Abstract
Immunoglobulin class-switch recombination (CSR) gives rise to looped-out circular DNA of a cleaved S segment, which is lost eventually after cell divisions. To understand the molecular mechanism of S region cleavage during CSR, we constructed artificial CSR substrates in which inversion-type CSR takes place to retain the cleaved S segment. Sequencing analyses of recombinant clones of these substrates revealed that varying degrees of deletions and duplications exist at CSR breakpoints, suggesting the involvement of staggered cleavage of the S region in CSR. In addition, mutations frequently found near junctions showed a similar profile of base replacement to Ig somatic hypermutation. These findings suggest that single-strand tails of staggered cleavage may be repaired by error-prone DNA synthesis.
Newly generated B cells expressing IgM and IgD migrate to peripheral lymphoid organs where they are activated by encounters with antigens, resulting in the clonal expansion of antigen-specific B cells. Activated B cell clones secrete not only IgM but also other isotypes of Ig molecules without altering the antigen specificity. This phenomenon, termed class switching, is mediated by DNA recombination in the Ig heavy (H) chain constant (C) region locus, which results in expression of a new transcription unit containing the same productively rearranged variable region (VH) gene with a downstream CH gene other than Cμ.
Class-switch recombination (CSR) from IgM to another isotype takes place anywhere in several-kilobase G-rich regions, termed switch (S) regions, which are located 5′ to each set of CH exons and consist of tandemly repetitive short sequences with many palindromes (1–9). CSR is accompanied by a looping-out deletion of intervening DNA sequences (10–12). Only limited sequence homology is found near the site of recombination between Sμ and other S sequences (13–15). Because the junctions of CSR are scattered over the S regions and in some instances in proximity to but outside the S regions, CSR is a region-specific recombination event.
It has been well established that the ability of a given cytokine to determine the target specificity of CSR is correlated with transcription induction (germline transcription) from a specific intron promoter located immediately upstream of each S region (16). The germline transcription is believed to regulate chromatin opening (accessibility) of the target S region to determine the isotype specificity of CSR (17, 18). Efficiency of CSR seems to be correlated quantitatively with the activity of germline transcription (19). In addition, subsequent splicing of the CSR germline transcripts seems important for CSR (20, 21), but the exact role of germline transcripts per se in CSR remains to be elucidated.
Previously, we described an artificial CSR substrate in which each of two S regions was directed by a different constitutive promoter, and transcripts were spliced (22). This study demonstrated that the isotype specificity of CSR was not determined by the nucleotide sequences of S regions. Subsequently, we also showed that inversion-type rather than deletion-type CSR takes place when the transcriptional orientations of two S regions are opposite, suggesting that the machineries for transcription and recombination may be coupled in CSR (23, 24). A recent report of genomic organization of chicken Ig heavy chain genes revealed that the transcriptional orientation of the Cα gene is inverted relative to the VH and Cμ genes, suggesting that inversion-type recombination probably occurs during IgA switching in chicken B cells (25).
More recently, activation-induced cytidine deaminase (AID) with potential RNA-editing activity (26) was found to be required for both CSR and somatic hypermutation (SHM; refs. 27 and 28). AID deficiency in mouse and human abolished CSR at the DNA level but did not affect germline transcription of S regions and V(D)J recombination. Because the universal nonhomologous end-joining mechanism is responsible for the repair of cleaved DNA ends in CSR as well as V(D)J recombination (29–31), AID may act at a step preceding nonhomologous end-joining repair, most likely the cleavage phase of CSR through mRNA editing (27, 28). The AID-regulated DNA cleavage is suspected in CSR as well as in SHM (32)
Despite these important insights into the molecular mechanism for CSR, it remains unknown how a particular S region DNA is recognized and cleaved by the CSR recombinase machinery. Although analysis of the total recombination products after CSR should provide some clues for the cleavage mechanism, the sequence alteration in the S regions at CSR junction sites has never been assessed, because the DNA sequence between the donor and acceptor S regions is deleted by looping out and inevitably lost after cell division (10–12).
To overcome this problem, we constructed the CSR substrates SCR2(μ,α) and SCR2(μ,Rα), which allowed us to detect efficiently an inversion-type CSR by surface expression of CD8α-green fluorescent protein (GFP) fusion protein. The advantage of the inversion-type substrate is that a pair of recombination junctions in S regions generated by a single CSR event is identifiable on the transgene substrate. The sequence analysis of 82 switch junctions revealed deletions and duplications with variable lengths exclusively at the switch junction ends. At least five junctions contained duplications of 9–266 bp, suggesting the occurrence of the staggered cleavage in double strands of the S region DNA. The single-strand tails of staggered cleavage ends may be processed by the chewing and/or fill-in, giving rise to deletion or duplication at junction ends in the S region after CSR.
Methods
Construction of Inversion-Type Class-Switch Substrates and Transfectants.
The extracellular (EC) portion and flanking introns of the mouse CD8α gene (33) were amplified by PCR from genomic DNA of CH12F3-2 cells using the primers 5′-CCT AGA GCC CTA GCT TGA CCT AAG CTG C-3′ and 5′-TTG TCG ACC CCA GGC TAT CTG CTT ATC-3′. This amplified EC portion of CD8α was linked to the splice acceptor signal (SS) from an expression vector, pcDL-SRα (34), on pGEM-T vector (Promega). The EC-SS fragment was then integrated into pEF-BOS (35), an expression vector with a promoter from the human EF-1α gene. From the resulting plasmid, a fragment containing the entire transcription unit was excised as BOS-EC-SS. The CD8α transmembrane (TM) exon with 5′ intron was amplified by genomic PCR using the primers 5′-TGG TCG ACT CCT GAT CTT GGA GGG AGA C-3′ and 5′-CTG GAT CCC TGT GGT AGC AGA TGA GAG TGA-3′. The GFP coding sequence was amplified by PCR using pEGFP-N1 (CLONTECH) as a template with the primers 5′-CGG GAT CCA CCG GTC GCC ACC GTG GTG-3′ and 5′-GTC GCG GCC GCT TTA CTT GTA CAG CTC GTC CAT GC-3′. This PCR was designed to introduce a replacement of the first methionine of GFP with valine to eliminate expression of GFP before CSR. The mutated GFP sequence was cloned in the pGEM-T vector, and the amplified TM exon was inserted in-frame upstream of GFP sequence. The TM-GFP sequence was excised and inserted with modified pcDL-SRα devoid of the splice acceptor signal. Transfection of COS7 cells with this plasmid resulted in expression of GFP despite the introduced mutation. This plasmid was digested with restriction enzymes to generate a fragment containing the whole transcription unit as SRα-TM-GFP. The BOS-EC-SS fragment and the SRα-TM-GFP were connected on a plasmid with the neomycin-resistance gene cassette pSMC-11 (22), which is derived from pMC1neopolyA (Stratagene). The recombinants with the two transcription units in the opposite directions were designated as pSCR(0,0). A stuffer fragment containing the splice acceptor sequence of exon 7 of the human IL-2Rα gene (36) was amplified from genomic DNA of Ramos cells by using the primers 5′-TAC ATC GAT TCC TGC TGC CCC CAT GCC AAG-3′ and 5′-AGC TTC GAA TTT ATT ACT GTC TCC GCT GCC AGG TGA G-3′ was inserted into the intron of the SRα transcription unit of pSCR(0,0) in the reverse orientation with respect to the SRα promoter to generate pSCR2(0,0).
A single copy of FSμ-4, a 1.3-kb fragment of the mouse Sμ core sequence (23), was integrated into the intron between EC and SS exons of pSCR2(0,0) to give rise to pSCR2(μ,0). The final pSCR2(μ,α) and pSCR2(μ,Rα) were generated by integrating a single copy of FSα-2, a 1.2-kb fragment of mouse Sα core sequence (22), into the intron of the SRα transcription unit of pSCR2(μ,0) in the same and opposite orientation, respectively. Sequences of FSμ-4 and FSα-2 are shown in Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org.
The linearized DNA constructs were introduced into CH12F3-2 cells by electroporation (250 V, 500 μF). Stable transfectants were selected by limiting dilution in the presence of G418. A single-copy transfectant clone was confirmed by Southern blotting.
Switch Induction and Isolation of Switched Clones.
The CH12F3-2 cell line was maintained as described (37). To induce in vitro CSR, cells were stimulated with 0.3 ng/ml transforming growth factor-β1, IL-4-containing supernatant, and 1 μg/ml monoclonal anti-mouse CD40 antibody (HM40-3, a kind gift from Dr. Yagita, Juntendo University) for 3 days.
Cells were double-stained with phycoerythrin-conjugated goat anti-mouse IgA polyclonal antibody and allophycocyanin-conjugated goat anti-mouse CD8α antibody and analyzed by FACScalibur and CELLQUEST software (Becton Dickinson).
CD8α+ cells were enriched by using a positive selection column. Briefly, the cells (107 cells in 90 μl of degassed medium) were labeled with 10 μl of anti-CD8α antibody-coupled magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and passed through a separation column (MS+/RS+), which was placed in a magnetic field. After being rinsed several times, the magnetically retained cells were eluted and collected.
The magnetic cell sorting-enriched cells were incubated briefly at 5% CO2 and 37°C for 2h and then diluted to 1, 3, and 9 cells per 0.1 ml per well in a 96-well plate. After a 7-day culture, CD8α-GFP-positive clones were obtained and expanded for further analysis.
Genomic PCR.
Upstream Sμ/Sα and downstream Sα/Sμ junctions in isolated CD8α-GFP-positive clones with SCR2(μ,α) or SCR2(μ,Rα) constructs were recovered by 30 cycles of genomic PCR by using primer pairs of 5′-TAA ATG CGG GCC AAG ATC TGC ACA CTG GTA TTT C-3′ and 5′-TGA ACA GCT CCT CGC CCT TGC TCA CCA C-3′ or 5′-ACG CTT TGC CTG ACC CTG CTT GCT CAA CTC TA-3′ and 5′-AAG GCA ACA TCC ACT GAG GAG CAG TTC TTT GAT TTG-3′, respectively. LA Taq (Takara Shuzo, Kyoto, Japan) was used for amplification according to manufacturer instructions except the inclusion of 2.5 mM Mg2+. Amplification products were visualized by electrophoresis on either 1 or 2% agarose gels containing ethidium bromide.
DNA Sequencing.
The PCR products were purified by using QIAquick PCR purification kit (Qiagen, Hilden, Germany) and either sequenced directly by using the primers 5′-GCT CAG GTT AGG TGC TCT CA-3′, 5′-CGT CTC CCG GTC CAG GTC TC-3′, 5′-TCT TTT TGT CTT TTA TTT CAG GTC CCG GAT CTA TG-3′, or 5′-CAA CAT CCA CTG AGG AGC AGT TCT TTG ATT TG-3′ or subcloned into a pBluescript KS vector (Stratagene) for sequencing with primer T7.
Results
Inversion-Type Class-Switch Substrates and Their Activities in CH12F3-2 Cells.
To investigate the cleavage mechanism during CSR, we constructed the inversion-type switch substrates SCR2(μ,α) and SCR2(μ,Rα), which were designed to induce expression of the CD8α-GFP fusion protein on cell membrane when the segment between the two S regions was inverted (Fig. 1A). The two switch substrates are identical except for the Sα orientation relative to the promoter. Because the inversion-type recombination retains two CSR junctions in the substrate DNA, one can assess precisely the sequence alteration at the junction sites before and after CSR. The linearized constructs were integrated stably into chromosomes of CH12F3-2 cells, and the G418-resistant transfectant clones were screened for integration of a single-copy transgene by Southern blotting (data not shown). Two and three single-copy transfectant clones were obtained for SCR2(μ,α) and SCR2(μ,Rα), respectively.
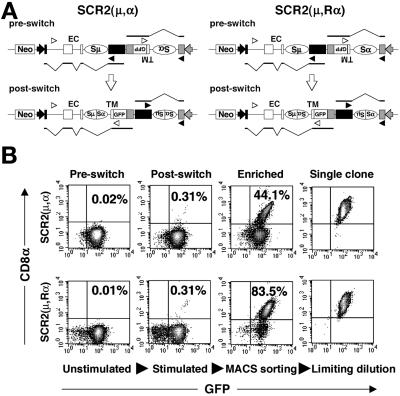
Figure 1.
Construction of inversion-type switch substrates and the detection of switch junctions. (A) Schematic diagram of inversion-type switch substrates SCR2(μ,α), in which the Sα region is the same orientation as the SRα promoter, and SCR2(μ,Rα), in which the Sα is inverted to the SRα promoter. EF-1α and SRα promoters are represented by closed and shaded arrows, respectively. Exons are shown by rectangles. EC and TM indicate two exons of the EC portion and the TM exon of the mouse CD8α gene, respectively. Two kinds of preswitch transcripts and postswitch transcripts are depicted below each substrate, and v-shaped lines indicate splicing. Arrowheads indicate primers for junction amplification. (B) Flow cytometry profiles of SCR2(μ, α)- and SCR2(μ, Rα)-transfectant clones before and after stimulation with IL-4, transforming growth factor-β, and anti-CD40 monoclonal antibody (HM40–3) mixture. The stimulated cells were enriched to select for the CD8α-GFP-positive population by magnetic cell sorting (MACS sorting). Clones of CD8α-GFP-positive cells were obtained by limiting dilution of the sorted cells.
To induce CSR in vitro, the single-copy transfectant clones were stimulated with a mixture of CD40L, IL-4, and transforming growth factor-β1 for 3 days. Flow cytometric analysis showed that a significant population (0.3%) of cells express CD8α-GFP in all the stimulated SCR2(μ,α)- and SCR2(μ,Rα)-transfected clones, whereas the unstimulated clones displayed no significant expression (0.01 ≈ 0.02%) of CD8α-GFP, indicating a very low background in this recombination system (Fig. 1B and data not shown).
Sequence Analysis of Inversion Switch Products.
A representative single transfectant clone for each construct was used for further analysis. To isolate switched clones, the CD8α-GFP-positive cells were enriched by using magnetic cell sorting. After one round of sorting, the CD8α-GFP-positive cell population increased 142- and 269-fold, reaching 44 and 83% for SCR2(μ,α) and SCR2(μ,Rα) transfectants, respectively (Fig. 1B). The high efficiency of magnetic cell sorting allowed us to isolate switched clones by limiting dilution. Hundreds of switched single clones were obtained for both transfectants. The upstream and downstream inversion switch junctions in the genomic DNA of each clone were amplified by PCR using two pairs of primers indicated in Fig. 1A. Identical clones were removed by comparing the PCR product in size and sequence. Nucleotide sequences of CSR junctions in 21 SCR2(μ,α) and 20 SCR2(μ,Rα) switched clones were determined.
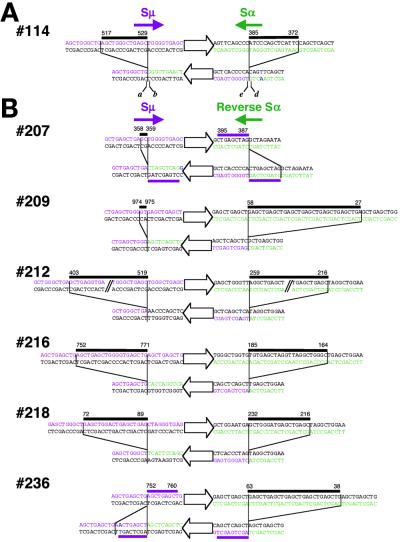
The sequences surrounding the upstream and downstream junctions of the 41 clones were aligned with Sμ or Sα sequences (Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). Sequence alignment was performed to minimize the number of mismatched bases and to maximize the length of homologous sequences surrounding each junction. Although the majority of junction points were not determined precisely at the nucleotide level because of sequence homology or short insertions near junctions, CSR junctions of seven clones were determined without ambiguity (as shown in Fig. 2). The aligned junctional sequences of these seven clones clearly showed that CSR was accompanied by either deletions (12 breakpoints) or duplications (2 breakpoints).
Figure 2.
Inversion-type class switching in SCR2(μ, α)- (A) and SCR2(μ, Rα)- (B) derived clones. For each clone, the upper and lower sequences represent preswitch and postswitch sequences, respectively. Open arrows in the center of each sequence indicate the orientation of a segment between the two S regions. The deletion of Sμ (magenta for nontemplate strand) and Sα (green for nontemplate strand) sequence is marked by black bars on the preswitch S regions. The duplication is marked with magenta bars on preswitch and postswitch sequences. Mutations after CSR are shown in blue letters. Magenta and green arrows indicate the orientation of transcription of Sμ and Sα, respectively. The numbers on the sequences indicate positions in S regions for the beginning and end of deletions or duplications. In A, the positions of nucleotides at Sμ and Sα side border of upstream junction are indicated by a (516 for clone no. 114, see Table 1) and b (386), respectively. Similarly, positions of the downstream junction are indicated by e (530) and d (371). The lengths of deletion in Sμ and Sα are calculated by e − a − 1 and b − d − 1, respectively.
With respect to other clones that possessed a region of junctional homology, decision of the junction points is elusive. Therefore, the difference of nucleotide lengths before and after CSR were estimated also with a range for Sμ and Sα. When a single clone has homology sequences at either or both junctions, the maximal and minimal differences in nucleotide lengths were estimated by choosing the extreme position of each junction. The extreme positions of junctions, lengths of junctional homology regions, and ranges of deletions or duplications of each clone are listed in Table 1.
Table 1.
Analysis of junctions in inversion-type class-switch substrate
| Clone | Sμ Junction
|
Sα junction
|
Homology
|
|||||
|---|---|---|---|---|---|---|---|---|
| Upstream end (a) | Downstream end (e) | Duplication/ deletion | Upstream end (d) | Downstream end (b) | Duplication/ deletion | Upstream junction (c) | Downstream junction (f) | |
| SCR2(μ,α) | ||||||||
| 101 | 189 | 520 | −330 ≈−320 | 533 | 539 | −5 ≈5 | 5 | 5 |
| 102 | 189 | 1029 | −839 ≈−825 | 299 | 533 | −233 ≈−219 | 0 | 14 |
| 105 | 520 | 682 | −161 ≈−150 | 586 | 548 | 39 ≈50 | 2 | 9 |
| 106 | 175 | 189 | −13 ≈−10 | 135 | 154 | −18 ≈−15 | 1 | 2 |
| 107 | 88 | 89 | 0 ≈3 | 833 | 841 | −7 ≈−4 | 3 | 0 |
| 108 | 374 | 905 | −530 ≈−526 | 336 | 347 | −10 ≈−6 | 2 | 2 |
| 109 | 490 | 532 | −41 ≈−26 | 292 | 329 | −36 ≈−21 | 1 | 14 |
| 110 | 584 | 1102 | −517 ≈−513 | 610 | 666 | −55 ≈−51 | 2 | 2 |
| 111 | 583 | 945 | −361 ≈−355 | 1028 | 1037 | −8 ≈−2 | 6 | 0 |
| 114 | 516 | 530 | −13 | 371 | 386 | −14 | 0 | 0 |
| 115 | 309 | 356 | −46 ≈−35 | 40 | 42 | −1 ≈10 | 0 | 11 |
| 116 | 309 | 356 | −46 ≈−24 | 40 | 52 | −11 ≈11 | 11 | 11 |
| 117 | 652 | 663 | −10 ≈0 | 365 | 367 | −1 ≈9 | 9 | 1 |
| 118 | 934 | 947 | −12 ≈7 | 598 | 611 | −12 ≈7 | 7 | 12 |
| 119 | 145 | 696 | −550 ≈−539 | 779 | 792 | −12 ≈−1 | 0 | 11 |
| 120 | 299 | 395 | −95 ≈−84 | 336 | 354 | −17 ≈−6 | 9 | 2 |
| 121 | 408 | 443 | −34 ≈−21 | 98 | 757 | −658 ≈−645 | 8 | 5 |
| 122 | 719 | 730 | −10 ≈−5 | 504 | 521 | −16 ≈−11 | 2 | 3 |
| 123 | 500 | 507 | −6 ≈2 | 898 | 925 | −26 ≈−18 | 0 | 8 |
| 125 | 442 | 181 | 262 ≈266 | 403 | 421 | −17 ≈−13 | 4 | 0 |
| 126 | 919 | 954 | −34 ≈−19 | 277 | 318 | −40 ≈−25 | 13 | 2 |
| SCR2(μ,Rα) | ||||||||
| 205 | 129 | 136 | −6 ≈−1 | 249 | 260 | −10 ≈−5 | 1 | 4 |
| 207 | 357 | 360 | −2 | 386 | 378 | 9 | 0 | 0 |
| 209 | 973 | 976 | −2 | 26 | 59 | −32 | 0 | 0 |
| 210 | 973 | 975 | −1 ≈0 | 24 | 54 | −29 ≈−28 | 0 | 1 |
| 211 | 416 | 422 | −5 ≈−4 | 348 | 588 | −239 ≈−238 | 1 | 0 |
| 212 | 402 | 520 | −117 | 215 | 260 | −44 | 0 | 0 |
| 213 | 777 | 795 | −17 ≈−14 | 254 | 257 | −2 ≈1 | 0 | 3 |
| 214 | 887 | 888 | 0 ≈1 | 25 | 48 | −22 ≈−21 | 0 | 1 |
| 215 | 755 | 756 | 0 ≈4 | 38 | 59 | −20 ≈−16 | 0 | 4 |
| 216 | 751 | 772 | −20 | 163 | 186 | −22 | 0 | 0 |
| 217 | 181 | 189 | −7 ≈−3 | 536 | 555 | −18 ≈−14 | 4 | 0 |
| 218 | 71 | 90 | −18 | 215 | 233 | −17 | 0 | 0 |
| 219 | 492 | 415 | 78 ≈79 | 683 | 837 | −153 ≈−152 | 0 | 1 |
| 220 | 889 | 916 | −26 ≈−21 | 58 | 83 | −24 ≈−19 | 1 | 4 |
| 222 | 151 | 166 | −14 ≈−11 | 459 | 466 | −6 ≈−3 | 1 | 2 |
| 229 | 683 | 705 | −21 ≈−19 | 292 | 317 | −24 ≈−22 | 1 | 1 |
| 230 | 172 | 447 | −274 ≈−272 | 405 | 414 | −8 ≈−6 | 2 | 0 |
| 233 | 256 | 974 | −717 ≈−713 | 24 | 283 | −258 ≈−254 | 3 | 1 |
| 235 | 50 | 938 | −887 ≈−886 | 264 | 768 | −503 ≈−502 | 1 | 0 |
| 236 | 760 | 752 | 9 | 37 | 64 | −26 | 0 | 0 |
A summary of 82 junctions from 41 postswitch clones derived from SCR2(μ,α) and SCR2(μ,Rα) transfectants is shown. Listed are nucleotide positions of upstream (a) and downstream (e) ends (Fig. 2) of Sμ junction relative to the promoter-proximal end of the S region. Ranges of duplication or deletion are shown, which were calculated from a and e, taking the length of junctional homology (c and f) into account. The plus and minus values indicate duplication and deletion, respectively. Similar analyses for Sα junctions (d and b) are shown also. Unequivocal cases for duplication are typed in bold.
Frequent Deletion or Duplication at CSR Junctions.
Analysis of deletion or duplication length in Sμ and Sα regions indicated that the majority of breakpoints (56 of 82, 68.3%) contained deletions of less than 50 bp, whereas deletion of more than 500 bp was found in 9.8% (8 of 82) of breakpoints. It is noteworthy that at least 5 junctions from clones 105, 125, 207, 219, and 236 contained unequivocal duplication, and 16 junctions at maximum might contain duplication. Duplications ranged from 1 to 266 bp.
Association of Breakpoints with Secondary Structure.
Previous reports have indicated that CSR breakpoints appear to be located in the proximity of transition points from single-stranded to double-stranded regions in predicted stem-loop structures in S regions (32, 38, 39). These observations led to a hypothesis that secondary structures transiently formed by the denaturation of S regions during transcription (germline transcription) might be a recognition target of CSR recombinase. To test this hypothesis, the seven pairs of junctions in which Sμ–Sα junctions had been determined precisely were mapped on predicted secondary structure of single-stranded S regions. Fig. 3 shows three possible structures of a part of nontemplate-strand Sμ (nucleotide number 670-1070), predicted under three different conditions at the salt concentration and distance constraint for base pairing. In this region, six junction ends were mapped close to transitions from single-stranded to double-stranded regions, which is consistent with the hypothesis above. This association of breakpoints with the secondary structure was observed irrespective of conditional parameters for other junctions as well (data not shown).
Figure 3.
Association of junction sites with predicted secondary structure of the S region. Sμ side ends (arrowheads) of three pairs of upstream (U) and downstream (D) junctions (clone nos. 209, 216, and 236 in Fig. 2) are mapped on three possible structures of the Sμ region predicted by MFOLD software (bioinfo.math.rpi.edu/∼mfold/dna/) under the different conditions indicated. The temperature parameter was set at 37°C. A and B mark nucleotides 670 and 1070, respectively, of the nontemplate strand of the Sμ sequence.
Frequent Point Mutations Near CSR Junctions.
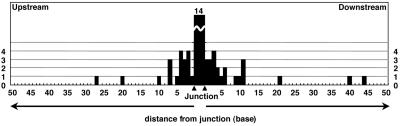
Seventeen of 21 SCR2(μ,α)-derived and 14 of 20 SCR2(μ,Rα)-derived switch clones were found to have point mutations in the proximity of recombination junctions, which were identified on either one (27 of 82 junctions, 33%) or both sides (6 of 82 junctions, 7.3%) of the junctions (Fig. 7). None of the 41 clones contained point mutations at both sides of the two junctions. The vast majority of point mutations were located within 10 bp from the junctions (as shown in Fig. 4), indicating that these mutations are unlikely to be caused by PCR artifacts. In fact, the mutation frequency in the 100-bp region surrounding the junctions was 1.4 × 10−2, which was at least 10 times higher than the PCR background under our conditions (data not shown). No strand bias was observed, because A and T nucleotides were targeted equally, and mutation frequencies in G and C nucleotides were similar also (data not shown). The G/C pair was mutated more frequently than the A/T pair (P = 0.030; Table 2). Transition was observed twice of transversion, which was 4-fold higher than random occurrence (P = 0.0023). The RGYW and its complementary WRCY motifs are reported to be the “hot spots” of the SHM of IgV regions (40, 41). These motifs appear very frequently (55.2 and 57.9% in the Sμ and Sα sequences used in the constructs, respectively; Fig. 6), and yet the mutation frequency in these motifs seemed to be higher than that expected for the random event (P = 0.055; Table 2).
Figure 4.
Positions of S region mutations relative to junction sites. The histogram indicates the numbers of mutated bases plotted to distance from the CSR junction. Junctions are defined as the range between upstream and downstream borders of junctional homology and are indicated by two arrowheads in the center. Fourteen mutations within the homologous region are plotted between the two arrowheads.
Table 2.
Mutation properties in the proximity of CSR junctions
| GC bias
|
Transition bias
|
RGYW/ WRCY bias
|
||||
|---|---|---|---|---|---|---|
| A/T | G/C | Transversion | Transition | Motif | Nonmotif | |
| Observed | 7 | 35 | 14 | 28 | 32 | 10 |
| Expected | 17 | 25 | 28 | 14 | 24 | 18 |
| Significance | P = 0.030 | P = 0.0023 | P = 0.055 | |||
Forty-two mutations are classified according to their targeted nucleotide (A/T or G/C), pattern (transversion or transition), and surrounding nucleotides (RGYW/WRCY hot spot motif or not). Expected values were calculated according to average frequency of G/C (60.5%), the hot spot motifs (56.5%) in Sμ and Sα regions. When mutations occur randomly, transversion occurs twice more frequently than transition. Statistical analysis was done by Fisher's exact probability test or χ2 test.
Discussion
We have analyzed the two recombination junctions of a single CSR event by using the artificial CSR substrates in which the cleaved segment between two S regions is retained by inversion after CSR. The system allowed us to assess precisely the deletion or duplication at the cleavage site accompanied by CSR. Sequence analysis of 82 switch junctions in 41 clones revealed variable lengths of deletions and duplications that occurred exclusively at the switch junction ends. Five unequivocal duplication events at CSR junctions (clones 105, 125, 207, 219, and 236) cannot be explained by double-stranded blunt cleavage. This finding suggests that the cleavage of the S region is most likely to be the staggered double-stranded cleavage generated by sequential nicking. Single-stranded DNA tails generated by such cleavage may be either chewed by exonuclease(s) and/or filled in by DNA polymerase(s) to be repaired by the nonhomologous end-joining repair system (Fig. 5). The chewing and fill-in processes may not be mutually exclusive, and the balance of the two events would depend on activities of the enzymes involved, leading to different outcomes—deletion, duplication, or no change in length—all of which were observed in this study. Point mutations introduced near the junction sites, usually within 10 bases from the junctions, are probably caused by error-prone DNA synthesis during the fill-in process, which is required for the processing of the 5′ overhang generated by either staggered cleavage or excess chewing of the 3′ overhang. The majority of clones displayed short lengths of deletions less than 50 bp (Table 1). Larger deletions observed in a small fraction of clones could be caused by multiple nicks on both strands or multiple cycles of recombination.
Figure 5.
Staggered double-strand DNA cleavage model of CSR. Two S regions indicated by ovals on double-stranded DNA (thick lines) are shown at the top with 5′ and 3′ polarity markers. Bumps on the thick lines indicate stem-loop structure induced by active transcription. The scissors represent a putative endonuclease that cleaves the stem-loop structure and produces staggered nicks in S regions. When 5′ overhang is generated (middle left), it may be double-stranded by error-prone polymerase(s), which introduces mutations and duplication. When a 3′ overhang is produced (middle right), chewing of the overhang by some exonucleases (pacman) will result in deletion. Note that fill-in and chewing are not mutually exclusive. The repaired ends are ligated by the nonhomologous end-joining (NHEJ) system. The ends of the middle segment are self-ligated to form circular DNA (bottom right). The outer segments are ligated to form the chromosomal junction (red arrowhead).
The next question is how staggered cleavage took place in the S region. The unique feature of S region sequences may provide some implication for this issue. The S region is composed of tandemly repetitive short sequences with many palindromes and inverted repeats (9, 13, 42). It has been shown that the primary sequences of S regions are not important for CSR (22). S region sequences can be replaced with those of different isotypes and inverted orientation. Furthermore, a multiple cloning site sequence rich in palindromes was shown to function in place of the S region, suggesting that the secondary structure of the S region rather than its primary sequence is critical for CSR (39). There are additional reasons to speculate that the stem-loop structures formed by palindromes or inverted repeats in both DNA strands may play an important role in staggered cleavage. First, stem-loop structures in the S region can be generated in single-stranded regions formed transiently during transcription (transcription bubble) of 20 up to 27 nucleotides in size (43), and transcription of the S region is shown to be essential for CSR (17, 18). Second, because formation of stem-loop structures can happen on each strand of S region DNA at different positions in the same or different bubbles, double-strand cleavage by staggered nicking seems to be possible. Third, the present and previous data showed that the switch junctions were clustered preferentially on the transition of predicted stem-loop structures, suggesting that such structures may be recognized in the process of CSR (38, 39). Taken together, it may be reasonable to speculate that stem-loop structures at different positions of two DNA strands may be the preferential recognition sites by some unknown nicking enzyme(s), leading to staggered cleavage of the S region (Fig. 5).
SHM is a process to introduce point mutations in the rearranged IgV genes at high frequency in germinal centers. Characterization of SHM reveals several common features with CSR (32). First, deletions, duplications, and strand breaks also are observed in addition to the introduction of point mutations in the V region, implicating DNA strand breaks for the mechanism of SHM (44–48). Second, similar to CSR, SHM is also a regionally specific mutational process with no clear primary sequence specificity. Third, both CSR and SHM depend on transcription (49–52). Fourth, the hot spots for SHM are found also on short repetitive or palindromic sequences and stem-loop structures (53). Fifth, the RGYW/WRCY motifs were targeted preferentially in mutations in S regions as well as those in V regions (40, 41). Although we cannot clearly demonstrate association between RGYW/WRCY motifs and stem-loop structure, frequent occurrence of AGCT, a quartet belonging to the RGYW/WRCY motifs, seems to be a major reason for stem-loop formation in S regions. Sixth, in mutations associating with CSR, the G/C base pairs in the S region are mutated more frequently than the A/T pairs (Table 2). Such G/C bias has been reported for SHM in the RGYW/WRCY motifs in normal mice (54) and mutant mice defective in the mismatch repair (55–58). Seventh, transition is favored over transversion in both CSR (Table 2) and SHM (59). Eighth, neither CSR (Table 2) nor SHM (60) shows strand bias in point mutations, indicating that the mutator machinery may target both DNA strands in CSR and SHM. Furthermore, studies on AID-deficient mice and humans (27, 28) demonstrated that AID is required for CSR as well as SHM but not for the formation of germinal centers, indicating that these two central events of the germinal center reaction may share a common machinery that is regulated by AID, a potential RNA-editing enzyme (26). These striking similarities suggest the presence of a common machinery for CSR and SHM. Elucidation of downstream events regulated by AID will clarify the mechanism underlying these fundamental processes for antibody diversity.
Supplementary Material
Acknowledgments
We acknowledge M. Muramatsu, C.-G. Lee, J. Tashiro, K. Yoshikawa, T. Muto, I.-M. Chung, S. H. Tian, and T. Eto for discussion and suggestion. We thank Drs. S. Takeda and Y. Sakakibara for critical reading of the manuscript. The authors also are grateful to Mses. Y. Tabuchi, T. Toyoshima, and K. Yurimoto for technical assistance and Mses. T. Nishikawa, K. Saito, and R. Yamasaki for secretarial assistance. This work was supported by the Center of Excellence Grant and Special Award Grant for Alien Co-Researcher from the Ministry of Education, Science, Sports, and Culture of Japan. X.C. was supported by the postdoctoral fellowship from the Japanese Society for Promotion of Science.
Abbreviations
- CSR
class-switch recombination
- S
switch
- AID
activation-induced cytidine deaminase
- SHM
somatic hypermutation
- GFP
green fluorescent protein
- EC
extracellular
- TM
transmembrane
- SS
splice acceptor signal
References
- 1.Honjo T, Kataoka T. Proc Natl Acad Sci USA. 1978;75:2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleclough C, Cooper D, Perry R P. Proc Natl Acad Sci USA. 1980;77:1422–1426. doi: 10.1073/pnas.77.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cory S, Jackson J, Adams J M. Nature (London) 1980;285:450–456. doi: 10.1038/285450a0. [DOI] [PubMed] [Google Scholar]
- 4.Davis M M, Kim S K, Hood L E. Science. 1980;209:1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- 5.Dunnick W, Rabbitts T H, Milstein C. Nature (London) 1980;286:669–675. doi: 10.1038/286669a0. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka T, Kawakami T, Takahashi N, Honjo T. Proc Natl Acad Sci USA. 1980;77:919–923. doi: 10.1073/pnas.77.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaoita Y, Honjo T. Nature (London) 1980;286:850–853. doi: 10.1038/286850a0. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka T, Miyata T, Honjo T. Cell. 1981;23:357–368. doi: 10.1016/0092-8674(81)90131-8. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido T, Nakai S, Honjo T. Nature (London) 1981;292:845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- 10.Iwasato T, Shimizu A, Honjo T, Yamagishi H. Cell. 1990;62:143–149. doi: 10.1016/0092-8674(90)90248-d. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka M, Yoshida K, Maeda T, Usuda S, Sakano H. Cell. 1990;62:135–142. doi: 10.1016/0092-8674(90)90247-c. [DOI] [PubMed] [Google Scholar]
- 12.von Schwedler U, Jack H M, Wabl M. Nature (London) 1990;345:452–456. doi: 10.1038/345452a0. [DOI] [PubMed] [Google Scholar]
- 13.Nikaido T, Yamawaki Kataoka Y, Honjo T. J Biol Chem. 1982;257:7322–7329. [PubMed] [Google Scholar]
- 14.Dunnick W, Hertz G Z, Scappino L, Gritzmacher C. Nucleic Acids Res. 1993;21:365–372. doi: 10.1093/nar/21.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C-G, Kondo S, Honjo T. Curr Biol. 1998;8:227–230. doi: 10.1016/s0960-9822(98)70087-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Alt F W, Honjo T. In: Immunoglobulin Genes. Honjo T, Alt F W, editors. London: Academic Limited; 1995. pp. 235–265. [Google Scholar]
- 17.Stavnezer N J, Sirlin S. EMBO J. 1986;5:95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancopoulos G D, DePinho R A, Zimmerman K A, Lutzker S G, Rosenberg N, Alt F W. EMBO J. 1986;5:3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C G, Kinoshita K, Arudchandran A, Cerritelli S M, Crouch R J, Honjo T. J Exp Med. 2001;194:365–374. doi: 10.1084/jem.194.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenz M, Jung S, Radbruch A. Science. 1995;267:1825–1828. doi: 10.1126/science.7892607. [DOI] [PubMed] [Google Scholar]
- 21.Hein K, Lorenz M G, Siebenkotten G, Petry K, Christine R, Radbruch A. J Exp Med. 1998;188:2369–2374. doi: 10.1084/jem.188.12.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita K, Tashiro J, Tomita S, Lee C G, Honjo T. Immunity. 1998;9:849–858. doi: 10.1016/s1074-7613(00)80650-0. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita K, Lee C-G, Tashiro J, Muramatsu M, Chen X-C, Yoshikawa K, Honjo T. Cold Spring Harbor Symposia on Quantitative Biology: Signaling & Gene Expression in the Immune System. Vol. 64. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 217–226. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita K, Honjo T. Curr Opin Immunol. 2000;12:195–198. doi: 10.1016/s0952-7915(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Rabbani H, Shimizu A, Hammarstrom L. Immunology. 2000;101:348–353. doi: 10.1046/j.1365-2567.2000.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muramatsu M, Sankaranand V S, Anant S, Sugai M, Kinoshita K, Davidson N O, Honjo T. J Biol Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 28.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 29.Rolink A, Melchers F, Andersson J. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 30.Casellas R, Nussenzweig A, Wuerffel R, Pelanda R, Reichlin A, Suh H, Qin X F, Besmer E, Kenter A, Rajewsky K, Nussenzweig M C. EMBO J. 1998;17:2404–2411. doi: 10.1093/emboj/17.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manis J P, Gu Y, Lansford R, Sonoda E, Ferrini R, Davidson L, Rajewsky K, Alt F W. J Exp Med. 1998;187:2081–2089. doi: 10.1084/jem.187.12.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita K, Honjo T. Nat Rev Mol Cell Biol. 2001;2:493–503. doi: 10.1038/35080033. [DOI] [PubMed] [Google Scholar]
- 33.Youn H J, Harriss J V, Gottlieb P D. Immunogenetics. 1988;28:345–352. doi: 10.1007/BF00364233. [DOI] [PubMed] [Google Scholar]
- 34.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima S, Nagata S. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard W J, Depper J M, Kanehisa M, Kronke M, Peffer N J, Svetlik P B, Sullivan M, Greene W C. Science. 1985;230:633–639. doi: 10.1126/science.2996141. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura M, Kondo S, Sugai M, Nazarea M, Imamura S, Honjo T. Int Immunol. 1996;8:193–201. doi: 10.1093/intimm/8.2.193. [DOI] [PubMed] [Google Scholar]
- 38.Mussmann R, Courtet M, Schwager J, Du Pasquier L. Eur J Immunol. 1997;27:2610–2619. doi: 10.1002/eji.1830271021. [DOI] [PubMed] [Google Scholar]
- 39.Tashiro J, Kinoshita K, Honjo T. Int Immunol. 2001;13:495–505. doi: 10.1093/intimm/13.4.495. [DOI] [PubMed] [Google Scholar]
- 40.Rogozin I B, Kolchanov N A. Biochim Biophys Acta. 1992;1171:11–18. doi: 10.1016/0167-4781(92)90134-l. [DOI] [PubMed] [Google Scholar]
- 41.Dorner T, Foster S J, Farner N L, Lipsky P E. Eur J Immunol. 1998;28:3384–3396. doi: 10.1002/(SICI)1521-4141(199810)28:10<3384::AID-IMMU3384>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi N, Kataoka T, Honjo T. Gene. 1980;11:117–127. doi: 10.1016/0378-1119(80)90092-x. [DOI] [PubMed] [Google Scholar]
- 43.Fiedler U, Timmers H T. Nucleic Acids Res. 2001;29:2706–2714. doi: 10.1093/nar/29.13.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goossens T, Klein U, Kuppers R. Proc Natl Acad Sci USA. 1998;95:2463–2468. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson P C, de Bouteiller O, Liu Y J, Potter K, Banchereau J, Capra J D, Pascual V. J Exp Med. 1998;187:59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bross L, Fukita Y, McBlane F, Demolliere C, Rajewsky K, Jacobs H. Immunity. 2000;13:589–597. doi: 10.1016/s1074-7613(00)00059-5. [DOI] [PubMed] [Google Scholar]
- 47.Papavasiliou F N, Schatz D G. Nature (London) 2000;408:216–221. doi: 10.1038/35041599. [DOI] [PubMed] [Google Scholar]
- 48.Kong Q, Maizels N. Genetics. 2001;158:369–378. doi: 10.1093/genetics/158.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters A, Storb U. Immunity. 1996;4:57–65. doi: 10.1016/s1074-7613(00)80298-8. [DOI] [PubMed] [Google Scholar]
- 50.Tumas-Brundage K, Manser T. J Exp Med. 1997;185:239–250. doi: 10.1084/jem.185.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukita Y, Jacobs H, Rajewsky K. Immunity. 1998;9:105–114. doi: 10.1016/s1074-7613(00)80592-0. [DOI] [PubMed] [Google Scholar]
- 52.Bachl J, Carlson C, Gray-Schopfer V, Dessing M, Olsson C. J Immunol. 2001;166:5051–5057. doi: 10.4049/jimmunol.166.8.5051. [DOI] [PubMed] [Google Scholar]
- 53.Storb U, Klotz E L, Hackett J, Jr, Kage K, Bozek G, Martin T E. J Exp Med. 1998;188:689–698. doi: 10.1084/jem.188.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster S J, Dorner T, Lipsky P E. Eur J Immunol. 1999;29:4011–4021. doi: 10.1002/(SICI)1521-4141(199912)29:12<4011::AID-IMMU4011>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 55.Frey S, Bertocci B, Delbos F, Quint L, Weill J C, Reynaud C A. Immunity. 1998;9:127–134. doi: 10.1016/s1074-7613(00)80594-4. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs H, Fukita Y, van der Horst G T, de Boer J, Weeda G, Essers J, de Wind N, Engelward B P, Samson L, Verbeek S, et al. J Exp Med. 1998;187:1735–1743. doi: 10.1084/jem.187.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phung Q H, Winter D B, Cranston A, Tarone R E, Bohr V A, Fishel R, Gearhart P J. J Exp Med. 1998;187:1745–1751. doi: 10.1084/jem.187.11.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rada C, Ehrenstein M R, Neuberger M S, Milstein C. Immunity. 1998;9:135–141. doi: 10.1016/s1074-7613(00)80595-6. [DOI] [PubMed] [Google Scholar]
- 59.Betz A G, Rada C, Pannell R, Milstein C, Neuberger M S. Proc Natl Acad Sci USA. 1993;90:2385–2388. doi: 10.1073/pnas.90.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milstein C, Neuberger M S, Staden R. Proc Natl Acad Sci USA. 1998;95:8791–8794. doi: 10.1073/pnas.95.15.8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.