Abstract
In this study, the effect of spheroid formation, as a model of three-dimensional (3D) culture systems, on the cancer stemness of human breast cancer (MCF-7) and human glioma (U87-MG) cell lines was analyzed. We compared the expression of pluripotency genes, the presence of various cancer stem cell populations, migration and proliferation capacities of cells cultured as monolayers or spheroids. MCF-7 cells formed uniform spheroids in vitro, upregulated the expression of stem cell markers both at gene and protein levels and increased their migration capacities when cultured in 3D systems. When a CSC targeting metabolic drug, metformin was used, multiple drug resistance genes (ABC transporters) were downregulated and the anti-cancer activity of 5-fluorouracil was enhanced. In summary, this study proved that the use of 3D culture systems such as spheroids can be used in CSC-related research. Therefore, studies involving 3D culture systems will help scientists to discover new CSC markers, show more realistic drug responses, and better evaluate tumor proliferation and morphology changes.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1412-y) contains supplementary material, which is available to authorized users.
Keywords: 3D cell culturing, Spheroids, Monolayer, Cancer stem cell, ABC transporter, Metformin
Introduction
The number of studies involving three-dimensional (3D) cell culture models has recently increased in the field of cancer research. More importantly, due to better mimicking of in vivo conditions, researchers have been able to study cancer stem cells (CSCs) in 3D culture models (Bielecka et al. 2016). Small subpopulation of cells within a tumor harboring ‘stem cell-like’ properties have been identified in various studies and these stem cell-like tumor cells have been called as cancer stem cells (CSCs) (Clarke et al. 2006; Clarke and Fuller 2006). CSCs have been shown to have distinctive characters as well as similar properties as non-malignant stem cells. Non-malignant stem cells with pluripotency or multipotency are vital during development and are also found in various tissues with high regenerative abilities (Trumpp and Wiestler 2008; Rojas-Ríos and González-Reyes 2014); whereas CSCs are involved during cancer progression and metastasis.
Cancer stem cells were originally discovered in leukemia research, and they have been shown to have unlimited self-renewal capacity and the ability to initiate and sustain tumor growth (Dean et al. 2005; Gupta et al. 2009; Economopoulou et al. 2012); however, there are still many unknowns in CSC research. Studies have reported that CSCs could be responsible for the development of chemotherapeutic drug resistance, as evident in various pre-clinical and clinical models (Dean et al. 2005). Therefore, a better understanding of CSCs’ properties may explain different patient responses to anti-neoplastic therapies, as well as provide improved insight into the life cycle of tumor cells (Bielecka et al. 2016).
Two-dimensional culture conditions, the so-called monolayers, are typically formed on polystyrene culture plates. In general, monolayers are inadequate to remodel the biological microenvironment of tumor cells; since they do not allow the establishment of proper cellular interactions due to unnatural growing conditions compared to natural in vivo environment (Cody et al. 2007; Li et al. 2008). On the other hand, 3D cell culture systems such as spheroids enable cells to grow in all three dimensions and form a complex system of extracellular matrix components as well as junctional complexes. Furthermore, these differences between2D and 3D culture systems may reflect the potentially different outcomes of in vitro and in vivo experimental findings (Li et al. 2008).
Although cancer cells can be propagated well under monolayer conditions—primarily due to their acquired resistance mutations and abnormal cell cycle control and uncontrolled checkpoints (Kastan and Bartek 2004; Ashwell and Zabludoff 2008)—researchers often encounter difficulties in capturing the effects of CSCs in 2D cultures. Therefore, there is an ongoing need for the development of 3D culture systems to study cancer biology and better identification of CSCs. During tumorigenesis, tumor growth, metastasis, and recurrence, CSCs have been shown to play an important role, therefore performing in vitro experiments in 3D culture models will allow to recreate conditions similar to in vivo tumorigenesis (Marotta and Polyak 2009; Oskarsson et al. 2014; Bielecka et al. 2016; Lv et al. 2017).
In this study, we aimed to evaluate the effect of spheroid formation as a model of 3D culture systems on the cancer stemness of two important cancer types which show the highest number of CSC populations in the literature: human breast cancer (MCF-7) and human glioma (U87MG) cell lines. We compared the expression of pluripotency genes, the presence of various cancer stem cell populations, and migration and proliferation capacities of cells cultured as monolayers or spheroids. Spheroids were assessed for the presence of five different CSC populations. We have also assessed the effect of CSC-targeting metabolic drug on the anti-cancer activity of chemotherapeutic agent on the MCF-7 spheroids and how this metabolic drug can be used to increase the sensitivity of cells to chemotherapeutic agents.
Materials and methods
Materials
Human glioma cell line U87-MG and human breast cancer cell line MCF-7 were purchased from ATCC (Rockville, MD, USA). Minimum essential medium, fetal bovine serum, penicillin, streptomycin, glutamine, non-essential amino acids, and alamar blue assay were obtained from Thermo Fisher (Massachusetts, USA). Metformin, 5-fluorouracil, PBS, ethanol, agar and propidium iodide were purchased from Sigma (Missouri, ABD). Anti-CD24-PE, CD44-APC, CD-117-PerCp, CD146-FITC, CD70-PE and CD-271-PE antibodies were ordered from BD (New Jersey, USA). Macherey Nagel RNA isolation kit was purchased from Macherey Nagel (Dure, Germany). cDNA synthesis kit and iO SYBR Green Supermix were obtained from Bio-Rad (California, USA). CIM plates were purchased from Acea Biosciences (California, USA).
Cell culture
Cells were maintained according to the manufacturer’s protocol in minimum essential medium (MEM), supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin, 50 µg/ml streptomycin, with some modifications including 1% l-glutamine and 1% non-essential amino acids (David and Gooderham 2016; Langan et al. 2016) at 37 °C in 5% CO2.
Spheroid formation and drug treatment
Healthy cells were cultured either as monolayers or spheroids in vitro. For monolayer culture, cells were either seeded on 6-well or 96-well plates. For spheroid formation, cells (10,000 cells) were seeded on 1% agar-coated 96-well plates. Prior to treatment or analyses, both monolayers or spheroids were kept for 15 days in culture. In monolayers, cells were passaged in every 2–3 days to achieve 70% confluency. In spheroids, culture media were exchanged in every 2 days to ensure 3D spheroids can get enough nutrients. Cultures were monitored every other day and representative images were taken on day 15 under the inverted microscope (Zeiss, Germany). When indicated, cells were incubated with metformin (1, 10 or 100 mM) and/or 5-fluorouracil (0.1, 1, 10 mM).
Flow cytometry analysis
For CSC marker analyses, cells cultured as monolayers or spheroids were collected by centrifugation, washed with ice cold 1X PBS followed by staining for CD24-PE, CD44-APC, CD-117-PerCp, CD146-FITC, CD70-PE and CD-271-PE. Cells were analyzed in BD Accuri Plus Flow Cytometer (BD, New Jersey, USA). For 20,000 events, percentages of positive populations were determined by using BD Accuri Plus software (BD, New Jersey, USA).
For cell cycle analysis, cells cultured as monolayers or spheroids were collected by centrifugation, washed with ice cold 1× PBS followed by fixation (70% cold ethanol) at − 20 °C for 15 min. After washing, cells were stained with PI and were analyzed in BD Accuri Plus Flow Cytometer. For 20,000 events, percentages of cells in different stages of cell cycle were determined using BD Accuri Plus software.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Cells cultured as monolayers or spheroids (5 × 105 cells) were collected and RNA was extracted with the Machery Nagel RNA isolation kit. cDNA synthesis from 1 µg of RNA sample was performed with iScript cDNA synthesis kit according to manufacturer’s instructions. 2 µl of each cDNA sample was used to perform real-time qPCR reactions with iO SYBR Green Supermix. Samples were run on CFX-96 Real Time System (Bio-Rad, UK) with the following protocol: 95 °C for 3 min, 1 cycle; 95 °C for 10 s, 60 °C for 30 s,—repeated for 40 cycles. GAPDH was used as a reference gene and gene expression levels were normalized to untreated control groups.
Migration analysis
Cells cultured as monolayers or spheroids were collected and single cell suspensions were formed. Cells (4 × 104 cells) were seeded on CIM plates. These plates have special structures and they have upper wells which contain only cells in media containing 0% serum. The lower chambers of these plates have media with 10% serum. This serum difference is driving cells to migrate from upper chamber to lower chamber. Cells were kept at CO2 incubator in the RTCP instrument (Acea Biosciences, USA) for 15 h. Cell index values, which are quantitative measures of cell number that shows migration, were plotted with RTCA software (Acea Biosciences, USA) over time.
Viability assay
Alamar blue cell viability kit was used to determine cell viability after drug treatment. Simply alamar Blue reagent was added as 10% of the well volume, followed by a 2 h incubation at 37 °C. The absorbance was read on a plate spectrophotometer (BMGLabtech, Germany) at 570 and 600 nm. Percentage of cell viability was calculated for each treatment group.
Statistical analysis
Experiments were performed with twelve different spheroids per condition and on at least three independent occasions. Triplicates containing required amount of cells were used during qRT-PCR, flow cytometry, and migration/invasion analyses. Statistical analysis was performed by analysis of variance and Tukey’s pairwise comparison using SPSS software, version 16.0.
Results
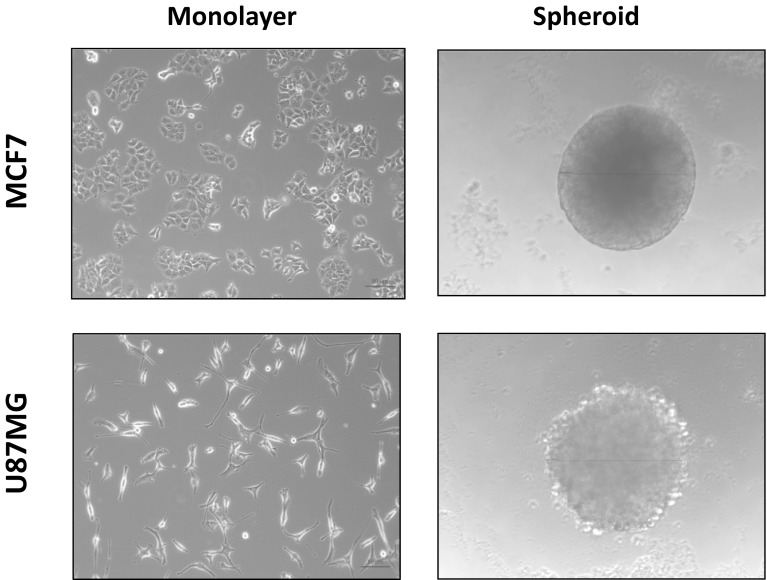
Cancer cell lines could be successfully cultured either as 2D monolayers or 3D spheroids. Figure 1 shows that healthy cells adhered to culturing surface and distinctive cell morphology for each cell line can be observed on monolayer cultures for both MCF-7 and U87MG cells. When the spheroids were formed, MCF-7 spheroids showed much more compact 3D structure compared to U87MG cells. For the following experiments where cancer stemness was analyzed, spheroids were cultured for 15 days. Figure S1 shows the development of spheroids and diameter measurements for both cell lines. This extended culturing protocol could give enough time and environment for CSCs to proliferate and it has been previously reported in 3D culture systems involving spheroids (Gong et al. 2015; Zanoni et al. 2016).
Fig. 1.
Images of monolayer and spheroid cultures. MCF-7 and U87-MG cells were cultured as monolayers and spheroids. Phase contrast images of cells were obtained with an inverted microscope (×20)
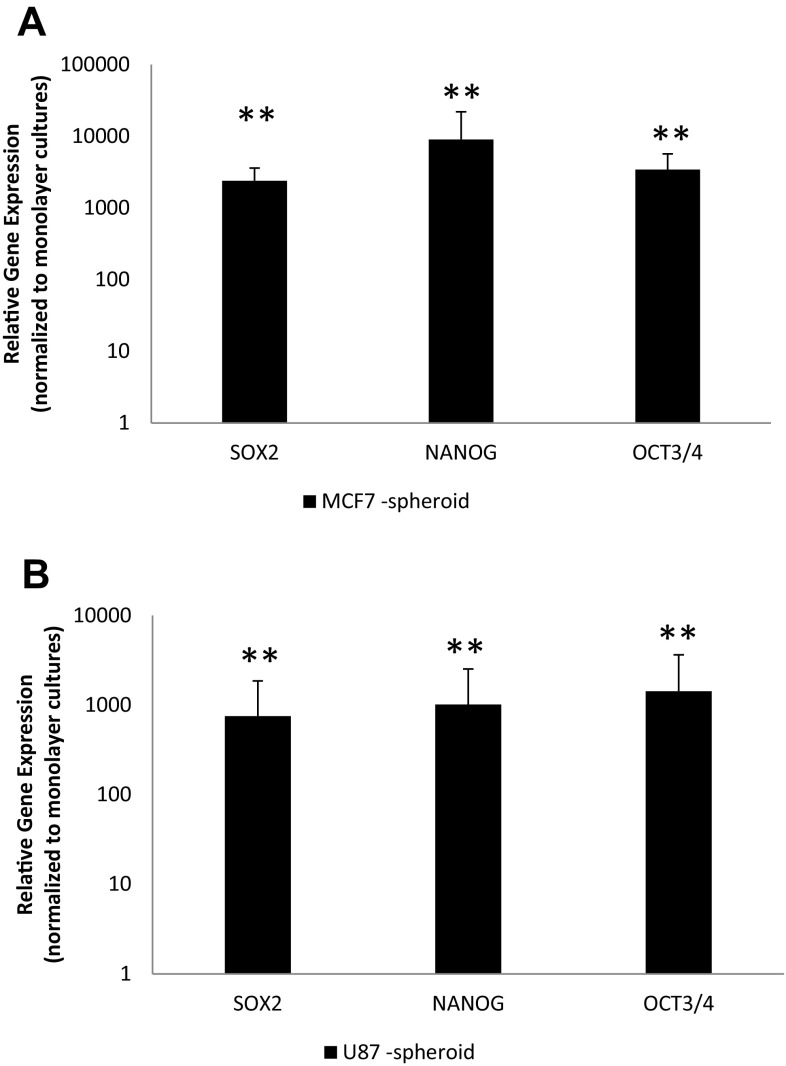
Following formation of spheroids, they were analyzed for the expression and presence of CSCs. It is well known that CSCs can express Nanog, Oct3/5 or Sox2 genes. For this reason, monolayers and spheroids were collected and expression of these genes was determined by qRT-PCR (Fig. 2a). Figure 2 shows the expression of Nanog, Oct3/5 or Sox2 genes in spheroid cultures compared to monolayer cultures. It is clear that cancer cell lines upregulated these genes when their three-dimensional culture systems, spheroids were formed.
Fig. 2.
Expression of stem cell markers. MCF-7 and U87-MG cells were cultured as monolayers and spheroids. RNA was isolated and qRT-PCR was performed for SOX2, NANOG and OCT4. Relative gene expression was plotted for a MCF-7 and b U87-MG cells cultured as spheroids, normalized to monolayers. **p < 0.01 compared to naïve samples
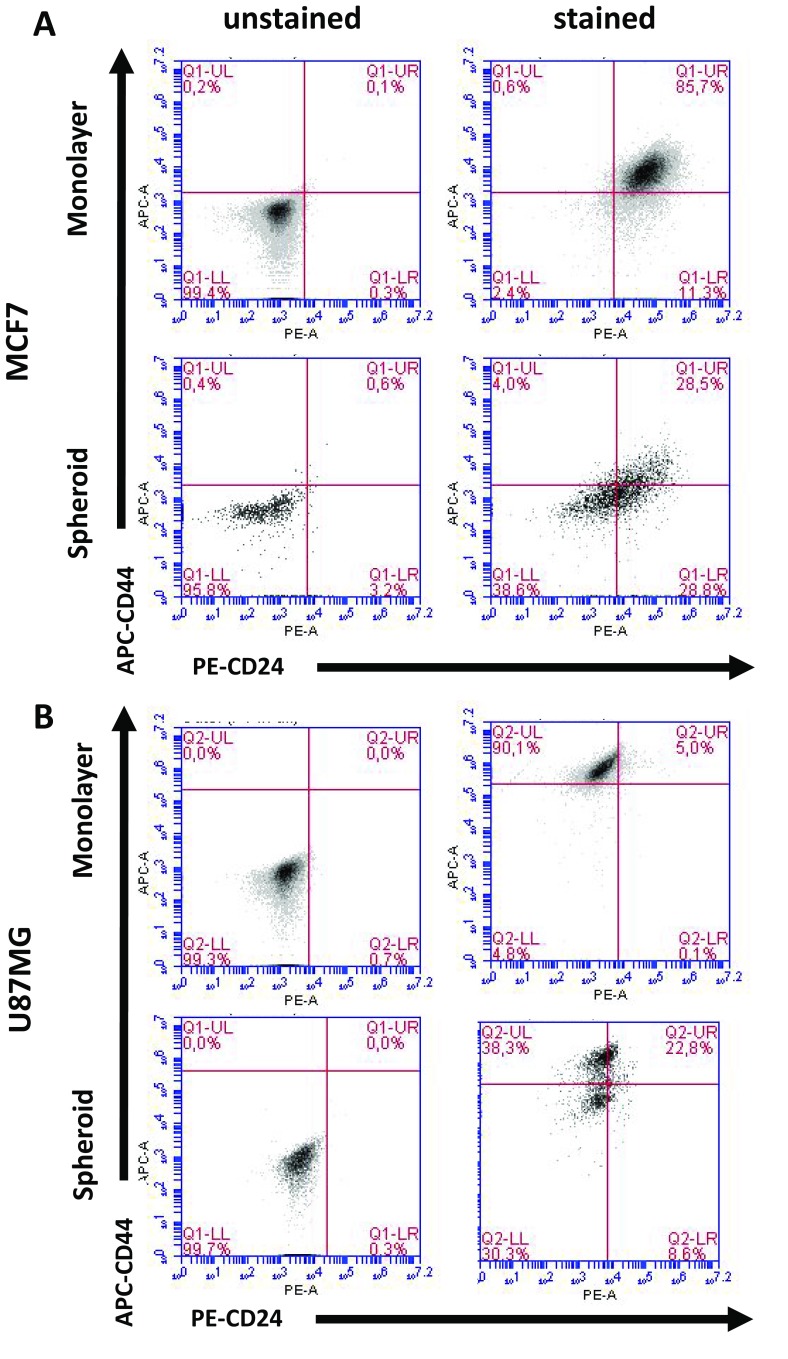
According to the literature, there are various well-established markers for CSCs. In this study, we investigated the following CSC markers: CD44−/CD24+ (Fig. 3, Table S1), CD70+ (Fig. 4, Table S4), CD117+ (Fig. 4, Table S3), CD146+ (Fig. 4, Table S5) and CD271+ (Fig. 4, Table S2). Flow cytometry analysis showed that when MCF-7 cells were cultured as monolayers, cells were mainly positive for CD146 (12.2%) and CD271 (22.6%). However, when spheroids were formed with MCF-7 cells, they stated to express CD117 (10.9%) and CD70 (21.3%). Furthermore, we were able to detect 4.0% CD44−/CD24+ cells. U87MG cells, on the other hand, did not change the expression of CD117 expression, and decreased the number of CD70+ or CD44−/CD24+ cells. Only CD271 positivity slightly increased following the spheroid formation in U87MG cells.
Fig. 3.
Percentage of CD24−/CD44+ CSC subpopulations. MCF-7 and U87-MG cells were cultured as monolayers and spheroids. Cells were collected by centrifugation, followed by staining with CD24-PE and CD44-APC. Cells were analyzed in BD Accuri Plus Flow Cytometer (BD)
Fig. 4.
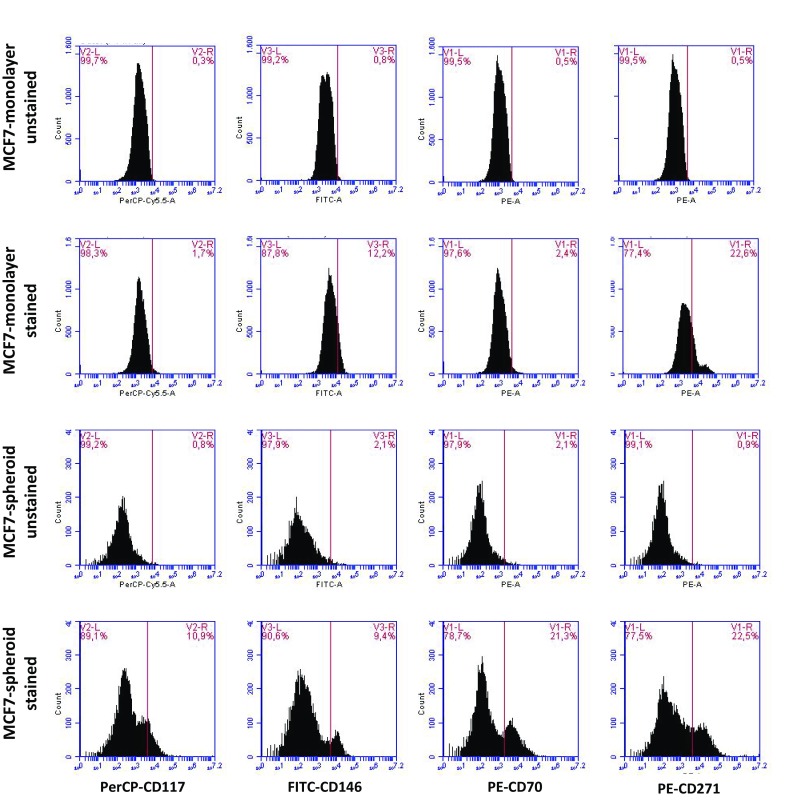
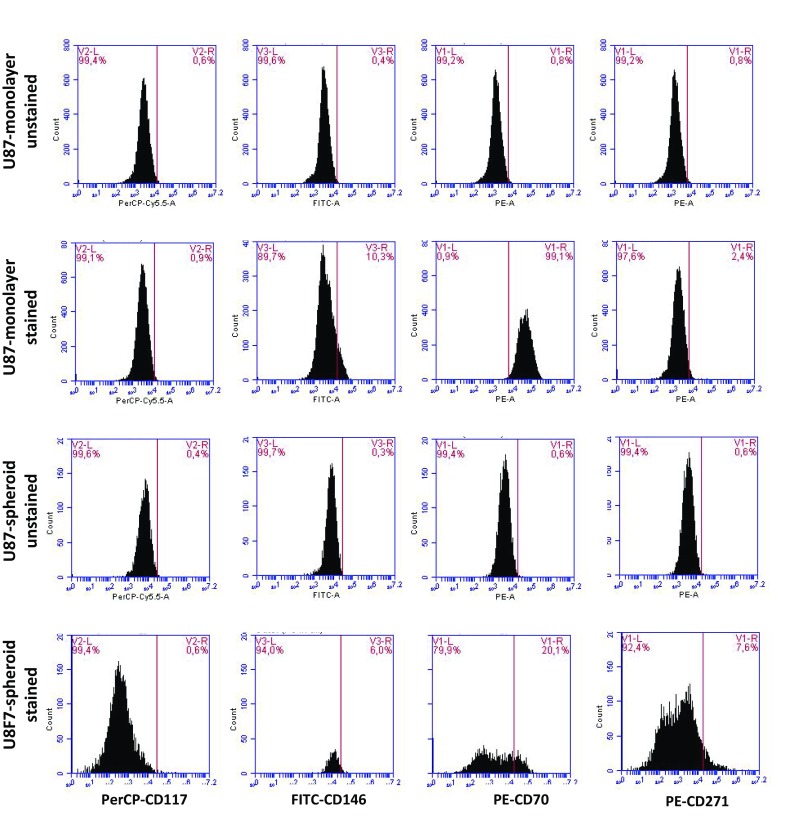
Expression of CD117, CD146, CD70 and CD271 CSC markers. a MCF-7 and b U87-MG cells were cultured as monolayers and spheroids. Cells were collected by centrifugation, followed by staining with CD-117-PerCP, CD146-FITC, CD70-PE and CD271-PE. Cells were analyzed in BD Accuri Plus Flow Cytometer (BD)
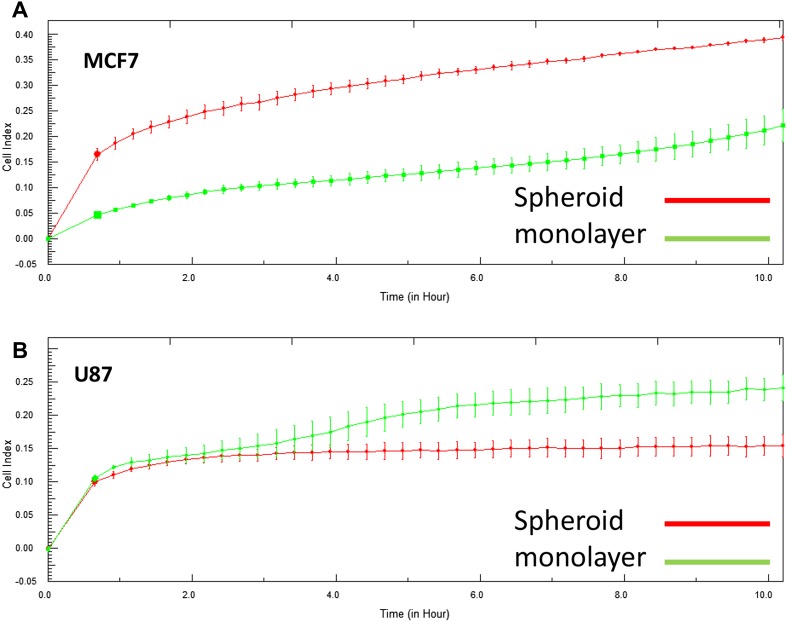
To understand the effect of spheroid formation on migration, single cell suspensions were obtained from spheroids after 15 days of culturing. These cells were tested for their migration capabilities (Fig. 5). MCF-7 cells obtained from spheroid cultures showed higher migration compared to cells obtained from monolayer cultures. On the other hand, U87MG cells showed similar migration rates for both monolayer and spheroid cultures, especially at early time points. After 2 h, U87MG cells which were previously cultured as monolayers showed higher cell index values compared to spheroid originated cells.
Fig. 5.
Migration capacities of cancer cells. MCF-7 and U87-MG cells were cultured as monolayers and spheroids. Cells were collected and seeded on CIM plates and cell index which is a value for migration was measured in RTCA instrument for 24 h
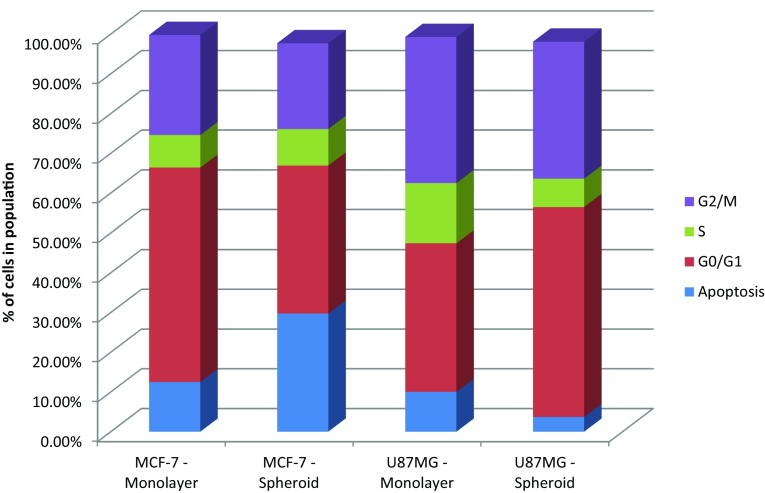
Cell cycle analysis was performed to compare the proliferation rates of cell lines cultured as monolayers or spheroids (Fig. 6). In MCF-7 cells, the number of subG0/G1 cells increased in spheroid cultures, possibly indicating the apoptotic cells found at the core of the spheroids. In U87-MG cells, spheroid formation increased the percentage of cells in the G0/G1 phase and reduced the S-phase.
Fig. 6.
Cell cycle analysis following spheroid formation. MCF-7 and U87-MG cells were cultured as monolayers and spheroids. Cell cycle analysis was carried out by PI staining. Data were analyzed using BD Accuri Plus flow cytometer and the number of cells in the subG1, G0/G1, S and G2/M phases were plotted
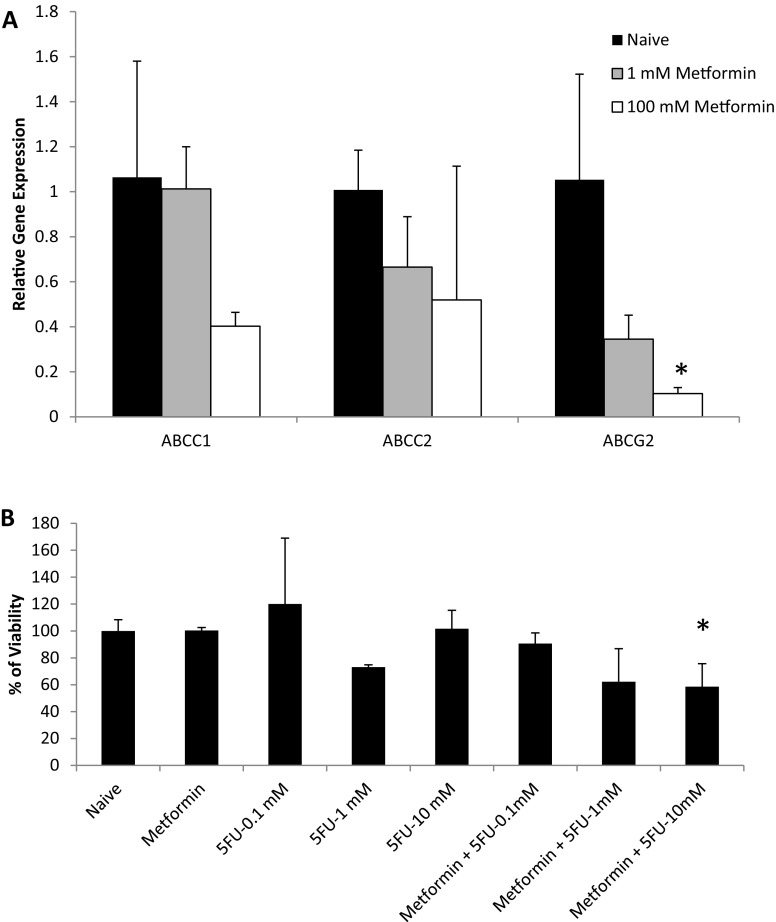
Finally, MCF-7 cells which showed higher cancer stemness upon spheroid formation was selected and used to evaluate the effect of chemotherapeutic drugs in these 3D culture systems. First, MCF-7 spheroids were analyzed for the expression of drug resistance genes (ABCC1, ABCC2 and ABCG2) in the presence of metformin. Figure 7a shows that when spheroids were treated with metformin, ABCC1, ABCC2 and ABCG2 genes were downregulated in a concentration-dependent manner. Later, cells were exposed to chemotherapeutic agent 5-fluorouracil (5-FU) in the presence of metformin. Metformin which is the most widely prescribed drug for treatment of type 2 diabetes has been shown to selectively target CSCs and enhance the efficacy of chemotherapy (Hirsch et al. 2009; Kim et al. 2014; Lee et al. 2014). Therefore, we tested the combinatorial effect of metformin and 5-FU in spheroids derived from MCF-7 cells. Figure 7 shows that MCF-7 spheroids were resistant to 5-FU at nearly all concentrations, whereas when spheroids were treated with both of the drugs, a dose-dependent cell killing activity could be observed.
Fig. 7.
5-FU response of MCF-7 spheroids. MCF-7 cells were cultured as spheroids. a RNA was extracted 2 and 15 days after spheroid formation. The relative gene expression levels of ABCC1, ABCC2 and ABCG2 genes were analyzed by qRT-PCR. GAPDH was used a reference gene and day 15 RNA samples were normalized against day 2 RNA samples. b To assess the effect of metformin on 5-FU response, spheroids were treated with metformin (1 mM) or 5-fluorouracil (0.1, 1, 10 mM) and viability was assessed by alamar blue assay 24 h after drug treatment. *p < 0.05 compared to naïve samples
Discussion
One of the ways to better mimic in vivo environment is the use of 3D culture systems. In this study, the effect of spheroid formation on CSC subpopulations has been investigated. Breast cancer (MCF-7) and glioma (U87-MG) cell lines cultured as monolayer or spheroid were compared in terms of expression of stem cell markers, cell migration and cell cycle profiles. In this study, we showed that MCF-7 cells were able to form much more uniform spheroids compared to U87-MG cells. Stem cell markers were upregulated at gene expression level in both cell lines. Among the five tested CSC subpopulations, formation of spheroids in MCF-7 cells increased the percentages of all CSCs compared to monolayer cultures. However, there was no upregulation of these CSC subpopulations in U87-MG spheroid cultures. These results indicate that spheroid formation provided a better microenvironment for the survival of CSC subpopulations in MCF-7 cells. These data were further supported by the enhanced migration capacities of MCF-7 cells.
In the literature, it has been reported that CSCs could be responsible for the drug resistance. Figure 7a showed that ABCC1, ABCC2 and ABCG2 genes which were known to be responsible for multiple drug resistance in cancer cells, were upregulated in cancer cells when spheroids were formed. Therefore, when 5-FU was used alone, spheroids were not responsive. However, when metformin was used to target CSCs through downregulating ABC transporters, the drug resistance could be eliminated, as can be seen from the concentration-dependent 5-FU drug response. These data prove that metformin can be used in combination with chemotherapeutic drugs to enhance drug efficacy. There are studies in the literature supporting these findings, however, our results and experimental design for the first time shows that 5-FU drug resistivity can be eliminated by metformin in MCF7 spheroids due to ABC transporter downregulation. In other studies, for example, Ramos Peñafiel et al. (2016) showed that the expression levels of multidrug-resistance genes were decreased in response to metformin. However, it was limited to only one cancer type and was not performed in spheroids. In another study by Li et al. (2018), metformin decreased the resistance of doxorubicin which is another chemotherapeutic drug on MCF7 cells cultured as monolayers. Therefore, as discussed above, our study is showing that the use of 3D culture systems leading to CSC proliferation could be a valuable tool to understand drug resistance. Furthermore, we believe it would be interesting to test different chemotherapeutic drugs including doxorubicin, docetaxel or tamoxifen which have been approved for breast cancer, to evaluate the effect of CSC populations during different therapeutic regimes.
In U87-MG cell line, even though there was an upregulation of stem cell markers at gene expression level, spheroid formation did not affect the tested CSC subpopulations. Considering that there are various identified CSC markers, and especially glioblastoma has been shown to involve CD133 + CSC subpopulations (Beier et al. 2007; Brescia et al. 2013; Wang et al. 2015). Therefore, future studies involving glioblastoma can focus on other CSC markers including CD133. Furthermore, apart from the 3D culture system, in the literature, different culture media and components have been suggested to result in higher levels of stem cell subpopulations while better recreating the tumor microenvironment (Bielecka et al. 2016).
Florcyzk et al. (2013) has reported that a 3D model involving scaffolds composed of chitosan and hyaluronic acid increased stem cell properties of glioblastoma. Similarly, epithelial to mesenchymal (EMT) transitions and CSC phenotypes (Nanog, SOX2, CD44, CD133, N-cadherin, and vimentin-positive cells) have been shown to increase in another 3D spheroid model for non-small cell lung cancer (Chang et al. 2013). In another study, MCF-7 cells cultured in 3D model were used to model cancer progression and assess anti-neoplastic drug resistance (Chen et al. 2012). The results showed that CSC properties were enhanced, EMT and stem cell markers were upregulated; however, only one CSC subpopulation has been considered and migration or proliferation capacities have not been discussed.
In summary, this study proved that in cancer cell lines where spheroid formation increases cancer stemness (as in the case of MCF-7 cells), the use of 3D culture systems could provide more information in CSC-related research. Future studies involving spheroid formation can help researchers to delineate the cancer stemness of other cancer types. It could help scientists to discover new CSC markers, show more realistic drug responses, and better evaluate cell proliferation and morphology. Therefore, 3D cultures should be preferred when trying to better understand tumor biology and develop novel therapeutic strategies for cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
AY acknowledges support by the Scientific and Technological Research Council of Turkey (TUBITAK, Grant Number 113S897).
Compliance with ethical standards
Conflict of interest
The author declares that there is no conflict of interest.
References
- Ashwell S, Zabludoff S. DNA damage detection and repair pathways—recent advances with inhibitors of checkpoint kinases in cancer therapy. Clin Can Res. 2008;14(13):4032–4037. doi: 10.1158/1078-0432.CCR-07-5138. [DOI] [PubMed] [Google Scholar]
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+)and CD133() glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Bielecka ZF, Maliszewska-Olejniczak K, Safir IJ, Szczylik C, Czarnecka AM. Three-dimensional cell culture model utilization in cancer stem cell research. Biol Rev Camb Philos Soc. 2016;92(3):1505–1520. doi: 10.1111/brv.12293. [DOI] [PubMed] [Google Scholar]
- Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G, Pelicci G. CD133 is essential for glioblastoma stem cell maintenance. Stem Cells. 2013;31(5):857–869. doi: 10.1002/stem.1317. [DOI] [PubMed] [Google Scholar]
- Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, Chen B, Dai J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012;33(5):1437–1444. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124(6):1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Cody NA, Ouellet V, Manderson EN, Quinn MC, Filali-Mouhim A, Tellis P, Zietarska M, Provencher DM, Mes-Masson AM, Chevrette M. Transfer of chromosome 3 fragments suppresses tumorigenicity of an ovarian cancer cell line monoallelic for chromosome 3p. Oncogene. 2007;26(4):618–632. doi: 10.1038/sj.onc.1209821. [DOI] [PubMed] [Google Scholar]
- David RM, Gooderham NJ. Using 3D MCF-7 mammary spheroids to assess the genotoxicity of mixtures of the food-derived carcinogens benzo[a]pyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Toxicol Res. 2016;5(1):312–317. doi: 10.1039/C5TX00343A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Economopoulou P, Kaklamani VG, Siziopikou K. The role of cancer stem cells in breast cancer initiation and progression: potential cancer stem cell-directed therapies. Oncologist. 2012;17(11):1394–1401. doi: 10.1634/theoncologist.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florczyk SJ, Wang K, Jana S, Wood DL, Sytsma SK, Sham JG, Kievit FM, Zhang M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials. 2013;34(38):10143–10150. doi: 10.1016/j.biomaterials.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Lin C, Cheng J, Su J, Zhao H, Liu T, Wen X, Zhao P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS One. 2015;10(6):e0130348. doi: 10.1371/journal.pone.0130348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15(9):1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69(19):7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kim TH, Suh DH, Kim M-K, Song YS. Metformin against cancer stem cells through the modulation of energy metabolism: special considerations on ovarian cancer. BioMed Res Int. 2014;2014:11. doi: 10.1155/2014/132702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan LM, Dodd NJF, Owen SF, Purcell WM, Jackson SK, Jha AN. Direct measurements of oxygen gradients in spheroid culture system using electron parametric resonance oximetry. PLoS One. 2016;11(2):e0149492. doi: 10.1371/journal.pone.0149492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park HJ, Park C-S, Oh E-T, Choi B-H, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One. 2014;9(2):e87979. doi: 10.1371/journal.pone.0087979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-L, Tian T, Nan K-J, Zhao N, Guo Y-H, Cui J, Wang J, Zhang W-G. Survival advantages of multicellular spheroids vs. monolayers of HepG2 cells in vitro. Oncol Rep. 2008;20(6):1465–1471. [PubMed] [Google Scholar]
- Li Y, Wang M, Zhi P, You J, Gao J-Q. Metformin synergistically suppress tumor growth with doxorubicin and reverse drug resistance by inhibiting the expression and function of P-glycoprotein in MCF7/ADR cells and xenograft models. Oncotarget. 2018;9(2):2158–2174. doi: 10.18632/oncotarget.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv D, Hu Z, Lu L, Lu H, Xu X. Three-dimensional cell culture: a powerful tool in tumor research and drug discovery. Oncology Lett. 2017;14(6):6999–7010. doi: 10.3892/ol.2017.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta LLC, Polyak K. Cancer stem cells: a model in the making. Curr Opin Genet Dev. 2009;19(1):44–50. doi: 10.1016/j.gde.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14(3):306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Peñafiel CO, Olarte Carrillo I, Cerón Maldonado R, Santoyo Sánchez A, Castellanos Sinco HB, Montaño Figueroa E, Queipo García G, Martínez Tovar A. Effect of the addition of metformin on the expression of multidrug-resistance genes (ABCB1, ABCG2) in acute lymphoblastic leukemia: in vitro and in vivo study. Blood. 2016;128(22):5142–5142. [Google Scholar]
- Rojas-Ríos P, González-Reyes A. Concise review: the plasticity of stem cell niches: a general property behind tissue homeostasis and repair. Stem Cells. 2014;32(4):852–859. doi: 10.1002/stem.1621. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Wiestler OD. Mechanisms of disease: cancer stem cells—targeting the evil twin. Nat Rev Clin Oncol. 2008;5(6):337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- Wang D, Guo Y, Li Y, Li W, Zheng X, Xia H, Mao Q. Detection of CD133 expression in U87 glioblastoma cells using a novel anti-CD133 monoclonal antibody. Oncol Lett. 2015;9(6):2603–2608. doi: 10.3892/ol.2015.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A, Tesei A. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016;6:19103. doi: 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.