine-rich peptide from Raphanus sativus seeds (Rs-AFP2) induces resistance to Alternaria longipes in transgenic tobacco. Expression of a pea defensin (DRR206) in canola resulted in transgenic plants becoming slightly resistant to Leptosphaeria maculans fungus (Wang et al. 1999). Gao et al. (2000) generated alfAFP-expressing transgenic potatoes that are resistant to Verticillium dahlia, the causal agent of vascular wilt in a wide range of plant species. In several studies, various plant defensins encoding genes have been introduced to crop plants to enhance resistance against devastating fungal pathogens (Kazan et al. 2002; Lee et al. 2008). More recently, Lee et al. (2018) expressed a pepper defensin (J1–1) gene in tobacco plants and demonstrated that Phytophthora parasitica and Pythium aphanidermatum disease symptoms were significantly suppressed. Therefore, it is evident from different studies in the literature that AMPs have considerable benefits in engineering durable resistance to plant fungal pathogens. To this end, the current research aimed to increase the density of alfAFP peptide molecules in the plasma membrane surface of the chitin-containing invasive fungi by fusing alfAFP peptide to the encoding sequence of a rice chitinase chitin-binding domain, CBD. By expression of CBD-alfAFP recombinant protein, the peptide-binding domain is expected (1) to facilitate alfAFP peptide accumulation on the plasma membrane surface through binding to the fungal chitin cell wall and (2) to prevent the degradation of the alfAFP peptide in the cytoplasm due to its small size.
Materials and methods
Synthesis and cloning of alfAFP-encoding DNA sequence
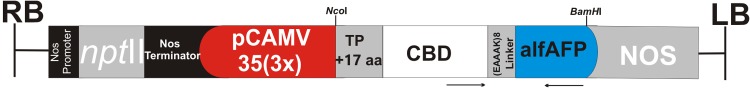
The DNA coding sequence of an alfalfa antimicrobial peptide (alfAFP) was fused to 395 nucleotides sequence of rice chitinase Chitin-Binding Domain (CBD), flanked by NcoI and BamHI restriction enzymes, and synthesized based on Nicotiana tobacco codon preferences (Biomatik, Canada). A helix-forming linker (EAAAK)8 was used to fuse alfAFP peptide to the C-terminal end of CBD. Next, the CBD-alfAFP recombinant DNA fragment was digested by NcoI and BamHI restriction enzymes and cloned in NcoI/BamHI site of the pGSA1285 binary expression vector, resulting in pGSA1285/CBD-alfAFP expression vector cassette (Fig. 1). The accuracy of the gene cassette was confirmed by Sanger sequencing and transferred to the Agrobacterium tumefaciens strain GV3101 for Agrobacterium-mediated transformation.
Fig. 1.
The schematic representation of pGSA1285/CBD-alfAFP binary expression vector used for Nicotiana tabacum transformation. The alfAFP peptide encoding sequence from alfalfa seeds was translationally fused to the C-terminal end of the rice chitinase chitin-binding domain (CBD) DNA sequence. The recombinant gene was driven by the cauliflower mosaic virus (CaMV) 35S (3×) promoter. The neomycin phosphotransferase II (nptII) gene was used to select in vitro transgenic plants. The arrows show the location of primers used for PCR-based screening of transgenic plants. The size of the elements shown in the figure does not match their actual size
Plant material and growth conditions
Nicotiana tabacum var. Xanthi was used to produce in vitro tobacco seedlings. Seeds were surface sterilized with 70% ethanol for 30 s following 1.5% sodium hypochlorite and Triton X100 surfactant for 15 min. The seeds were then washed five times with sterile distilled water. Next, they were filtered, paper dried, and then cultured on MS medium (Murashige and Skoog 1962) containing 20 g L− 1 sucrose and incubated at 25 ± 2 °C. The tobacco seedlings were transferred to glass jars for further growth.
Tobacco transformation and regeneration
Agrobacterium-mediated transformation was used to introduce pGSA1285/CBD-alfAFP binary vector to leaf disc explants. Briefly, young tobacco leaves were excised and punched by a 1 cm− 1 sterile cork borer. A total of 260 leaf disc explants were co-cultivated with A. tumefaciens strain GV3101 harboring a pGSA1285/CBD-alfAFP binary vector for 10 min. Inoculated leaf discs were later transferred to basal MS medium containing BA (2 mg L− 1), NAA (0.1 mg L− 1), and 30 g L− 1 sucrose for three days in the dark at 25 ± 2 °C. After three days, explants were transferred to a fresh co-culture medium containing kanamycin (50 mg L− 1) and cefotaxime (200 mg L− 1) at 25 ± 2 °C for regeneration and subcultured once every 2 weeks for 1 month. Adventitious shoots were excised and transferred to an MS medium supplemented with NAA (0.1 mg L− 1), cefotaxime (200 mg L− 1), and 100 mg L− 1 kanamycin until roots were induced from regenerated adventitious shoots. The plantlets were transferred to a pot containing a mixture of 50% perlite plus 50% peat moss bagged with plastic bags and watered using half-liquid MS medium. Finally, the plantlets were transplanted in sterile soil and transferred to the greenhouse for disease analysis.
Analysis of transgenic plants
To confirm the presence of recombinant CBD-alfAFP gene, genomic DNA was extracted from putative transgenic and a non-transgenic control plants, using the CTAB method (Murray and Thompson 1980). To investigate the insertion of the CBD-alfAFP gene, the forward and reverse primers were designed from CBD sequence (AFP1-F:5′-TGTAGTCAATATGGCTGGTGTG-3′) and alfAFP peptide sequence (AFP1-R: 5′-GCATGGTCCTCTGTACTTATCG-3′), respectively (Fig. 1). The PCR reaction conditions were as follows: pre-denaturation at 94 °C (5 min), then 35 cycles at 94 °C (30 s)/60 °C (30 s)/72 °C (30 s), and a final extension at 72 °C for 10 min.
Semi-quantification of mRNA expression
Total RNA was extracted from young leaves of selected transgenic and non-transgenic plants (EURx Ltd. 80–297 Gdansk Poland) according to the manufacturer’s instructions. The quality and quantity of extracted RNA were determined using 2% agarose gel electrophoresis as well as spectrophotometry. The cDNA was synthesized from total RNA by reverse transcription using a reverse transcriptase (Fermentas, St. Leon-Rot, Germany). The semi-quantitative RT-PCR reaction was carried out using specific primers (AFP2-Forward: 5′-GTCAAAGTCAGTGTTCCGGT-3′ and AFP2-Reverse: 5′-CACCTCTTTGTACACCAACACC-3′). As an internal control, the same cDNA was also used to amplify elongation factor-1α (elf1α) (elf-Forward: 5′-TGAACCATCCAGGACAGATTG-3′, elf-Reverse:5′-TCTTAACCATACCAGCATCACC-3′). The semi-quantitative RT-PCR reaction was performed as follows: pre-denaturation at 94 °C (5 min), then 30 cycles at 94 °C (60 s)/60 °C (30 s)/72 °C (30 s), and a final extension at 72 °C for 5 min. RT-PCR products were electrophoresed and compared on 2% agarose gel.
SDS–PAGE analysis
The total protein was extracted from the selected transgenic and control plants according to Fantozzi and Sensidoni (1983). Briefly, 1 g of young leaves from transgenic and control plants were ground in liquid nitrogen and homogenized with 3 mL of extraction buffer (2 mM EDTA and 50 mM Tris–HCl, pH 8). The samples were shaken at 300 rpm at 4 °C for 10 min and then centrifuged at 15,000g at 4 °C for 15 min. The supernatant containing total protein was transferred to new tubes and passed through 0.2 µm filters. The total protein content was quantified using Bradford compared with BSA as a standard protein (Bradford 1976). The SDS–PAGE analysis was done to separate proteins in a 15% acrylamide gel electrophoresis followed by Coomassie brilliant blue staining according to Laemmli (1970).
Bioassays and determination of minimal inhibitory concentration (MIC)
About 1 g of young (5-week-old) leaf tissue from selected transgenic lines and a control were ground in liquid nitrogen and immediately homogenized in the same volume (v/v) of potassium phosphate buffer (pH 7) complemented with a complete protease inhibitor cocktail (Roche, Mannheim Germany) and then centrifuged at 1000g for 30 min at 4 °C. The supernatant was then collected carefully, kept on ice and protein by Bradford assay (Bradford 1976). To determine the lowest concentration of the protein extracts containing CBD-alfAFP peptide inhibiting the visible growth of pathogens, the minimal inhibitory concentration (MIC) was determined using Broth Macro dilution susceptibility assay (Vanden and Vlietinck 1991). A total of 24 tubes were used to test the transgenic plants protein extract at different dilutions (200, 100, 50, 5, 25, 12, 6, 3.12, and 1.5 µg mL− 1) in three replicates; one tube as the negative control (containing diluted extract plus the medium) and a tube as a positive control (containing microbial suspension plus culture medium). Pseudomonas syringae pv tabaci (ATCC 17914), a devastating plant pathogen with economic importance (Mansfield et al. 2012) was cultured overnight on King’s medium B (King et al. 1954) at 26 °C for 24 h. Cells were washed in sterile water and suspended at a concentration of 1.5 × 108 cfu/mL in phosphate buffer (10 mM; pH 7.0) prior to the assay. About 50 µL suspension of 1.5 × 108 cfu/mL equal to the standard of half-McFarland from overnight cultures was added to all tubes except for the negative control tube. All test tubes were incubated at 37 °C for 24 h. After incubation, tubes were examined for turbidity caused by inoculated bacterial growth. After observing the turbidity in each tube, the previous tube was considered as the minimal inhibitory concentration (MIC). In the case of positive control tube, turbidity showed sufficient bacterial growth. The negative control tube was later examined for the absence of growth. Here, the tube should be free of growth and clear.
The MIC index for Candida albicans (PTCC5027), a human fungal pathogen, and Fusarium solani (PTCC 5284) was determined. Candida albicans were obtained in lyophilized form from Iranian Scientific and Industrial Research Organization, Tehran, Iran. Fusarium solani was obtained from the Faculty of Agriculture, Bu-Ali Sina University, Hamedan, Iran. Briefly, both fungi were cultured in PDA medium at 30 °C for 48 h. A fungal suspension was prepared in a physiology serum and concentration of fungal cells equal to 1 × 106 spores per mL was obtained using a neobar lam. About 200 µL of the above fungal suspension was poured in each tube. Protein extract (200 µg) of the transgenic and untransformed control plants were added to each tube. The tubes were placed in a shaking incubator at 30 °C at 150 rpm for 24 h. The growth rate of the fungi was evaluated based on the turbidity of the wells (Vanden and Vlietinck 1991; Sindambiwe et al. 1999).
Recombinant CBD-alfAFP antibacterial analysis
Escherichia coli (ATCC 8739) and P. syringae (ATCC 17914) bacteria were kindly provided by the Faculty of Medicine, Hamadan University of Medical Sciences, Iran. Disc diffusion method on Müller–Hinton agar medium was used to measure the antimicrobial susceptibility of pathogens using protein extracts (200 µg) of selected transgenic plants (Bauer et al. 1966; Mangena and Muyima 1999). To prepare the test discs, blank discs (6 mm in diameter) were saturated with protein extracts containing CBD-alfAFP recombinant protein. In each test series, a disc containing protein extract of the non-transgenic plant was used as the negative control. To compare inhibition zones, gentamicin (10 µg) and cefotaxime (30 µg) antibiotics were used to prevent bacterial growth as the positive control. Next, 100 µL of 21 h culture of a bacterial suspension of half-MacFarland was poured onto the medium and uniformly inoculated from the suspension of bacteria at the surface of the Müller–Hinton agar culture medium using a sterilized cotton swab. Then, discs prepared from different extracts were placed on the surface of the culture medium using sterilized forceps and incubated at 37 °C for 24 h. Finally, the diameter of the inhibition zones was measured in three replicates and the mean values were reported.
The antifungal effect of CBD-alfAFP recombinant protein on Fusarium solani (PTCC 5284) and Candida albicans was investigated in vitro. Fusarium solani was obtained from the Faculty of Agriculture, Bu-Ali Sina University, Hamedan, Iran. One milliliter of phosphate saline buffer (PBS, pH 7.2) was added to a sterile 1.5 ml microtube. Then, a small amount of cultured colonies of Candida albicans of Potato Dextrose Agar (PDA) medium was removed using sterile forceps and mixed in a PBS buffer. After mixing the fungus in the buffer, a suspension of 1 × 106 cells/mm was counted with a neobar (Furletti et al. 2011). PDA plates were inoculated with 100 µL of fungal suspension (1 × 106 cells per ml). After 20 min, the discs saturated with protein extract of transgenic plants (200 µg mL− 1) along with fluconazole discs (25 µg) were placed on the plates, as controls. Plates were incubated in an incubator for 24 h and then the inhibition zone diameter was measured in comparison with the control group, in three replicates and their mean was recorded.
Fusarium solani inhibition assay on transgenic tobacco plants
Fusarium solani, a ubiquitous soil-borne pathogen that causes vascular wilt, is one of the most frequently isolated soil-borne fungi associated with the greatest economic losses in many crop plants. Fusarium species are among top ten devastating fungal pathogens in plant pathology (Dean et al. 2012). First, Fusarium solani was cultured in the PDA medium to prepare a fresh culture. Then, Fusarium solani was transferred to the Agar and Carnation Leaf (CLA) medium and placed in UV incubator for the sporulation. The sporodochium began to grow on the carnation leaves after 3 weeks. One-month-old alfAFP-01 transgenic and control tobacco plants were grown in MS medium challenged with Fusarium solani. A small block of agar (8 mm) with good Fusarium solani mycelial growth was placed at the base of the transgenic and control plants inside the jar. The jars were placed in a growth chamber at 25 ± 2 °C under 16/8 photoperiod for 4 weeks and the experiment was repeated in triplicate. Transgenic and control plants were observed for any disease symptoms, survival of seedlings, 9, 16, and 30 days after the inoculation (dpi).
Statistical analysis
All data were analyzed in three replicates. One-way ANOVA was performed using SAS ver.9.1 and mean were compared by Duncan’s multiple range test.
Results
For the first time, the encoding sequence of one of the strongest antimicrobial peptides found in alfalfa seeds was fused to the rice chitinase chitin-binding domain (CBD) encoding sequence using a helix-forming linker (EAAAK)8. The recombinant CBD-alfAFP gene (Fig. 1) was introduced to tobacco plants by Agrobacterium-mediated transformation. PCR-based analysis of putative transgenic plants showed that out of 260 transformed explants, 21 lines (transformation efficiency of 8%) were able to grow in an antibiotic-containing medium, and CBD-alfAFP recombinant gene was integrated into their genome. From now on, transgenic and control plants are referred to as alfAFP-XX and Ut, respectively. Also, XX denotes the transgenic line number.
Expression of CBD-alfAFP recombinant protein
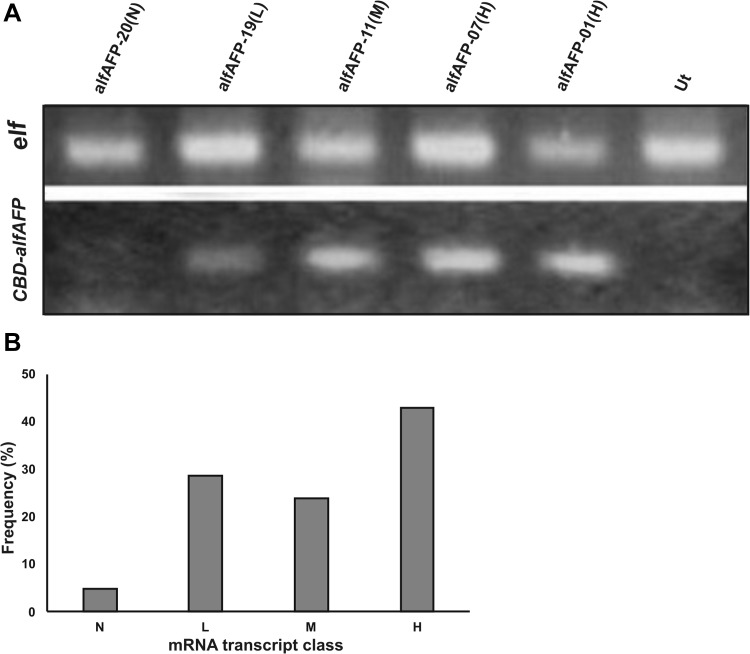
To determine the expression of recombinant CBD-alfAFP gene in transgenic plants, the semi-quantitative RT-PCR analysis was performed on 21 PCR positive transgenic lines and an untransformed control plant. The expression level of the recombinant CBD-alfAFP gene was relatively compared based on the specific PCR products and compared to the expression of the elongation factor 1-alpha (elf1α), as a housekeeping gene. Selected transgenic plants were divided into four classes (Fig. 2a) based on the level of mRNA abundance. The (L), (M), (H), and (N) classes represent plants with low, intermediate, high and no CBD-alfAFP mRNA abundance. Figure 2b shows the percentage of transgenic lines over different levels of CBD-alfAFP expression. From various expression classes, a number of transgenic lines were selected for further characterization.
Fig. 2.
a Semi-quantitative RT-PCR analysis of the selected transformants; The upper panel shows the PCR product using the primers designed for the replication of the housekeeping gene, elf1α, served as the internal control, and the lower panel shows a 120 bp PCR product of CBD-alfAFP recombinant gene in selected transgenic plants; b distribution of individual transformants over different classes of transgenic mRNA expression; The panel defines four different mRNA expression levels in transformants: none (N), low (L), medium (M), and high (H)
Accumulation of recombinant CBD-alfAFP protein in transformants
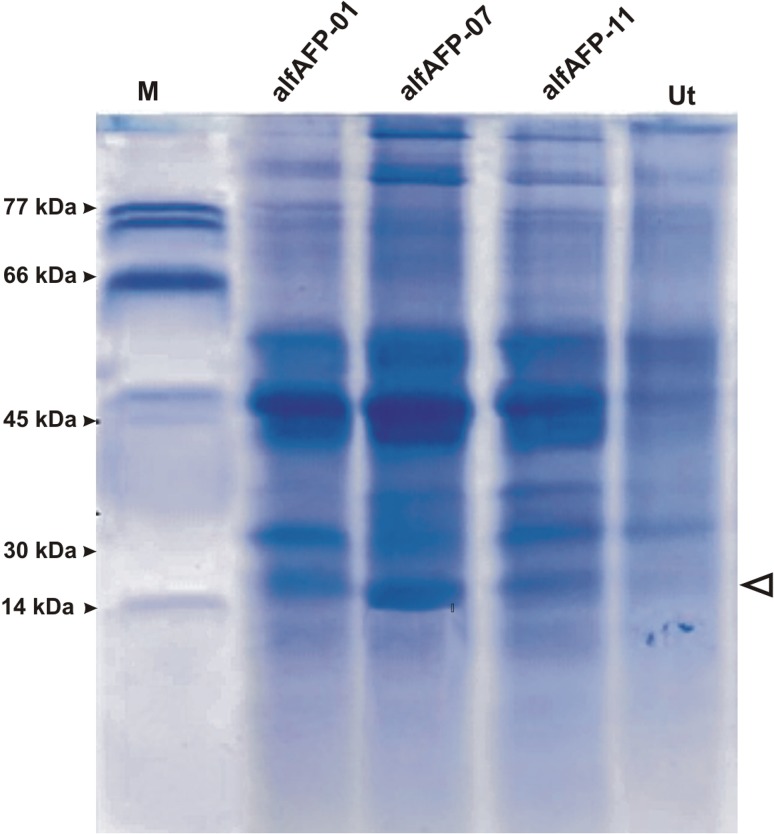
To verify that the recombinant CBD-alfAFP protein of correct size is present in transgenic plants, the total protein was extracted from selected transgenic and the untransformed control plants and subjected to Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) following Coomassie brilliant blue staining. Figure 3 shows the presence of a band approximately 14 kDa, which corresponded well with the predicted molecular mass of CBD-alfAFP recombinant protein (14.4 kDa) in selected transgenic lines. No protein band was detected in the control plant as well as alfAFP-19 transgenic line (Fig. 3).
Fig. 3.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of CBD-alfAFP recombinant protein in selected transgenic plants followed by Coomassie Brilliant Blue staining. Lane M shows the migration of the marker proteins. The molecular mass of the proteins is expressed in kDa. Ut untransformed control plant; the arrowhead shows the migration of CBD-alfAFP recombinant protein
Antibacterial activity of crude transgenic plant protein in vitro
To evaluate the antibacterial potential of CBD-alfAFP recombinant protein of selected transgenic lines, some important pathogens were challenged with the extracted protein. The one-way analysis of variance (ANOVA) showed a significant (P < 0.01) difference among transgenic lines and untransformed control with respect to the antibacterial activity of recombinant CBD-alfAFP protein (Table 1). Transgenic line alfAFP-01 had the highest activity in comparison with the other transgenic lines. As can be seen, the crude protein extract (200 µg) of transgenic plants inhibited P. syringae growth, but not E.coli. There was no significant growth inhibitory difference (P < 0.01) between the effect of protein extract from alfAFP-07 and alfAFP-11 on P. syringae. Protein extract from non-transgenic control tobacco plants did not show any growth inhibitory effect on both bacteria tested.
Table 1.
The antibacterial activity of transgenic protein extracts
| Mean diameter (± SD) of the inhibition zone (mm) | ||
|---|---|---|
| Transgenic line/antibiotics | Pseudomonas syringae | E. coli |
| alfAFP-01 | 20.00 ± 0.5a | 4.10 ± 0.3b |
| alfAFP-07 | 15.00 ± 0.6b | 5.10 ± 0.5b |
| alfAFP-11 | 13.03 ± 0.5b | 4.40 ± 0.5b |
| Ut | 0 | 0 |
| Gentamicin(10 µg) | 25.00 ± 0.5a | 30.00 ± 0.5a |
| Cefotaxime(30 µg) | – | – |
Mean inhibition zones diameter are recorded in triplicate and have been statistically compared with Duncan’s multiple range test
Ut untransformed control
Antifungal activity of the recombinant protein
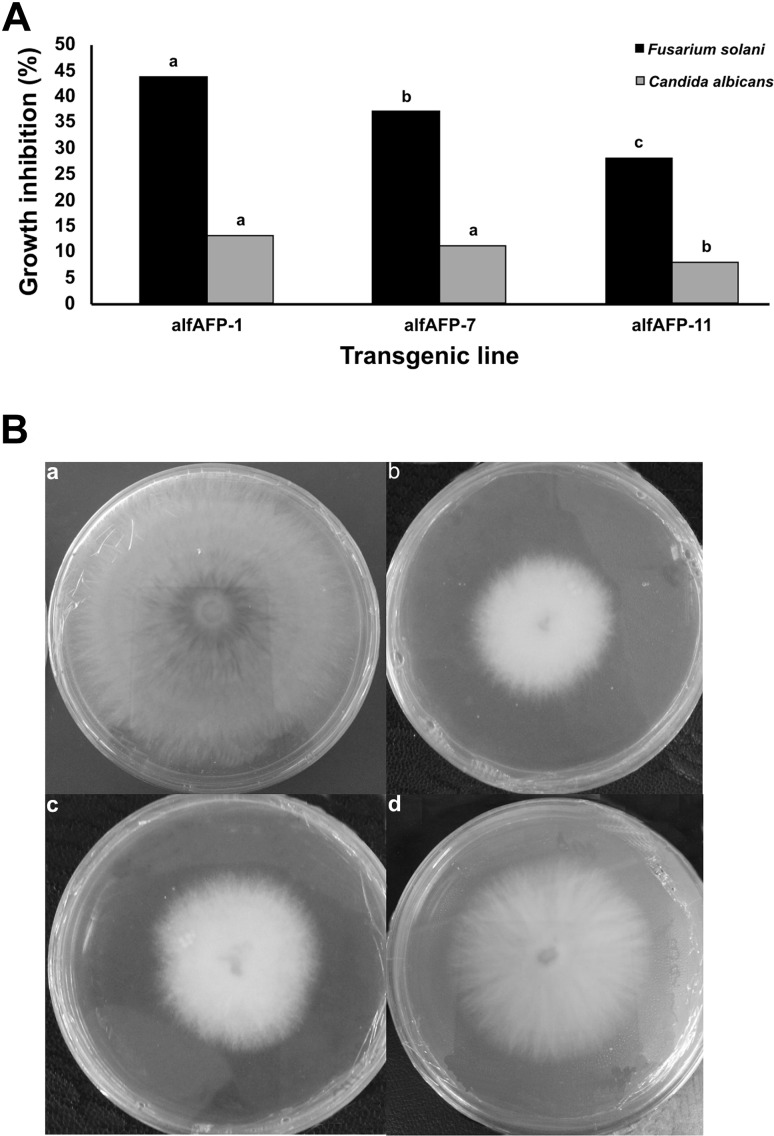
Considering the affinity of the chitin-binding domain (CBD) for chitin-containing cell wall components, the antifungal activity of the CBD-alfAFP recombinant protein was tested on Candida albicans and Fusarium solani pathogens. The protein extract (100 µg mL− 1) of transgenic lines had a significant effect (P < 0.05) on both fungi growth in vitro (Fig. 4a). The lower concentration of protein extracts (< 100 µg mL− 1) did not have a significant (P > 0.05) inhibitory effect on both fungi (data not shown). At 200 µg mL− 1 protein extracts, Fusarium solani and Candida albicans growth were significantly hampered. As can be seen, protein extract from transgenic plants had a higher inhibitory effect on Fusarium solani than Candida albicans. The total protein extract of both alfAFP-07, alfAFP-01 lines had the highest activity in controlling both fungi, especially Fusarium solani (Fig. 4b).
Fig. 4.
a The diagram showing the effect of protein extract (200 µg) of the selected transgenic tobacco plants on inhibiting Candida albicans and Fusarium solani growth. b Antifungal activity of non-transgenic control (a) alfAFP-07 (b), alfAFP-01 (c), and alfAFP-11 (d) protein extract on inhibition of Fusarium solani mycelial growth. PDA plates were incubated in dark at 30 °C for 7 days
Fusarium solani resistant transgenic plants
The 4-week-old transgenic alfAFP-01 plant, showing the maximum antifungal activity (Table 1), was analyzed for disease resistance to the infection by Fusarium solani. Transgenic tobacco alfAFP-01 line expressing CBD-alfAFP recombinant protein showed disease symptoms with a 2-week delay. The first symptoms of the disease, including brown spots in the roots and stems, appeared in the untransformed control plant 9 dpi. Sixteen days after the inoculation, due to necrosis and damage to the vascular systems and the main roots, all leaves in the untransformed control plant completely turned yellow and the whole plant collapsed, as a result of a Fusarium solani infection (Fig. 5). In contrast, the first symptoms of disease in the alfAFP-01 plant were noticed a week later compared to the untransformed control plants. The alfAFP-01 transgenic plant remained green and healthy despite limited fungal infection. Interestingly, alfAFP-01 survived Fusarium solani infection and did not develop disease symptoms over a 30-day period of observation and disease assessment.
Fig. 5.
Enhanced resistance of CBD-alfAFP transgenic tobacco plants to Fusarium solani. Transgenic (alfAFP-01) and untransformed control plants were inoculated with PDA-plug colonized with Fusarium solani. Photographs were taken 9, 16, and 30 days after the inoculation (dpi)
Discussion
The conventional plant breeding methods have not been successful to produce resistance crop plants to survive biotic stresses, especially devastating pathogenic fungi. Instead, genetic engineering techniques seem to hold great promise to cope with the damages caused by plant diseases by producing transgenic lines acquiring resistance toward a particular pathogen.
The expression of antimicrobial peptides and resistance genes (R-genes) such as chitinases and glucanases in plants is the most appropriate method to produce resistance crop plants. For instance, expression of cecropin and sarcotoxin peptides in tobacco (Jan et al. 2010; Jaynes et al. 1993), MsrA3 in tomato (Osusky et al. 2004), and the cecropin-melittin chimeric peptide in potato (Osusky et al. 2000) led to the production of transgenic plants resistant to some plant diseases. Expression of different antimicrobial peptides in tomato resulted in increased resistance to Xanthomonas campestris, Phytophthora infestance, and Fusarium solani (Diaz et al. 2016). Although in all cases transgenic plants showed a significant increase in resistance to pathogens in comparison with non-transgenic plants, no genetically modified crop plant cultivar has been released in the market to resist fungal pathogens.
In the present study, to target the plasma membrane of chitin-containing pathogenic fungi, an alfalfa defensin (alfAFP) was fused to the rice chitinase CBD using a helix-forming linker and its antibacterial and antifungal activity was evaluated. We report for the first time the successful generation of transgenic tobacco plants’ resistance to a number of pathogens tested including an economically important plant pathogen, Fusarium solani. The antifungal activity of selected tobacco transgenic lines in vitro showed a strong potential of the CBD-alfAFP recombinant protein against P. syringae, Fusarium solani, and Candida albicans. Although defenins mainly have antifungal activity and show less antibacterial potential, the significant activity of protein extract from transgenic plants against P. syringae was surprising, suggesting a further future antibacterial evaluation. In contrast to antibacterial activity, recombinant protein showed a significant inhibitory effect on chitin-containing human and plant fungi, suggesting that CBD is a cooperative partner to antifungal peptides. In addition to the cell wall penetration, CBD may facilitate alfAFP entering fungal cells and identifying its intracellular targets (Brogden 2005). It is worth noting that some defensins may bind to specific plasma membrane sphingolipids in susceptible fungi because a mutant of Fusarium graminearum defective in the GlcCer synthesis gene showed a high resistance to alfAFP antifungal activity. Therefore, it can be inferred that the binding of alfAFP to glucosylceramide (GlcCer) is necessary for antifungal activity (Sagaram et al. 2011; Spelbrink et al. 2004).
The introduction of individual rice chitinase (CHI) gene, alfAFP, and the recombinant gene (CHI-alfAFP) in transgenic tomato plants protected the vegetative tissues against pathogens attack (Chen et al. 2009). In line with the present study, Chen et al. (2009) obtained a higher resistance to Botrytis cinerea in some of the CHI-alfAFP transgenic lines compared to the expression of individual genes. So, it can be concluded that the synergistic cooperation of two genes may provide more effective resistance against the disease. In the present study, the N-termianl CBD domain of a rice chitinase fused to alfAFP peptide significantly delayed the onset of disease symptoms and ultimately resulted in the resistance of transgenic plants to Fusarium solani. The symptoms of Fusarium solani disease on tobacco transgenic lines appeared 2 weeks later compared to untransformed control plants. The delay in onset of the disease in transgenics lines can be attributed to the fact that CBD provides alfAFP peptide with access to the fungal cell wall through binding to cell wall chitin, chitin oligomers, and N-acetylglucosamine. Therefore, the alfAFP peptide can possibly penetrate fungal cells and target the plasma membrane efficiently (Fig. 6). In addition, plant defensins alone have the ability to bind to the cell wall glucans (Yokoyama et al. 2009). The presence of Knottin motifs that contain conserve aromatic and serine amino acids helps defensin peptides to bind to the cell wall of the fungi pathogens facilitating the antibacterial activity through an unknown mechanism (Yokoyama et al. 2009).
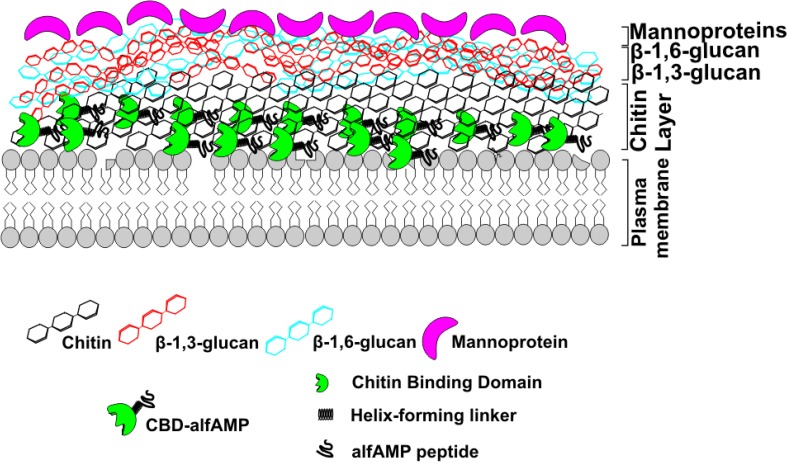
Fig. 6.
The proposed mechanism of CBD-alfAFP recombinant antimicrobial peptide; Chitin-binding domain (CBD) aids alfAFP peptide to aggregate on the fungal cell wall. Both CBD and alfAFP have an affinity for chitin and glucans. CBD may anchor alfAFP peptide in the cell wall permeabilizing the plasma membrane and possibly lead to pore formation and cell leakage
In conclusion, this study for the first time showed that the CBD may increase the antifungal activity of the alfAFP peptide (Suarez et al. 2001). These results demonstrate that the introduction of this recombinant gene to other crop plants, such as potatoes, may lead to transgenic crop plant cultivars with elevated resistant against fungal pathogens, especially chitin-containing fungi.
Acknowledgements
We would like to show our gratitude to the member of Dr. Mostafa Darvishnia Plant disease LAB for in vitro fungal tests.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no competing interest in the publication of this manuscript.
References
- Alastruey-Izquierdo A, Cuenca-Estrella M, Monzón A, Mellado E, Rodríguez-Tudela JL. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61(4):805–809. doi: 10.1093/jac/dkn022. [DOI] [PubMed] [Google Scholar]
- Aoki T, O’Donnell K, Homma Y, Lattanzi AR. Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex—F. virguliforme in North America and F. tucumaniae in South America. Mycologia. 2003;95(4):660–684. [PubMed] [Google Scholar]
- Bauer A, Kirby W, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- Becker-Ritt AB, Carlini CR. Fungitoxic and insecticidal plant polypeptides. J Pept Sci. 2012;98(4):367–384. doi: 10.1002/bip.22097. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28(4):1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Chen S, Liu A, Wang F, Ahammed G. Combined overexpression of chitinase and defensin genesin transgenic tomato enhances resistance to Botrytis cinerea. Afr J Biotechnol. 2009;8:20. [Google Scholar]
- Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13(4):414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz AH, Kovacs I, Lindermayr C. Inducible expression of the de-novo designed antimicrobial peptide SP1-1 in tomato confers resistance to Xanthomonas campestris pv. vesicatoria. PloS One. 2016;11(10):e0164097. doi: 10.1371/journal.pone.0164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantozzi P, Sensidoni A. Protein extraction from tobacco leaves: technological, nutritional and agronomical aspects. Plant Food Hum Nutr J (Formerly Qualitas Plantarum) 1983;32(3):351–368. doi: 10.1007/BF01091194. [DOI] [Google Scholar]
- FAO (2017) FAOSTAT data production, trade, food balance, food security. http://www.faoorg/faostat/en/#home. Accessed 2 Aug 2017
- Free SJ. Fungal cell wall organization and biosynthesis. Adv Genet. 2012;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- Furletti V, Teixeira I, Obando-Pereda G, Mardegan R, Sartoratto A, Figueira G, Duarte R, Rehder V, Duarte M, Höfling J. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/985832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A-G, Hakimi SM, Mittanck CA, Wu Y, Woerner BM, Stark DM, Shah DM, Liang J, Rommens CM. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotech. 2000;18(12):1307. doi: 10.1038/82436. [DOI] [PubMed] [Google Scholar]
- Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol. 2012;15(4):406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135(1):1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Jan P-S, Huang H-Y, Chen H-M. Expression of a synthesized gene encoding cationic peptide cecropin B in transgenic tomato plants protects against bacterial diseases. J Appl Environ Microbiol. 2010;76(3):769–775. doi: 10.1128/AEM.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes JM, Nagpala P, Destéfano-Beltrán L, Hong Huang J, Kim J, Denny T, Cetiner S. Expression of a Cecropin B lytic peptide analog in transgenic tobacco confers enhanced resistance to bacterial wilt caused by Pseudomonas solanacearum. Plant Sci. 1993;89(1):43–53. doi: 10.1016/0168-9452(93)90169-Z. [DOI] [Google Scholar]
- Kaur J, Sagaram US, Shah D. Can plant defensins be used to engineer durable commercially useful fungal resistance in crop plants? Fungal Biol Rev. 2011;25(3):128–135. doi: 10.1016/j.fbr.2011.07.004. [DOI] [Google Scholar]
- Kazan K, Rusu A, Marcus JP, Goulter KC, JM M. Enhanced quantitative resistance to Leptospharia maculans conferred by expression of a novel antimicrobial peptide in canola (Brassica napus L.) Mol Breed. 2002;10:63–70. doi: 10.1023/A:1020354809737. [DOI] [Google Scholar]
- Khan RS, Nakamura I, Mii M. Development of disease-resistant marker-free tomato by R/RS site-specific recombination. Plant Cell Rep. 2011;30(6):1041–1053. doi: 10.1007/s00299-011-1011-4. [DOI] [PubMed] [Google Scholar]
- King EO, Ward MK, Raney DE. Two simple media for the demonstration of phycocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- Komárek M, Čadková E, Chrastný V, Bordas F, Bollinger J-C. Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Environ Int. 2010;36(1):138–151. doi: 10.1016/j.envint.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee SC, Hwang IS, Choi HW, BK H. Involvement of the pepper antimicrobial protein CaAMP1 gene in broad spectrum disease resistance. Plant Physiol. 2008;148:1004–1020. doi: 10.1104/pp.108.123836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-H, Kim J-S, Hoang QT, Kim J-I, Kim YS. Root-specific expression of defensin in transgenic tobacco results in enhanced resistance against Phytophthora parasitica var. nicotianae. Eur J Plant Pathol. 2018;151(3):811–823. doi: 10.1007/s10658-018-1419-6. [DOI] [Google Scholar]
- Li Z, Zhou M, Zhang Z, Ren L, Du L, Zhang B, Xu H, Xin Z. Expression of a radish defensin in transgenic wheat confers increased resistance to Fusarium graminearum and Rhizoctonia cerealis. Funct Integr Genomics. 2011;11(1):63–70. doi: 10.1007/s10142-011-0211-x. [DOI] [PubMed] [Google Scholar]
- Mangena T, Muyima N. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected bacteria and yeast strains. Lett Appl Microbiol. 1999;28(4):291–296. doi: 10.1046/j.1365-2672.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13(6):614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S. Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat Biotech. 2000;18(11):1162–1166. doi: 10.1038/81145. [DOI] [PubMed] [Google Scholar]
- Osusky M, Osuska L, Hancock RE, Kay WW, Misra S. Transgenic potatoes expressing a novel cationic peptide are resistant to late blight and pink rot. Transgenic Res. 2004;13(2):181–190. doi: 10.1023/B:TRAG.0000026076.72779.60. [DOI] [PubMed] [Google Scholar]
- Parashina E, Serdobinskii L, Kalle E, Lavrova N, Avetisov V, Lunin V, Naroditskii B. Genetic engineering of oilseed rape and tomato plants expressing a radish defensin gene. Russ J Plant Physiol. 2000;47(3):417–423. [Google Scholar]
- Sagaram US, Pandurangi R, Kaur J, Smith TJ, Shah DM. Structure-activity determinants in antifungal plant defensins MsDef1 and MtDef4 with different modes of action against Fusarium graminearum. PLoS One. 2011;6(4):e18550. doi: 10.1371/journal.pone.0018550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas CE, Badillo-Corona JA, Ramírez-Sotelo G, Oliver-Salvador C. Biologically active and antimicrobial peptides from plants. BioMed Res Int. 2015;2015:1–11. doi: 10.1155/2015/102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoseyov O, Shani Z, Levy I. Carbohydrate binding modules: biochemical properties and novel applications. Microbiol Mol Biol Rev. 2006;70(2):283–295. doi: 10.1128/MMBR.00028-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindambiwe J, Calomme M, Cos P, Totte J, Pieters L, Vlietinck A, Berghe DV. Screening of seven selected Rwandan medicinal plants for antimicrobial and antiviral activities. J Ethnopharmacol. 1999;65(1):71–77. doi: 10.1016/S0378-8741(98)00154-8. [DOI] [PubMed] [Google Scholar]
- Spelbrink RG, Dilmac N, Allen A, Smith TJ, Shah DM, Hockerman GH. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004;135(4):2055–2067. doi: 10.1104/pp.104.040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez V, Staehelin C, Arango R, Holtorf H, Hofsteenge J, Meins F. Substrate specificity and antifungal activity of recombinant tobacco class I chitinases. Plant Mol Biol. 2001;45(5):609–618. doi: 10.1023/A:1010619421524. [DOI] [PubMed] [Google Scholar]
- Terras F, Schoofs H, De Bolle M, Van Leuven F, Rees SB, Vanderleyden J, Cammue B, Broekaert WF. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem. 1992;267(22):15301–15309. [PubMed] [Google Scholar]
- Vanden B, Vlietinck A. Screening methods for antibacterial and antiviral agents from higher plants. In: Dey PM, Harborne JB, editors. Methods in plant biochemistry. London: Academic press; 1991. [Google Scholar]
- Wang Y, Nowak G, Culley D, Hadwiger LA. Constitutive expression of pea defense gene DRR206 confers resistance to blackleg (Leptosphaeria maculans) disease in transgenic canola (Brassica napus) Mol Plant-Microbe Interact. 1999;12:410–418. doi: 10.1094/MPMI.1999.12.5.410. [DOI] [Google Scholar]
- Yokoyama S, Iida Y, Kawasaki Y, Minami Y, Watanabe K, Yagi F. The chitin-binding capability of Cy-AMP1 from cycad is essential to antifungal activity. J Peptide Sci. 2009;15(7):492–497. doi: 10.1002/psc.1147. [DOI] [PubMed] [Google Scholar]
- Zaccardelli M, Vitale S, Luongo L, Merighi M, Corazza L. Morphological and molecular characterization of Fusarium solani isolates. J phytopathol. 2008;156(9):534–541. doi: 10.1111/j.1439-0434.2008.01403.x. [DOI] [Google Scholar]