Abstract
Purpose
Pneumonia is the third leading cause of death in children under 5 years of age worldwide. In pediatrics, both the accuracy and safety of diagnostic tools are important. Lung ultrasound (LUS) could be a safe diagnostic tool for this reason. We searched in the literature for diagnostic studies about LUS to predict pneumonia in pediatric patients using systematic review and meta-analysis.
Methods
The Medline, CINAHL, Cochrane Library, Embase, SPORTDiscus, ScienceDirect, and Web of Science databases from inception to September 2017 were searched. All studies that evaluated the diagnostic accuracy of LUS in determining the presence of pneumonia in patients under 18 years of age were included.
Results
1042 articles were found by systematic search. 76 articles were assessed for eligibility. Seventeen studies were included in the systematic review. We included 2612 pooled cases. The age of the pooled sample population ranged from 0 to about 21 years old. Summary sensitivity, specificity, and AUC were 0.94 (IQR: 0.89–0.97), 0.93 (IQR: 0.86–0.98), and 0.98 (IQR: 0.94–0.99), respectively. No agreement on reference standard was detected: nine studies used chest X-rays, while four studies considered the clinical diagnosis. Only one study used computed tomography.
Conclusions
LUS seems to be a promise tool for diagnosing pneumonia in children. However, the high heterogeneity found across the individual studies, and the absence of a reliable reference standard, make the finding questionable. More methodologically rigorous studies are needed.
Electronic supplementary material
The online version of this article (10.1007/s40477-018-0306-5) contains supplementary material, which is available to authorized users.
Keywords: Lung ultrasound, Pneumonia, Childhood, Pediatric, Diagnosis, Meta-analysis
Sommario
Obiettivo
La polmonite è la terza causa di morte nel mondo al di sotto dei 5 anni. In campo pediatrico, sia l’accuratezza che la sicurezza degli strumenti diagnostici sono importanti. In questo senso l’ecografia polmonare (LUS) potrebbe essere uno strumento diagnostico sicuro. Abbiamo analizzato la letteratura sull’accuratezza della LUS nel diagnosticare la polmonite nei pazienti pediatrici attraverso una revisione sistematica e una meta-analisi.
Metodi
Sono stati consultati i database Medline, CINAHL, Cochrane Library, Embase, SPORTDiscus, ScienceDirect e Web of Science dal loro avvio alla fine di settembre 2017. Sono stati inclusi tutti gli studi che hanno valutato l’accuratezza diagnostica della LUS nel determinare la presenza di polmonite nei pazienti di età inferiore ai 18 anni. 1.042 articoli sono stati trovati dalla ricerca sistematica. 76 articoli sono stati valutati per l’ammissibilità. 17 studi sono stati inclusi nella revisione sistematica.
Risultati
Abbiamo incluso 2.612 casi raggruppati. L’età della popolazione campione raggruppata varia da 0 a circa 21 anni. Sensibilità, specificità e AUC sono rispettivamente 0,94 (IQR: 0,89–0,97); 0,93 (IQR: 0,86–0,98) e 0,98 (IQR: 0,94–0,99). Non è stato rilevato accordo sullo standard di riferimento: 9 studi hanno usato la radiografia del torace; 4 studi hanno considerato la diagnosi clinica. Solo uno studio ha utilizzato la tomografia computerizzata.
Conclusioni
La LUS sembra essere uno strumento promettente per diagnosticare la polmonite nei bambini. Tuttavia l’elevata eterogeneità riscontrata nei singoli studi e l’assenza di uno standard di riferimento affidabile rendono discutibile il risultato. Sono necessari studi metodologicamente più rigorosi.
Introduction
Rationale
Of the 6 million infant deaths under 5 years of age which occur worldwide every year, about half of them are caused by infectious diseases [1]. Pneumonia, in particular, is one of the three major causes of death, along with intrapartum-related and birth weight complications [1]. Pneumonia is responsible for the deaths of about a million children under 5 years of age annually [2]. It still constitutes a significant health expenditure in developed countries, both in economic terms and in terms of the exposure of the pediatric population to antibiotic drugs [2]. In developing countries, improvement of the population’s nutrition conditions has led to a reduction of pneumonia-related mortality [1]. Although the incidence rate of pediatric pneumonia is declining, there are still issues related to diagnosis [3]. In fact, currently, there is no gold standard that can diagnose pneumonia with a high degree of accuracy in the pediatric population [4]. To avoid indiscriminate exposure to ionizing radiation as well as unavailability of advanced imaging devices in developing countries, computerized tomography (CT) is scarcely used for this purpose. On the other hand, the plain chest radiograph (CXR), which is often used as a silver standard, has shown little accuracy in distinguishing alveolar from interstitial pneumonia [5]; furthermore, inter-observer agreement on CXR results is at most moderate [6–8]. Lung ultrasound (LUS) has been shown to be a very accurate diagnostic tool in diagnosing pneumonia in adults [9, 10]. The LUS seems to be able to overcome the diagnostic limits of CXR [11–14]. In some studies, the LUS has been used in pediatric populations. The potential advantage of the LUS is to be a fast bedside imaging diagnostic tool which could preserve pediatric patients from exposure to ionizing radiation.
Objectives
We systematically reviewed studies in the literature addressing the role of the LUS in diagnosis of pneumonia in pediatric populations.
Methods
Eligibility criteria and information sources
We searched the Medline, CINAHL, Cochrane Library, Embase, SPORTDiscus, ScienceDirect, and Web of Science databases from inception to September 2017. The searched item consisted of terms related to pneumonia, ultrasound, and pediatrics ((“pneumonia” [MeSH Terms] OR “pneumonia” [All Fields]) AND (“ultrasound” [MeSH Terms] OR (“ultrasound” [All Fields]) AND (“pediatric” [MeSH Terms] OR “pediatric” [All Fields]) OR (“pediatric” [MeSH Terms] OR “pediatric” [All Fields]) OR (“childhood” [MeSH Terms] OR “childhood” [All Fields])).
All studies that evaluated the diagnostic accuracy of LUS in determining the presence of pneumonia in patients under 18 years of age were included. Studies in any hospital departments or settings were included. We included only published articles.
Conference abstracts, review articles, studies written in languages other than English, non-human studies, protocols or policy statements, and guidelines were excluded. We excluded studies that dealt with other diagnoses besides pneumonia.
Two authors (DO and AB) recovered the full texts of the relevant articles. All relevant titles and abstracts were retrieved and searched for the full text. References from included studies and review articles were hand-searched to identify any additional relevant studies not screened in the systematic search.
Study selection and risk of bias in individual studies
Two independent, trained reviewers (DO and AB) read all papers and scored them according to the QUADAS2 checklist [15]. Any disagreement was discussed between the two reviewers. If no agreement was reached even after the discussion, a third author (NG) was involved. An agreement between two out of three reviewers was considered sufficient to include the study under discussion. The studies which passed the reviewers’ quality selection were considered in the systematic review.
Data about publication year, sampling, target population, clinical setting, role and experience of the sonographer, reference standard device, considered diagnostic ultrasound pattern, sample size, prevalence of pneumonia, sensitivity, and specificity were extracted from studies.
Synthesis of results
A funnel plot was planned to investigate the presence of any publication bias. Sensitivity and specificity values of individual studies were summarized and compared through forest plots. Sub-analyses were conducted to verify any sources of heterogeneity. The diagnostic accuracy of LUS in predicting pneumonia in pediatric patients was planned to be summarized using the receiver operating characteristic (ROC) curve. Results were expressed as percentages and interquartile ranges between the 25 and 75% quartile (IQR).
All statistical analyses were performed using the R-CRAN project ver. 3.4.2. Meta4diag and INLA packages were implemented.
Results
Study selection
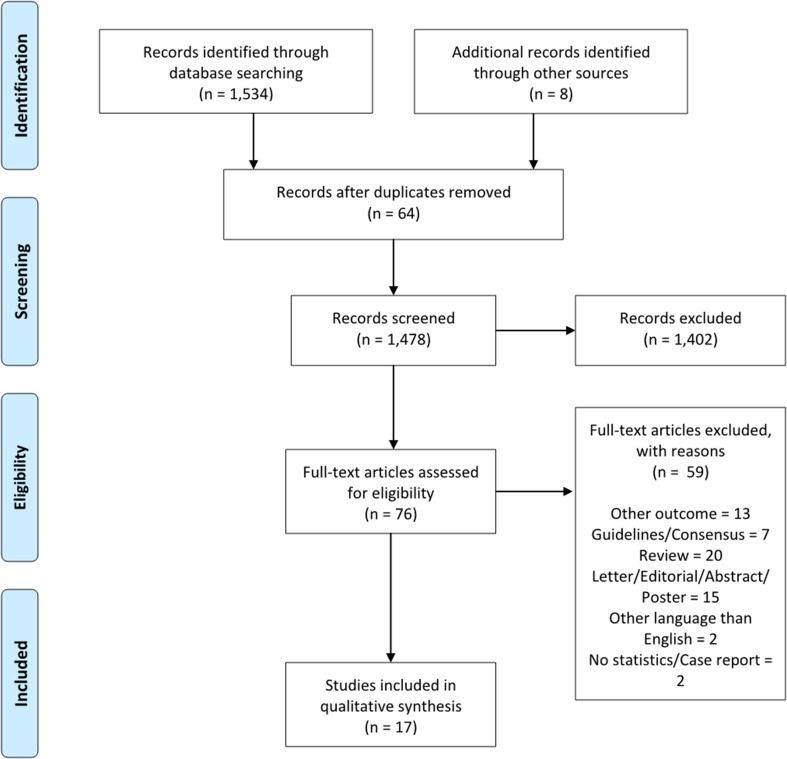
One thousand forty-two articles were found by systematic search. Of these, 64 were excluded because they were duplicated; 1402 were excluded because they did not fulfill the inclusion criteria. So, 76 articles were assessed for eligibility. Of these, 13 articles were excluded because they also examined other diagnoses than pneumonia alone; 7 articles were excluded because they were guidelines or consensus papers; 20 because they were review articles; 15 because they were letters, editorials, abstracts or conference posters; 2 because they were written in languages other than English; and 2 because they were case reports or because no statistics were reported. Finally, 17 studies were included in the systematic review [16–32] (Fig. 1).
Fig. 1.
PRISMA 2009 flow diagram for the article search and selection process
Study characteristics
2612 pooled cases were included. Sample sizes ranged from 36 patients in the study by Ambroggio and colleagues [21] to 812 in the study by Ellington and colleagues [17]. A wide age range was observed: one study considered only the neonatal population, while several studies also considered young adults (the upper limit was 21 years of age). Six studies were totally or at least partly conducted in a pediatric emergency department (PED); two studies took place in a general emergency department (ED); seven studies enrolled patients in a pediatric ward (PW); one study considered patients hospitalized in a pediatric intensive care unit (PICU); one study was performed in a neonatal intensive care unit (NICU). One study considered patients in a radiology department as well and one study included also outpatients. One study was carried out in a pediatric pneumology department. One study did not specify the setting in which it was carried out.
All studies except two enrolled patients prospectively. The inclusion criterion for all studies except one was the suspicion (or formal diagnosis in retrospective studies) of community-acquired pneumonia (CAP) or neonatal pneumonia. Only one study considered any respiratory symptoms.
The prevalence of pneumonia in the considered populations spread over a very wide range: from 18% in the study by Shah, Tunik and Tsung [30] to 96% in the study by Boursiani and colleagues [16].
Regarding the reference standard, the included studies considered a wide range of methods: nine studies used CXR as a reference standard, while four studies considered the attending physicians’ clinical diagnoses. In three studies, the final diagnoses were established by external adjudication committees. Only one study used CT as a reference standard.
In eight studies, the sonographer was a pediatrician or neonatologist; in two studies, he was an emergency physician; in five studies, he was a radiologist; and finally, in one study, the sonographer was a pediatric pneumologist. One study did not specify the sonographer’s role. The level of experience of the sonographers in the considered studies was rather variable: it ranged from a 1-h training course up to 25 years of professional experience.
In almost all the studies the sonographer was unaware of the results of the reference standard. There was no blindness at all just in one study. However, this parameter was not reported in 5 studies.
In all studies the chest posterior regions were also evaluated sonographically. Almost all studies considered consolidation (as a hypoechoic subpleural area) as a sonographic pattern of pneumonia, associated with some other patterns (e.g., B lines, interstitial pattern, and bronchograms) as well (Table 1).
Table 1.
Summary characteristics of the included studies
| ID | Study | Setting | Sampling | Age | Inclusion criteria | St. Ref. | Sonographer | Son. exp. | Blinding | Explored regions | Diagnostic criteria | Sample Size | Prevalence | TP | FP | FN | TN | Sens | Spec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Boursiani 2017 | PED | Prospective | 6–24 | Suspected CAP | Adjudication | Radiologist | 25 yy | Yes | Ant-Post | Consolidation/interstitial pattern | 69 | 0.96 | 62 | 0 | 4 | 3 | 0.92 | 1 |
| 2 | Ellington 2017 | PED. PW and OP | Prospective | 2–59 | Suspected CAP | CXR | Pediatrician | 7 dd | Yes | Ant-Post | Consolidation | 812 | 0.52 | 345 | 11 | 37 | 419 | 0.89 | 1 |
| 3 | Man 2017 | PW | Retrospective | Nr | Suspected CAP | CXR | Radiologist | Nr | Nr | Ant-Post | Consolidation | 81 | 0.89 | 57 | 5 | 15 | 4 | 0.79 | 0.44 |
| 4 | Yadav 2017 | PED/PW/Rad | Prospective | 2–59 | Suspected CAP | CXR | Radiologist | 10 dd | Yes | Ant-Post | Consolidation/bronchogram/focal B lines | 118 | 0.85 | 99 | 6 | 2 | 11 | 0.98 | 0.65 |
| 5 | Yilmaz 2017 | PED | Prospective | 1–216 | Suspected CAP | CXR | Pediatrician | > 100 exams | Yes | Ant-Post | Consolidation/bronchogram | 160 | 0.93 | 127 | 15 | 5 | 2 | 0.96 | 0.12 |
| 6 | Ambroggio 2016 | Nr | Prospective | 3–18 | Respiratory symptoms | CT | Radiologist | 1 h | Yes | Ant-Post | Consolidation/interstitial pattern | 36 | 0.34 | 7 | 6 | 5 | 18 | 0.63 | 0.75 |
| 7 | Claes 2016 | PW | Prospective | 0–192 | Suspected CAP | CXR | Radiologist | Nr | Nr | Ant-Post | Nr | 143 | 0.31 | 44 | 8 | 1 | 90 | 0.98 | 0.93 |
| 8 | Samson 2016 | PED | Prospective | 0–180 | Suspected CAP | CXR | Pediatrician | 3 h | Yes | Ant-Post | Consolidation/bronchogram | 200 | 0.43 | 74 | 6 | 11 | 109 | 0.87 | 0.95 |
| 9 | Zhan 2016 | PED | Prospective | 0–180 | Suspected CAP | CXR | Pediatrician | 3 dd | Yes | Ant-Post | Consolidation | 164 | 0.5 | 33 | 7 | 49 | 75 | 0.40 | 0.91 |
| 10 | Iorio 2015 | PW | Retrospective | Nr | CAP | Clin_diagn | Nr | Nr | Nr | Ant-Post | Consolidation/bronchogram | 52 | 0.56 | 28 | 1 | 1 | 22 | 0.97 | 0.96 |
| 11 | Urbankowska 2015 | Ped Pulm Dept | Prospective | 1–? | Suspected CAP | Clin_diagn | Pediatrician | Nr | No | Ant-Post | Consolidation/interstitial pattern | 106 | 0.71 | 71 | 0 | 5 | 30 | 0.98 | 0.95 |
| 12 | Esposito 2014 | Ped ICU | Prospective | 1–168 | Suspected CAP | CXR | Pediatrician | 7 h | Yes | Ant-Post | Consolidation/bronchogram/focal B lines | 103 | 0.47 | 47 | 3 | 1 | 52 | 0.98 | 0.95 |
| 13 | Liu 2014 | NICU | Prospective | 0–1 | Neonatal Pneumonia | Clin_diagn | Neonatologist | Nr | Nr | Ant-Post | Consolidation/interstitial pattern | 80 | 0.5 | 40 | 0 | 0 | 40 | 1 | 1 |
| 14 | Reali 2014 | PW | Prospective | 0–192 | Suspected CAP | Adjudication | Pulmonologist | > 100 exams | Yes | Ant-Post | Consolidation/interstitial pattern | 107 | 0.76 | 76 | 1 | 5 | 25 | 0.94 | 0.96 |
| 15 | Shah 2013 | ED | Prospective | 0–252 | Suspected CAP | CXR | EP | 1 h | Yes | Ant-Post | Consolidation/bronchogram/focal B lines | 200 | 0.18 | 31 | 18 | 5 | 146 | 0.86 | 0.89 |
| 16 | Caiulo 2012 | PW | Prospective | 12–192 | Suspected CAP | Adjudication | Pediatrician | Nr | Yes | Ant-Post | Consolidation/interstitial pattern | 102 | 0.87 | 88 | 0 | 1 | 13 | 0.99 | 1 |
| 17 | Copetti 2008 | ED | Prospective | 6–192 | Suspected CAP | Clin_diagn | EP | Nr | Nr | Ant-Post | Consolidation/bronchogram/focal B lines | 79 | 0.76 | 60 | 0 | 0 | 19 | 1 | 1 |
PED Pediatric Emergency Department, PW pediatric ward, OP outpatients, Rad radiology department, ED emergency department, ICU Intensive Care Unit, NICU Neonatal Intensive Care Unit, nr not reported. Age: in months. CAP community-acquired pneumonia, St. Ref. Standard Reference, CXR chest X-ray, CT computed tomography, EP emergency physician, yy years, dd days, hh hours, TP true positives, FP false positives, FN false negatives, TN true negatives, Sens sensitivity, Spec specificity
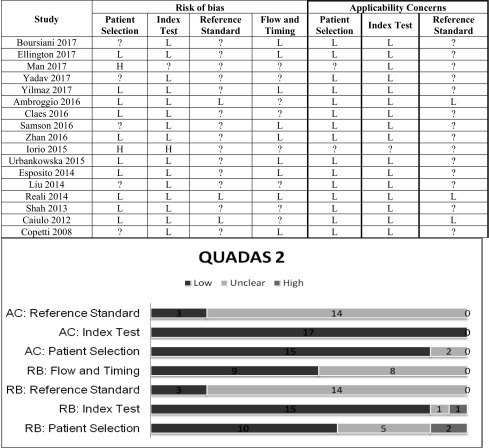
Risk of bias within studies
The retrospective selection of patients was judged to be at a high risk of bias. Four studies considered a convenience sample: we felt uncertain about the risk of bias [16, 23, 28, 32]. Most studies used CXR as a reference standard. We are not sure that it is the most accurate imaging method against which to compare LUS. We therefore considered these studies’ risk of bias to be uncertain. The clinical diagnosis of the attending physicians as the only reference standard was considered a potential source of bias. Some studies did not specify whether LUS was performed before or after other imaging techniques. Some studies considered LUS within a 24- up to 36-h period from the first patient evaluation. This wide range of time could be a source of bias.
With respect to applicability concerns, diagnosis by CXR or by the clinical evaluation of attending physicians alone could be a bias source (Table 2).
Table 2.
QUADAS 2 results for the risk of bias and applicability
L low risk; ? Uncertain risk, H high risk
Synthesis of results
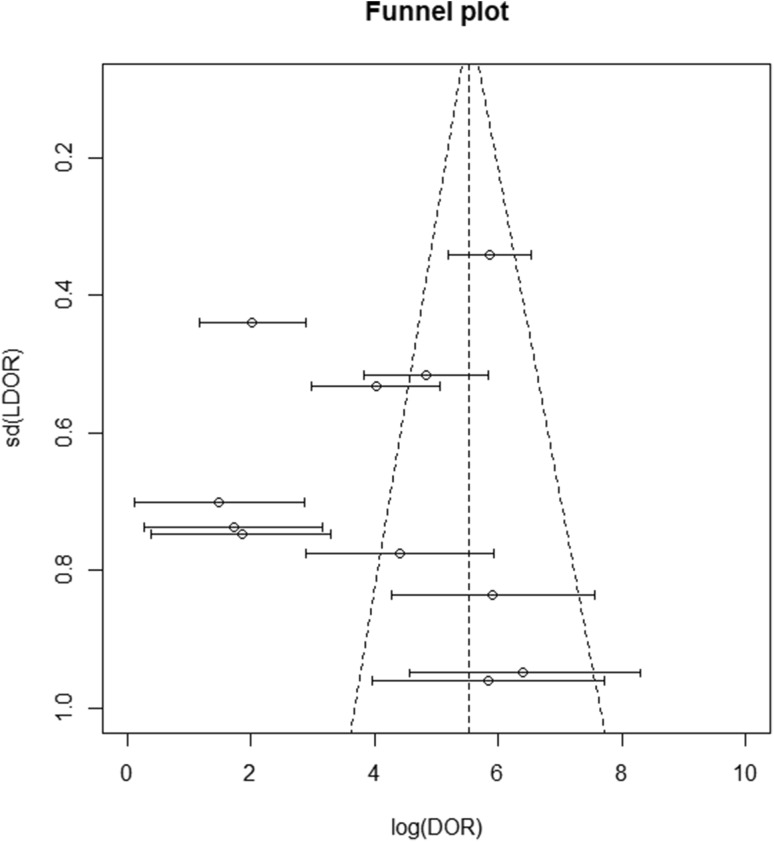
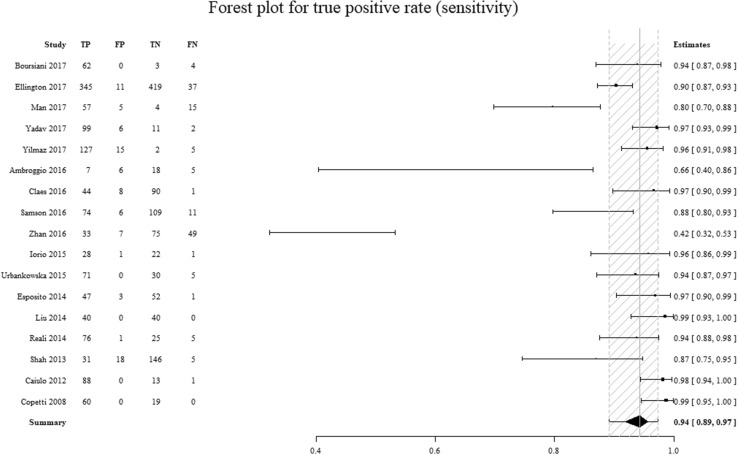
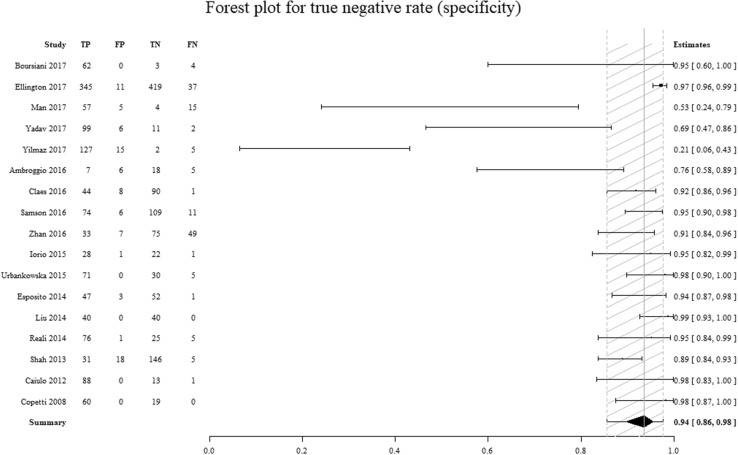
The sensitivity and specificity of the considered studies are displayed in Table 1. The individual studies’ sensitivity ranged from 40 to 100% (sensitivity summary: 94%; IQR: 89–97%) and specificity ranged from 12 to 100% (specificity summary: 94%; IQR: 86–98%). The funnel plot shows that studies with a small sample size achieved a considerably better effect than studies with a wider sample size, in terms of a possible publication bias (Fig. 2).
Fig. 2.
Funnel plot for the studies regarding diagnostic accuracy of LUS in predicting pneumonia. A scatter plot of the effect estimates from individual studies against LDOR. Standard deviation is plotted on the vertical axis. The effects estimated by smaller studies spread lower on the bottom, while larger studies are distributed along the narrower, triangular upper part
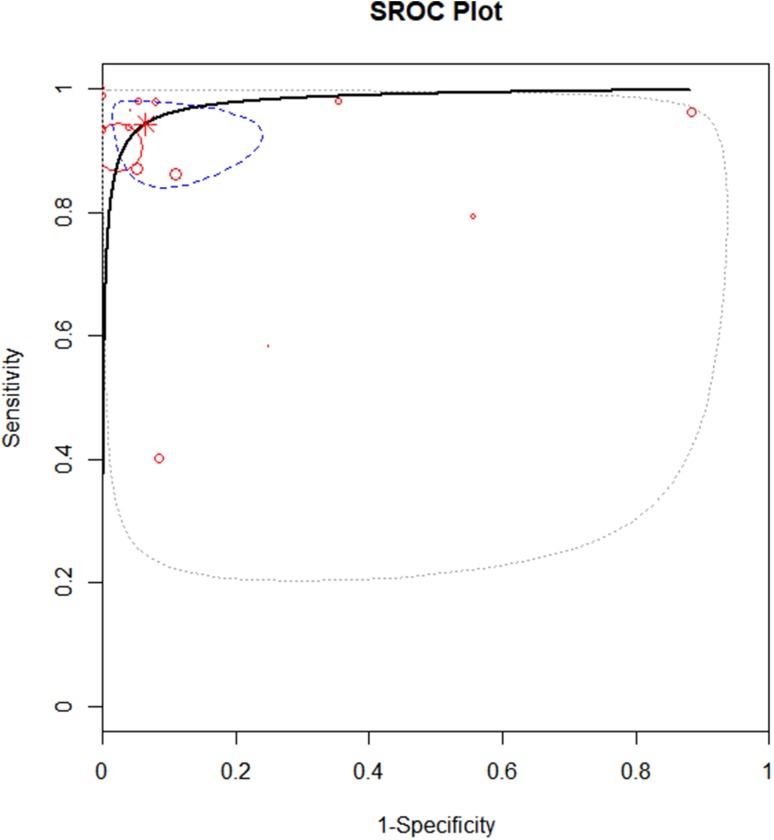
The overall diagnostic accuracy of LUS was expressed as a summary AUC of 0.98 (IQR: 0.94–0.99) (Fig. 3).
Fig. 3.
Summary ROC curve of LUS in diagnosing pneumonia in childhood. Every circle represents a study, the sample size of which is proportional to the circle. The dashed line represents IQR between 25 and 75%. Summary AUC = 0.98; IQR: 0.94–0.99
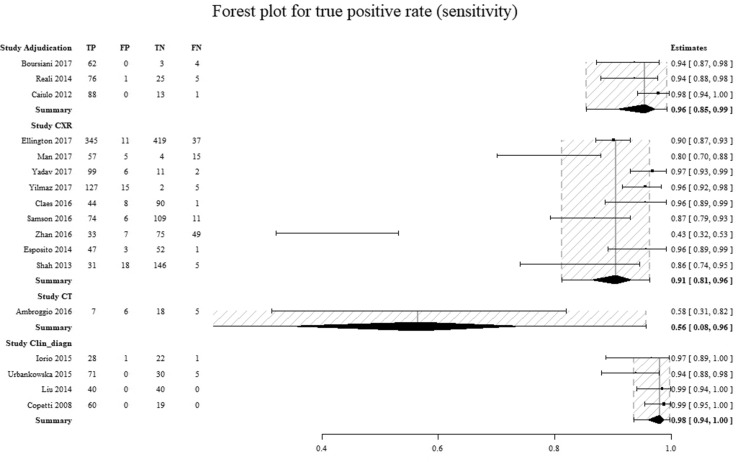
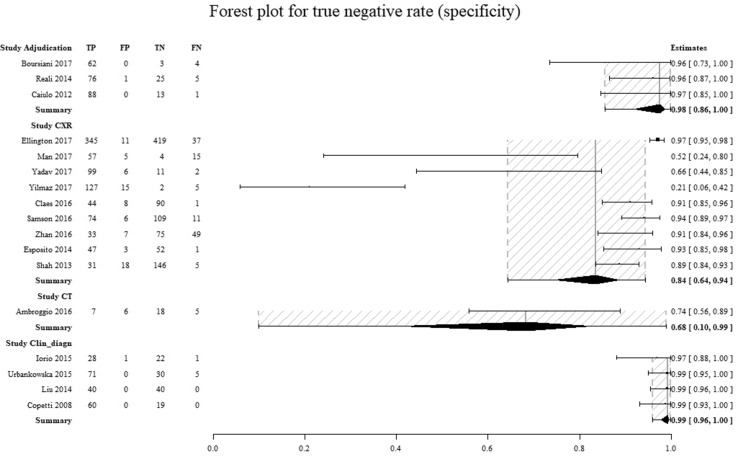
To determine the components most responsible for the high degree of heterogeneity found, the data were analyzed with forest plots (Figs. 4, 5 and Figures 8 and 9 in Supplemental Materials). A significant difference between the amount of specificity in the group in which the CXR was used as the reference standard and the group in which it was considered to be only the clinical diagnosis by the attending physician was found, as highlighted by the non-overlapped confidence intervals (Figs. 6, 7).
Fig. 4.
Comparison of the sensitivity values for individual studies. On the left column are the individual studies. On the right column is the sensitivity (95% CI). TP true positives, FP false positives, TN true negatives, FN false negatives. The dashed area represents the 95% confidence interval
Fig. 5.
Comparison of the specificity values for individual studies. On the left column are the individual studies. On the right column is the specificity (95% CI). TP true positives, FP false positives, TN true negatives, FN false negatives. The dashed area represents the 95% confidence interval
Fig. 6.
Comparison of the sensitivity for individual studies for every reference standard group. On the left column are the individual studies. On the right column is the sensitivity (95% CI). Adjudication diagnosis by an adjudication committee, CXR diagnosis by chest x-ray, CT diagnosis by computed tomography, Clin_diagn diagnosis by clinical evaluation of the attending physician(s), TP true positives, FP false positives, TN true negatives, FN false negatives. The dashed area represents the 95% confidence interval
Fig. 7.
Comparison of the specificity for individual studies for every reference standard group. On the left column are the individual studies. On the right column is the specificity (95% CI). Adjudication diagnosis by an adjudication committee, CXR diagnosis by chest x-ray, CT diagnosis by computed tomography, Clin_diagn diagnosis by clinical evaluation of the attending physician(s), TP true positives, FP false positives, TN true negatives, FN false negatives. The dashed area represents the 95% confidence interval
Discussion
Summary of evidence
Although pneumonia remains a major cause of childhood morbidity and mortality, there is no clinical sign or symptom capable of diagnosing it alone, at least without the concurrence of some imaging tools [3]. In adult patients’ studies, CT showed a high degree of accuracy as a gold standard for imaging in case of pneumonia. However, there is no justification for indiscriminately exposing a pediatric patient to ionizing radiation, unless there are clinical reasons such as respiratory complications [33, 34]. Currently, the most commonly used imaging exam is standard CXR. CXR is a relatively inexpensive and sufficiently quick test. These two features are particularly useful in the evaluation of pediatric patients, both in the emergency department and in a hospital ward. However, CXR shows a non-optimal ability to distinguish alveolar pneumonia from interstitial pneumonia (a sensitivity of about 43%); it generates ionizing radiation as well and, moreover, inter-observer agreement is not perfect [7, 35–38]. So, an inexpensive, accurate and fast (possibly bedside) diagnostic tool that can be used for this purpose is needed. LUS, at least in adult patients, has proven to possess all these features [9, 10].
LUS is still poorly studied in pediatric medicine, compared to the adult setting. Pereda and colleagues noted that the literature contains few studies about LUS in pediatric populations. However, from the data collected, the authors concluded that LUS seemed to be a sufficiently accurate technique to be used in diagnosing pneumonia, even in pediatric patients [39]. Even from our findings, LUS shows a fair level of accuracy despite the different ages considered in the included studies. In this regard, we must note that there are still few studies on neonatal populations in the literature. In any case, there seems to have been a fairly high degree of diagnostic accuracy in many prospective studies.
Nevertheless, a possible source of bias could be the extreme range of the sonographer’s experience. Adult population studies show that LUS can be learned quickly; however, a minimal learning curve seems to be necessary [40]. This aspect does not seem sufficiently considered in the current pediatric literature. A recent systematic review noted that the sonographer’s experience, as well as the clinical severity of pneumonia in the studied population, was among the factors most responsible for a considerable share of heterogeneity [41].
As far as we could analyze, the main source of bias seems to be the choice of reference standard. While it is unethical to use CT just for methodological reasons, especially without any clinical reason, both standard CXR and exclusively clinical evaluation by attending physicians, on the other hand, do not seem to be sufficiently accurate methods [42, 43]. While the use of CXR as a reference standard seems to determine an excessive rate of false positives, a mere clinical evaluation of the attending physician, on the contrary, seems to determine an excessive rate of true positives. This effect could be linked, on the one hand, to the CXR’s lack of sensitivity, as already highlighted in the literature, and, on the other hand, to a suspected confirmation bias. As with the diagnosis of sepsis (although for other reasons), there is no gold standard to compare to any other diagnostic technique [44], even in this case. In any case, a possible solution could be that the final diagnosis is established by one or more external physicians not involved in the clinical case. These physicians could assess, using all findings and available data (and possibly microbiological results), the probability of diagnosis of pneumonia in pediatric patients [45, 46].
Limitations
Our review had some limitations. First, only studies that presented the diagnostic accuracy of LUS were considered, rather than those which contained any agreement with other imaging techniques. Furthermore, a meta-analysis was difficult to execute due to the high heterogeneity found. We did not consider our findings totally reliable, given the controversies related to the choice of the studied population, the extreme range of the sonographers’ experience, and, above all, the absence of a real gold standard. Confirming our findings, a recent meta-analysis showed a high rate of heterogeneity [41].
Conclusions
LUS seemed to be a promising method to diagnose pneumonia in the pediatric population; however, the high heterogeneity found across the individual studies and the absence of a reliable reference standard make the pooled results questionable. More methodologically rigorous studies are needed. More stringent criteria are also required for the reference standard and the diagnostic definition of pneumonia through LUS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 8 Comparison of the sensitivity for individual studies for every sampling method. On the left column are the individual studies. On the right column is the sensitivity (95%CI). Prospective = prospective enrolment; Retrospective = retrospective enrolment; TP = true positives; FP = false positives; TN = true negatives; FN = false negatives. The dashed area represents the 95% confidence interval (JPEG 224 kb)
Figure 9 Comparison of the specificity for individual studies for every sampling method. On the left column are the individual studies. On the right column is the specificity (95%CI). Prospective prospective enrolment; Retrospective retrospective enrolment; TP true positives; FP false positives; TN true negatives; FN false negatives. The dashed area represents the 95% confidence interval (JPEG 220 kb)
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SN, Bachur RG, Simel DL, Neuman MI. Does this child have pneumonia? The rational clinical examination systematic review. JAMA. 2017;318:461–471. doi: 10.1001/jama.2017.9039. [DOI] [PubMed] [Google Scholar]
- 4.Lynch T, Bialy L, Kellner JD, Osmond MH, Klassen TP, Durec T, Leicht R, Johnson DW. A systematic review on the diagnosis of pediatric bacterial pneumonia: when gold is bronze. PLoS One. 2010;5:e11989. doi: 10.1371/journal.pone.0011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Shimol S, Dagan R, Givon-Lavi N, Tal A, Aviram M, Bar-Ziv J, Zodicov V, Greenberg D. Evaluation of the World Health Organization criteria for chest radiographs for pneumonia diagnosis in children. Eur J Pediatr. 2012;171:369–374. doi: 10.1007/s00431-011-1543-1. [DOI] [PubMed] [Google Scholar]
- 6.Williams GJ, Macaskill P, Kerr M, Fitzgerald DA, Isaacs D, Codarini M, McCaskill M, Prelog K, Craig JC. Variability and accuracy in interpretation of consolidation on chest radiography for diagnosing pneumonia in children under 5 years of age. Pediatr Pulmonol. 2013;48:1195–1200. doi: 10.1002/ppul.22806. [DOI] [PubMed] [Google Scholar]
- 7.Levinsky Y, Mimouni FB, Fisher D, Ehrlichman M. Chest radiography of acute paediatric lower respiratory infections: experience versus interobserver variation. Acta Paediatr. 2013;102:e310–e314. doi: 10.1111/apa.12249. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J, Kline JA. Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs. Emerg Radiol. 2010;17:285–290. doi: 10.1007/s10140-009-0854-2. [DOI] [PubMed] [Google Scholar]
- 9.Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Accuracy of lung ultrasound in the diagnosis of pneumonia in adults: systematic review and meta-analysis. Chest. 2017;151:374–382. doi: 10.1016/j.chest.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9:6. doi: 10.1186/s13089-017-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testa A, Soldati G, Copetti R, Giannuzzi R, Portale G, Gentiloni-Silveri N. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012;16:R30. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reissig A, Copetti R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration. 2014;87:179–189. doi: 10.1159/000357449. [DOI] [PubMed] [Google Scholar]
- 13.Asano M, Watanabe H, Sato K, Okuda Y, Sakamoto S, Hasegawa Y, Sudo K, Takeda M, Sano M, Kibira S, Ito H (2017) Validity of ultrasound lung comets for assessment of the severity of interstitial pneumonia. J Ultrasound Med. 10.1002/jum.14497(Epub ahead of print) [DOI] [PubMed]
- 14.Perrone T, Quaglia F. Lung US features of severe interstitial pneumonia: case report and review of the literature. J Ultrasound. 2017;20:247–249. doi: 10.1007/s40477-017-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Boosuyt PM, Quadas-2 Group QUADAS-2: a revised tool for the qualify assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Boursiani C, Tsolia M, Koumanidou C, Malagari A, Vakaki M, Karapostolakis G, Mazioti A, Alexopoulou E. Lung ultrasound as first-line examination for the diagnosis of community-acquired pneumonia in children. Pediatr Emer Care. 2017;33:62–66. doi: 10.1097/PEC.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 17.Ellington LE, Gilman RH, Chavez MA, Pervaiz F, Marin-Concha J, Compen-Chang P, Riedel S, Rodriguez SJ, Gaydos C, Hardick J, Tielsch JM, Steinhoff M, Benson J, May EA, Figueroa-Quintanilla D, Checkley W, Lung Ultrasound for Pneumonia Assessment (LUPA) Study Investigators Lung ultrasound as a diagnostic tool for radiographically-confirmed pneumonia in low resource settings. Respir Med. 2017;128:57–64. doi: 10.1016/j.rmed.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man SC, Fufezan O, Sas V, Schnell C. Performance of lung ultrasonography for the diagnosis of community-acquired pneumonia in hospitalized children. Med Ultrason. 2017;19:276–281. doi: 10.11152/mu-1027. [DOI] [PubMed] [Google Scholar]
- 19.Yadav KK, Awasthi S, Parihar A. Lung ultrasound is comparable with chest roentgenogram for diagnosis of community-acquired pneumonia in hospitalised children. Indian J Pediatr. 2017;84:499–504. doi: 10.1007/s12098-017-2333-1. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz HL, Özkaya AK, Gökay SS, Kendir ÖT, Şenol H. Point-of-care lung ultrasound in children with community acquired pneumonia. Am J Emer Med. 2017;35:964–969. doi: 10.1016/j.ajem.2017.01.065. [DOI] [PubMed] [Google Scholar]
- 21.Ambroggio L, Sucharew H, Rattan MS, O’Hara SM, Babcock DS, Clohessy C, Steinhoff MC, Macaluso M, Shah SS, Coley BD. Lung ultrasonography: a viable alternative to chest radiography in children with suspected pneumonia? J Pediatr. 2016;176:93–98. doi: 10.1016/j.jpeds.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Claes AS, Clapuyt P, Menten R, Michoux N, Dumitriu D. Performance of chest ultrasound in pediatric pneumonia. Eur J Radiol. 2017;88:82–87. doi: 10.1016/j.ejrad.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 23.Samson F, Gorostiza I, Gonzalez A, Landa M, Ruiz L, Grau M. Prospective evaluation of clinical lung ultrasonography in the diagnosis of community-acquired pneumonia in a pediatric emergency department. Eur J Emer Med. 2018;25:65–70. doi: 10.1097/MEJ.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 24.Zhan C, Grundtvig N, Klug BH. Performance of bedside lung ultrasound by a pediatric resident: a useful diagnostic tool in children with suspected pneumonia. Pediatr Emer Care. 2016 doi: 10.1097/PEC.0000000000000888. [DOI] [PubMed] [Google Scholar]
- 25.Iorio G, Capasso M, De Luca G, Prisco S, Mancusi C, Laganà B, Comune V. Lung ultrasound in the diagnosis of pneumonia in children: proposal for a new diagnostic algorithm. Peer J. 2015;3:e1374. doi: 10.7717/peerj.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbankowska E, Krenke K, Drobczyński L, Korczyński P, Urbankowski T, Krawiec M, Kraj G, Brzewski M, Kulus M. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir Med. 2015;109:1207–1212. doi: 10.1016/j.rmed.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Esposito S, Sferrazza Papa S, Borzani I, Pinzani R, Giannitto C, Consonni D, Principi N. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital J Pediatr. 2014;40:37. doi: 10.1186/1824-7288-40-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Liu F, Liu Y, Wang HW, Feng ZC. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. 2014;146:383–388. doi: 10.1378/chest.13-2852. [DOI] [PubMed] [Google Scholar]
- 29.Reali F, Sferrazza Papa GF, Carlucci P, Fracasso P, Di Marco F, Mandelli M, Soldi S, Riva E, Centanni S. Can lung ultrasound replace chest radiography for the diagnosis of pneumonia in hospitalized children? Respiration. 2014;88:112–115. doi: 10.1159/000362692. [DOI] [PubMed] [Google Scholar]
- 30.Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167:119–125. doi: 10.1001/2013.jamapediatrics.107. [DOI] [PubMed] [Google Scholar]
- 31.Caiulo VA, Gargani L, Caiulo S, Fisicaro A, Moramarco F, Latini G, Picano E, Mele G. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatr Pulmonol. 2013;48:280–287. doi: 10.1002/ppul.22585. [DOI] [PubMed] [Google Scholar]
- 32.Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol Med. 2008;113:190–198. doi: 10.1007/s11547-008-0247-8. [DOI] [PubMed] [Google Scholar]
- 33.Bradley JS, Byngton CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH jr, Moore MR, St Peter SD, Stockwell Ja, Swanson JT; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A; British Thoracic Society Standard of Care Committee (2011) British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66 Suppl 6: ii1-23 [DOI] [PubMed]
- 35.Nascimento-Carvalho CM, Araùio-Neto CA, Ruuskanen O. Association between bacterial infection and radiologically confirmed pneumonia among children. Pediatr Infect Dis J. 2015;34:490–493. doi: 10.1097/INF.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 36.Self WH, Courtney DM, McNaughton CD, Wunderink RG, Kline JA (2014) High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 31:401–405 [DOI] [PMC free article] [PubMed]
- 37.Maughan BC, Asseling N, Carey JL, Sucov A, Valente JH. False-negative chest radiographs in emergency department diagnosis of pneumonia. R I Med J. 2013;97:20–23. [PubMed] [Google Scholar]
- 38.Simbalista R, Andrade DC, Borges IC, Araùjo M, Nascimento-Carvalho CM. Diferrences upon admission and in hospital course of children hospitalized with community-acquired pneumonia with or without radiologically-confirmed pneumonia: a retrospective color study. BMC Pediatr. 2015;15:166. doi: 10.1186/s12887-015-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereda MA, Chavez MA, Hooper-Miele CC, Gilman RH, Steinhoff MC, Ellington LE, Gross M, Price C, Tielsch JM, Checkley W. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714–722. doi: 10.1542/peds.2014-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackstock U, Munson J, Szyld D. Bedside ultrasound curriculum for medical students: report a blended learning curriculum implementation and validation. J Clin Ultrasound. 2015;43:139–144. doi: 10.1002/jcu.22224. [DOI] [PubMed] [Google Scholar]
- 41.Xin H, Li J, Hu HY (2017) Is lung ultrasound useful for diagnosing pneumonia in children?: a meta-analysis and systemic review. Ultrasound Q 10.1097/ruq.0000000000000330(Epub ahead of print) [DOI] [PubMed]
- 42.Wingerter SL, Bachur RG, Monuetaux MC, Neuman MI. Application of the World Health Organization criteria to predict radiograph pneumonia in a US-based pediatric emergency department. Pediatr Infect Dis J. 2012;31:561–564. doi: 10.1097/INF.0b013e31824da716. [DOI] [PubMed] [Google Scholar]
- 43.O’Grady KAF, Torzillo PJ, Ruben AR, Taylor-Thomson D, Valery PC, Chang AB. Identification of radiological alveolar pneumonia in children with high rates of hospitalized respiratory infections: comparison of WHO-defined and pediatric pulmonologist diagnosis in the clinical context. Pediatr Pulmonol. 2012;47:386–392. doi: 10.1002/ppul.21551. [DOI] [PubMed] [Google Scholar]
- 44.Mearelli F, Orso D, Fiotti N, Altamura N, Breglia A, De Nardo M, Paoli I, Zanetti M, Casarsa C, Biolo G. Sepsis outside intensive care unit: the other side of the coin. Infection. 2015;43:1–11. doi: 10.1007/s15010-014-0673-6. [DOI] [PubMed] [Google Scholar]
- 45.Bedford Russell AR, Kumar R. Early onset neonatal sepsis: diagnostic dilemmas and practical management. Arch Dis Child Fetal Neonatal Ed. 2015;100:F350–F354. doi: 10.1136/archdischild-2014-306193. [DOI] [PubMed] [Google Scholar]
- 46.Neuman MI, Monuteaux MC, Scully KJ, Bachur RG. Prediction of pneumonia in a pediatric emergency department. Pediatrics. 2011;128:246–253. doi: 10.1542/peds.2010-3367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 8 Comparison of the sensitivity for individual studies for every sampling method. On the left column are the individual studies. On the right column is the sensitivity (95%CI). Prospective = prospective enrolment; Retrospective = retrospective enrolment; TP = true positives; FP = false positives; TN = true negatives; FN = false negatives. The dashed area represents the 95% confidence interval (JPEG 224 kb)
Figure 9 Comparison of the specificity for individual studies for every sampling method. On the left column are the individual studies. On the right column is the specificity (95%CI). Prospective prospective enrolment; Retrospective retrospective enrolment; TP true positives; FP false positives; TN true negatives; FN false negatives. The dashed area represents the 95% confidence interval (JPEG 220 kb)