Abstract
Tissue homeostasis depends on the ability of tissue-specific adult stem cells to maintain a balance between proliferation and differentiation, as well as ensure DNA damage repair. Here, we use the Drosophila male germline stem cell system to study how a chromatin factor, enhancer of polycomb [E(Pc)], regulates the proliferation-to-differentiation (mitosis-to-meiosis) transition and DNA damage repair. We identified two critical targets of E(Pc). First, E(Pc) represses CycB transcription, likely through modulating H4 acetylation. Second, E(Pc) is required for accumulation of an important germline differentiation factor, Bag-of-marbles (Bam), through post-transcriptional regulation. When E(Pc) is downregulated, increased CycB and decreased Bam are both responsible for defective mitosis-to-meiosis transition in the germline. Moreover, DNA double-strand breaks (DSBs) accumulate upon germline inactivation of E(Pc) under both physiological condition and recovery from heat shock-induced endonuclease expression. Failure of robust DSB repair likely leads to germ cell loss. Finally, compromising the activity of Tip60, a histone acetyltransferase, leads to germline defects similar to E(Pc) loss-of-function, suggesting that E(Pc) acts cooperatively with Tip60. Together, our data demonstrate that E(Pc) has pleiotropic roles in maintaining male germline activity and genome integrity. Our findings will help elucidate the in vivo molecular mechanisms of E(Pc).
Introduction
Stem cell proliferation and differentiation require precise regulation to achieve balanced tissue homeostasis. Drosophila spermatogenesis provides a great model system to study the molecular mechanisms of stem cell proliferation versus differentiation decision under physiological conditions [1]. Spermatogenesis initiates from the asymmetric cell division of germline stem cells (GSCs), generating a self-renewed GSC and a gonialblast, which undergoes four-round mitosis as the transit-amplifying spermatogonia. After mitosis, 16 fusome-connected germ cells enter meiosis with a prolonged G2-phase as spermatocytes, followed by two rounds of meiotic divisions. The transition from mitotic spermatogonia to meiotic spermatocytes requires a differentiation factor called Bag-of-marbles (Bam), the loss of which leads to continuously dividing spermatogonia [2–7].
Chromatin regulators that change the epigenetic status of target genes without altering their primary DNA sequences play critical roles in GSC lineages [8]. High-throughput mRNA sequencing (RNA-seq) revealed that chromatin remodeling factors and histone-modifying enzymes are enriched in mitotic germline-enriched bam mutant testes compared with meiotic germline-containing wild-type testes [9], suggesting their potential roles in regulating the mitosis-to-meiosis transition.
During the mitotic stage, approximately 20% of spermatogonial cysts, mainly at the 4- to 16-cell stage, undergo spontaneous cell death, which could serve as a quality control [10–12] to maintain germline genome integrity by eliminating cells with unrepaired DNA damage [13]. During DNA repair, chromatin modification is essential to allow repair proteins to access the damage sites. For example, phosphorylation of histone variant H2AX at DNA double-strand break (DSB) sites facilitates recognition by repair proteins [14].
Among known chromatin regulators acting in DNA damage response, Tip60 contributes to DNA repair through its ATPase activity [15]. Tip60 has dual roles as both ATP-dependent chromatin remodeling enzyme and histone acetyltransferase (HAT) [16]. Tip60 can acetylate lysine residues of H4, H2A, and transcription factors, to activate gene expression by decondensing chromatin and allowing transcriptional machinery to access DNA [17].
E(Pc) has been characterized as one component of the Drosophila Tip60 (dTip60) complex biochemically [18]. Genetic assays identified E(Pc) mutant as an enhancer of Polycomb mutant and a suppressor of position-effect variegation [19, 20]. Here we report that E(Pc) promotes mitosis-to-meiosis transition through repressing CycB transcription and promoting Bam protein accumulation in the Drosophila male germline. Moreover, inactivating E(Pc) in germ cells leads to increased DSBs and cell death. E(Pc) is required for the HAT activity of dTip60. Compromising HAT activity of dTip60 phenocopies E(Pc) loss-of-function defects. Together, our findings reveal heretofore poorly understood in vivo pleiotropic roles of E(Pc).
Results
E(Pc) is required cell autonomously for germline survival and differentiation
E(Pc) is highly expressed in Drosophila adult testes with enrichment in bam mutant compared with wild-type testes, based on RNA-seq analysis (Supplementary Fig. 1a) [9]. A GFP (Green Fluorescent Protein)-tagged genomic-rescuing E(Pc) transgene [21] showed nuclear localization of E(Pc)–GFP fusion protein in GSCs, spermatogonia, and spermatocytes (Supplementary Fig. 1b, c), consistent with previous findings that E(Pc) encodes a chromatin factor and binds to multiple polytene chromosomal sites [19, 20].
To explore E(Pc)’s functions in germ cells, a short hairpin RNA (shRNA) targeting its coding sequences was driven by an early-stage germline driver nanos-Gal4 (nos-Gal4) for knockdown (KD) experiments. In nos > E(Pc) shRNA testes, the E(Pc)-GFP signal was diminished in germ cells from GSCs to spermatogonia, but still detectable in somatic gonadal cells (Supplementary Fig. 1d, e), suggesting a germline-specific KD.
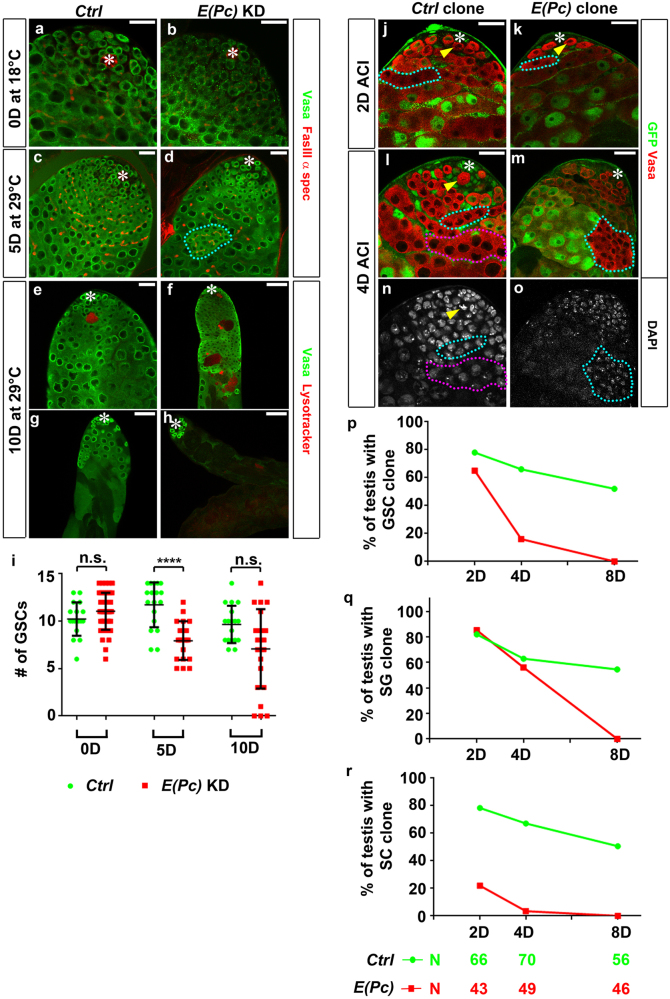
To investigate germline defects specifically in adult testes, the temperature-sensitive Gal80 controlled by the tubulin promoter (tub-Gal80ts) was applied to temporally express E(Pc) shRNA. At the permissive temperature, both tub-Gal80ts, nos-Gal4 (Ctrl), and tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes had germ cells at all stages, including GSCs, spermatogonia, and spermatocytes (Figs. 1a, b). However, 5 days after shifting to the restrictive temperature, cysts with >16 germ cells were found in 55% of E(Pc) KD (N = 20) (cyan outline, Fig. 1d) but not in Ctrl (N = 28, Fig. 1c) testes. Such phenotype became more severe in 39% of E(Pc) KD testes (N = 23) 10 days after temperature shift (Fig. 1f, average spermatogonial tumors/testis was 5 at day 10 vs. 2.4 at day 5).
Fig. 1.
E(Pc) is required cell autonomously for GSC maintenance and germ cell differentiation. tub-Gal80ts, nos-Gal4 (Ctrl), and tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes at a, b permissive temperature (18 °C) and c–h restrictive temperature (29 °C), immunostained with Vasa to label germ cells, FasIII for hub (asterisk) and α-spectrin for spectrosome and fusome. i Quantification of GSCs number (average ± SD). Mann–Whitney U-test. n.s. nonsignificant, ****P < 0.0001. j–o GFP was used to identify Ctrl and E(Pc) mutant clones. j–k 2D after clonal induction (ACI), GFP-negative Ctrl GSC clone (yellow arrowhead in j), and E(Pc) mutant GSC clone (yellow arrowhead in k); GFP-negative Ctrl spermatogonial (SG) clone (cyan outline in j) and E(Pc) mutant SG clone (cyan outline in k). l, n 4D ACI, Ctrl GSC clone (yellow arrowhead), SG clone (cyan outline), and spermatocyte (SC) clone (magenta outline). m, o 4D ACI, E(Pc) mutant SG clone with bright DAPI intensity o has >16 germ cells within one cyst (cyan outline). No GSC clone or SC clone could be found for testes shown. p–r Percentage of testes with GSC clone p, SG q, SC r clones. N at the bottom represents the number of total testes from three independent clonal induction experiments. For each experiment, number of testes with at least one GFP-negative clone/total testes number was calculated and presented as % of testes with the corresponding type of clone. Asterisk: hub. Scale bar for f, g, h: 50 μm. Scale bar for other panels: 20 μm.
Furthermore, when using lysotracker to detect germ cell death [10, 11], more than three lysotracker-positive cysts could be found in ~30% of E(Pc) KD testes (Fig. 1f) but in only 5% of Ctrl testes (N = 18, Fig. 1e). This increased cell death might explain germ cell loss in E(Pc) KD testes (Figs. 1g, h). A time-course quantification (Fig. 1i) revealed that GSC number between Ctrl (N = 18, 10.22 ± 1.77, mean ± SD) and E(Pc) KD (N = 45, 11.04 ± 1.95) testes was comparable at the permissive temperature. But a significant GSC decrease (P < 10−4) was found in E(Pc) KD (N = 20, 7.95 ± 2.04) compared with Ctrl (N = 18, 11.72 ± 2.37) testes 5 days after temperature shift. At 10 days after temperature shift, GSC number in E(Pc) KD (N = 23, 7.09 ± 4.18) became more comparable to Ctrl (N = 18, 9.67 ± 1.97) testes. However, some of these E(Pc) KD testes were devoid of late-stage germ cells (Fig. 1h). Together, these results indicate that E(Pc) is required cell autonomously for germ cell survival, the mitosis-to-meiosis transition, and GSC maintenance.
Next, we generated E(Pc) mutant clones using the Flippase/Flippase Recognition Target system with a strong loss-of-function E(Pc)w3 allele [22]. At 2 days after clonal induction (ACI), 65% of testes (N = 43) had at least one E(Pc) GSC clone, comparable to Ctrl testes with wild-type GSC clones (77%, N = 66; Fig. 1p), suggesting similar clonal induction efficiency. However, the percentage of testes with E(Pc) GSC clones declined much faster than that of Ctrl testes (Fig. 1p). No testis with E(Pc) GSC clone could be found 8 days ACI (Fig. 1p). These data confirmed that E(Pc) is required cell autonomously for GSC maintenance.
Although no obvious difference was found for the percentage of testes with spermatogonial clones 4 days ACI, 17.5% of E(Pc) spermatogonial clones (N = 97) had >16 germ cells (cyan outline, Figs. 1m, o), consistent with the spermatogonial tumor found in E(Pc) KD testes (cyan outline, Fig. 1d). Moreover, the E(Pc) spermatogonial clone was undetectable 8 days ACI (Fig. 1q), likely attributed to decreased GSCs and increased spermatogonial cell death. Furthermore, at any time point ACI, very few E(Pc) spermatocyte clones was found (Figs. 1k, m, r), consistent with previous KD results (Figs. 1d, f).
Furthermore, clonal induction using another loss-of-function E(Pc)1 allele [19] showed similar germline phenotypes (Supplementary Fig. 2). Together, both germline KD and clonal analyses demonstrate that E(Pc) acts cell autonomously to maintain germline including GSCs and promote the mitosis-to-meiosis transition.
E(Pc) represses CycB transcription to restrict spermatogonial overproliferation
To understand the molecular mechanisms underlying spermatogonial tumor phenotype, we examined a critical cell cycle regulator CycB by immunostaining. In Ctrl testes, CycB was detected in the mitotic germ cells (yellow solid line, Figs. 2a–c and Supplementary Fig. 3a-c, 3d-f) but downregulated upon completion of mitosis and became undetectable in meiotic spermatocytes (white solid line, Fig. 2c). CycB was detectable again at the G2/M transition of meiosis I (magenta solid line, Supplementary Fig. 3a-c), as reported previously [23, 24]. By contrast, in 66% of E(Pc) KD testes (N = 21), CycB-positive cells were separated from the apical tip (cyan outline, Figs. 2d–f) with bright 4,6-diamidino-2-phenylindole (DAPI) staining and condensed nuclear morphology, suggesting their identity as overproliferating spermatogonia. Noticeably, the CycB signal in early-stage germ cells did not change dramatically (Figs. 2f’ vs. c’), suggesting that misexpression of CycB upon E(Pc) KD is stage specific.
Fig. 2.
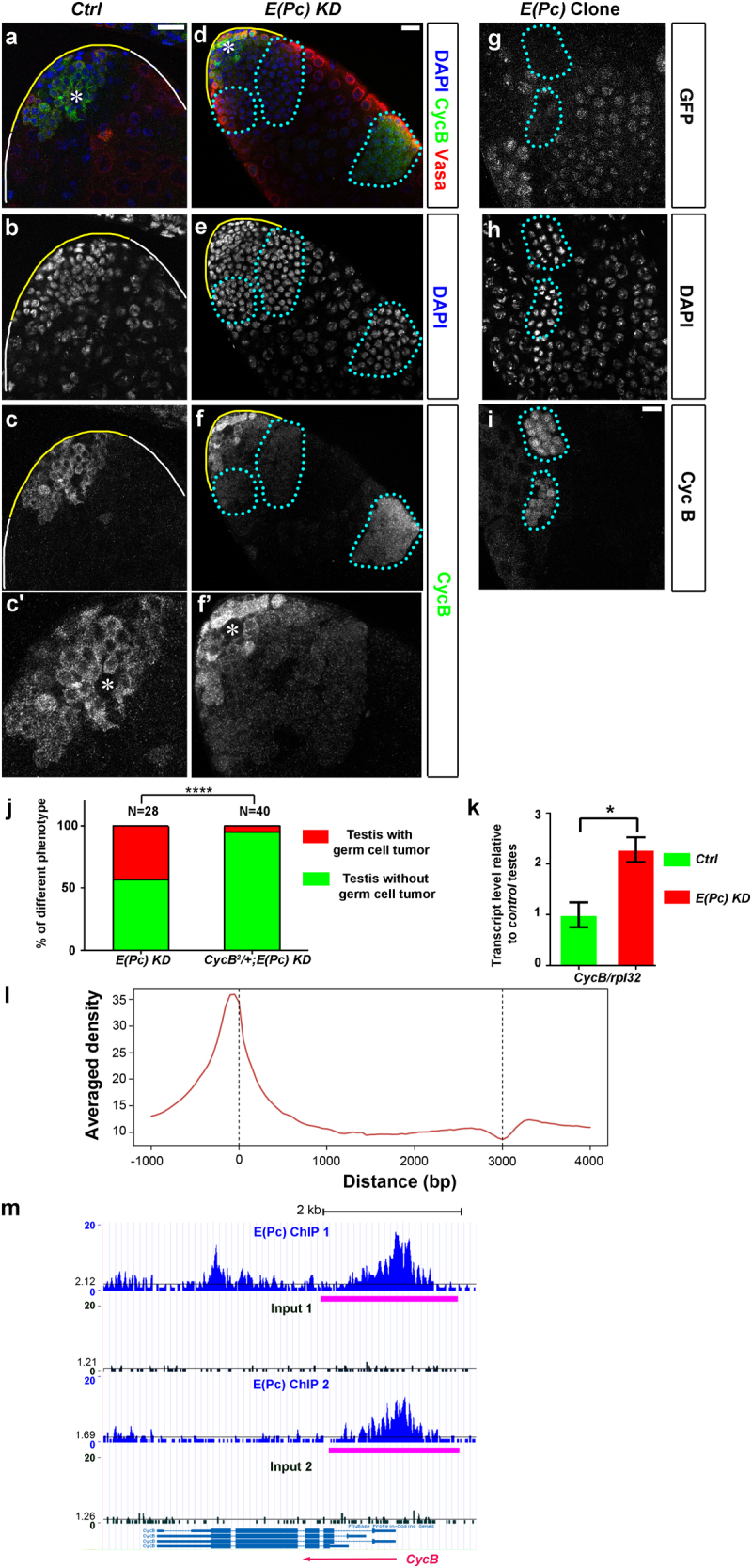
E(Pc) in germ cells negatively regulates CycB transcription. tub-Gal80ts, nos-Gal4 (Ctrl) testes a–c and tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes d–f immunostained with CycB, Vasa and DAPI. c’, f’ Higher magnification of CycB staining at the apical tip. g–i GFP-negative E(Pc) mutant (cyan outline) clones have CycB expression. j Quantification of germline tumor phenotype at different genetic backgrounds. ****P < 0.0001, Chi-square test. k Quantitative RT-PCR of CycB mRNA in tub-Gal80ts, nos-Gal4 (Ctrl) and tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes. Mean ± SD (standard deviation) based on three independent biological replicates. Transcript level in Ctrl testes set to 1. Student’s t-test. P = 0.02. l ChIP-seq was performed with GFP antibody in S2 cells transfected with Actin-Gal4 and UAS-E(Pc) cDNA-GFP plasmids. Two independent ChIP experiments were performed. Average E(Pc) signal profile of 5629 target genes over a -1-kb to +4-kb region with respect to the TSSs. m A genome browser snapshot of the CycB gene region. Compared with input, enriched E(Pc) at genomic region of CycB. Peaks are indicated by magenta lines. The black lines indicate the average read density at chromosome 2R. Two independent replicates are shown here. Asterisk: hub. Scale bar: 20 μm.
In addition, nucleoside analog 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay identified cysts with 32 EdU-positive spermatogonia in 21.9% of E(Pc) KD testes (N = 32). However, only ⩽16 EdU-positive spermatogonia within one cyst could be detected in Ctrl testes, confirming spermatogonial overproliferation in E(Pc) KD testes. Finally, most E(Pc) spermatogonial clones (cyan outline, Figs. 2g–h, 92%, N = 26) showed increased CycB signal (N = 26, Fig. 2i).
Next, we halved CycB level using a null CycB2 allele [25]. We found decreased percentage of testes with spermatogonial tumors from 43% in E(Pc) KD testes (N = 28) to 5% in CycB2/+, E(Pc) KD testes (N = 40) (Fig. 2j, P < 10−4). These results suggest that enhanced CycB levels upon inactivating E(Pc) does, at least partially, contribute to the spermatogonial overproliferation phenotype.
Then, CycB transcript level was measured using quantitative reverse transcriptase-PCR (RT-PCR). An approximate twofold increase of CycB mRNA was detected in E(Pc) KD testes (tub-Gal80ts, nos > E(Pc) shRNA) compared with Ctrl testes (tub-Gal80ts, nos-Gal4) (Fig. 2k, P = 0.02), suggesting that E(Pc) likely regulates CycB transcription. Next, chromatin immunoprecipitation (ChIP)-seq experiments were performed to investigate whether E(Pc) binds to the CycB genomic region in Drosophila S2 cells, which has high E(Pc) expression based on ModENCODE data. A ChIP-grade GFP antibody [21] was used to pull down E(Pc)-GFP-bound DNA followed by high-throughput sequencing, which identified 5629 E(Pc)-bound genes. Enrichment of E(Pc) was detected within a 600-bp region upstream of transcription start sites (TSSs) (Fig. 2l), confirming E(Pc) as a chromatin factor [19–21, 26]. Based on ChIP-seq result, enrichment of E(Pc) was near the TSS of CycB locus (Fig. 2m). Together, E(Pc) likely downregulates CycB transcription in spermatogonia for their mitosis-to-meiosis transition. Meanwhile, it is very likely that the pleiotropic E(Pc) phenotypes reflect its regulation of many targets including CycB.
E(Pc) acts with dTip60 to maintain H4 acetylation levels in the germline
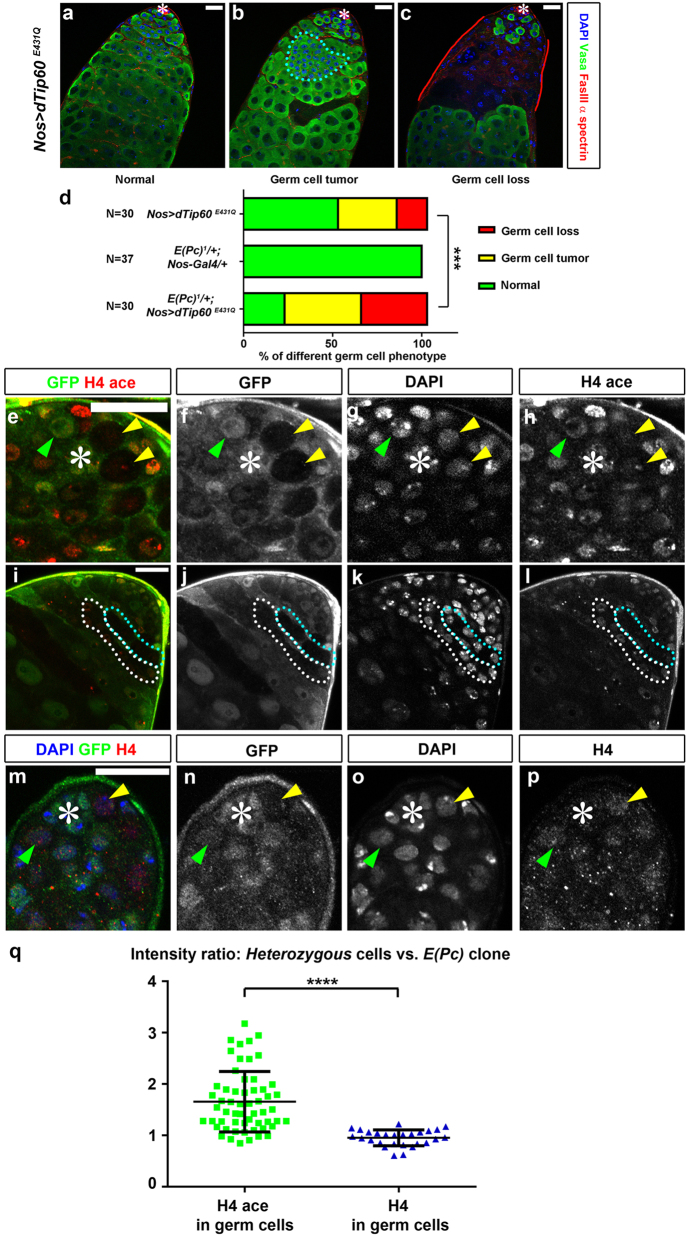
Previous biochemical studies have shown that E(Pc) is a component of the dTip60 HAT complex [18]. The dTip60 has been shown to acetylate both H4 and H2A, an activity conserved from yeast [27] to human [15]. Indeed, expression of a dominant-negative HAT-deficient dTip60E431Q form [28] led to excess spermatogonia (cyan outline, Fig. 3b) and severe germ cell loss (red solid line, Fig. 3c) in 33% and 17% of nos > dTip60E431Q testes (N = 30), respectively. These defects recapitulated E(Pc) loss-of-function phenotypes but were not fully penetrant, since ~50% of nos > dTip60E431Q testes showed apparently normal morphology (Fig. 3a). However, both germline defects were significantly enhanced upon compromising E(Pc) with the strong loss-of-function E(Pc)1 allele [19, 29] (Fig. 3d). Together, these data suggest that E(Pc) acts cooperatively with dTip60, whose HAT activity is required in germ cells. Consistently, tetra-acetylated lysines 5, 8, 12, and 16 of H4 had reduced levels in E(Pc) GSC clones (N = 24, Figs. 3e–h) and spermatogonial clones (N = 33, Figs. 3i–l). Nevertheless, reduced H4 tetra-acetylation in E(Pc) mutant germ cells did not result from the change of the overall H4 level (N = 27; compare the GSCs indicated by yellow vs. green arrowheads in Figs. 3m–p, q). Together, these data suggest that downregulation of E(Pc) compromised the HAT activity of dTip60. Furthermore, ChIP-qPCR experiments using anti-tetra-acetylated H4 in wild-type testes showed its enrichment near the TSS of CycB locus (Supplementary Fig. 4), suggesting E(Pc) might regulate CycB transcription through H4 acetylation.
Fig. 3.
E(Pc) cooperates with histone acetyltransferase dTip60 to regulate germ cell differentiation through H4 acetylation. a–c nos > dTip60E431Q testes stained with DAPI, germ cell marker Vasa, hub marker FasIII and fusome marker α-spectrin. Normal morphology a, spermatogonial overproliferation (cyan outline in b), and germ cell loss (red solid line in c) phenotypes were detected with different penetrance. d Quantification of germ cell phenotype. Heterozygous E(Pc)1/+ enhances germ cell phenotype in nos > dTip60E431Q testes. ***P < 0.005. Chi-square test. e–p Apical tip of testes immunostained with GFP f, j, n to identify GFP-negative E(Pc) mutant clones and DAPI g, k, o. q Quantification of intensity of H4 tetra-acetylation (ace) and H4 immunostaining signals between GFP-positive control cells and GFP-negative E(Pc) mutant clones. H4 acectrl/H4 aceclone = 1.66 ± 0.60. H4ctrl/H4clone = 1.00 ± 0.17 (mean ± SD). Two-tailed t-test. ****P < 0.0001. Asterisk: hub. Scale bar: 20 μm.
Finally, neither an active H3K4me3 mark [30] (N = 29, Supplementary Fig. 5a-c, g) nor a repressive H3K27me3 mark [31] (N = 33, Supplementary Fig. 5d-f, g) showed detectable difference between E(Pc) and Ctrl GSC clones, indicating the specificity of changed H4 acetylation in E(Pc) mutant cells. Interestingly, depletion of Epl1, the yeast homolog of E(Pc), also leads to global loss of H4 acetylation [27]. Therefore, the role of E(Pc) in maintaining H4 acetylation levels is likely conserved.
E(Pc) regulates Bam protein accumulation for the mitosis-to-meiosis transition
As E(Pc) loss-of-function phenotypes (Figs. 1d, m, Supplementary Fig. 2d) resemble the bam mutant phenotype [2–5, 7], we next examined Bam expression in E(Pc) KD testes. Cytoplasmic Bam signal was detected in 4–16 cell spermatogonia close to the apical tip, but not much farther away in meiotic spermatocytes in wild-type (N = 18, Figs. 4a–d) and mcherry KD control (N = 22, Supplementary Fig. 3g-i) testes, as reported previously [2–4, 6, 7]. In E(Pc) KD testes (N = 31), germ cells located at the apical tip with normal morphology (Figs. 4e–h) still had detectable Bam (yellow solid line in Fig. 4g’; red signal in Fig. 4h). However, excess germ cells located away from the tip with condensed nuclei (cyan outline, Fig. 4e) and small sizes (cyan outline, Fig. 4f) had reduced Bam in 48% of E(Pc) KD testes (N = 31, cyan outline, Fig. 4g). Spermatogonial tumors (Fig. 1d) with reduced Bam were found in ~55% E(Pc) KD testes. Consistently, Bam was greatly reduced in overproliferative E(Pc) mutant spermatogonial clones (N = 21, cyan outline, Figs. 4i–l). These data suggest that the regulation of E(Pc) on Bam could be stage specific, because Bam level seemed to normal in early-stage germline (Figs. 4c’ vs. g’ or k’).
Fig. 4.
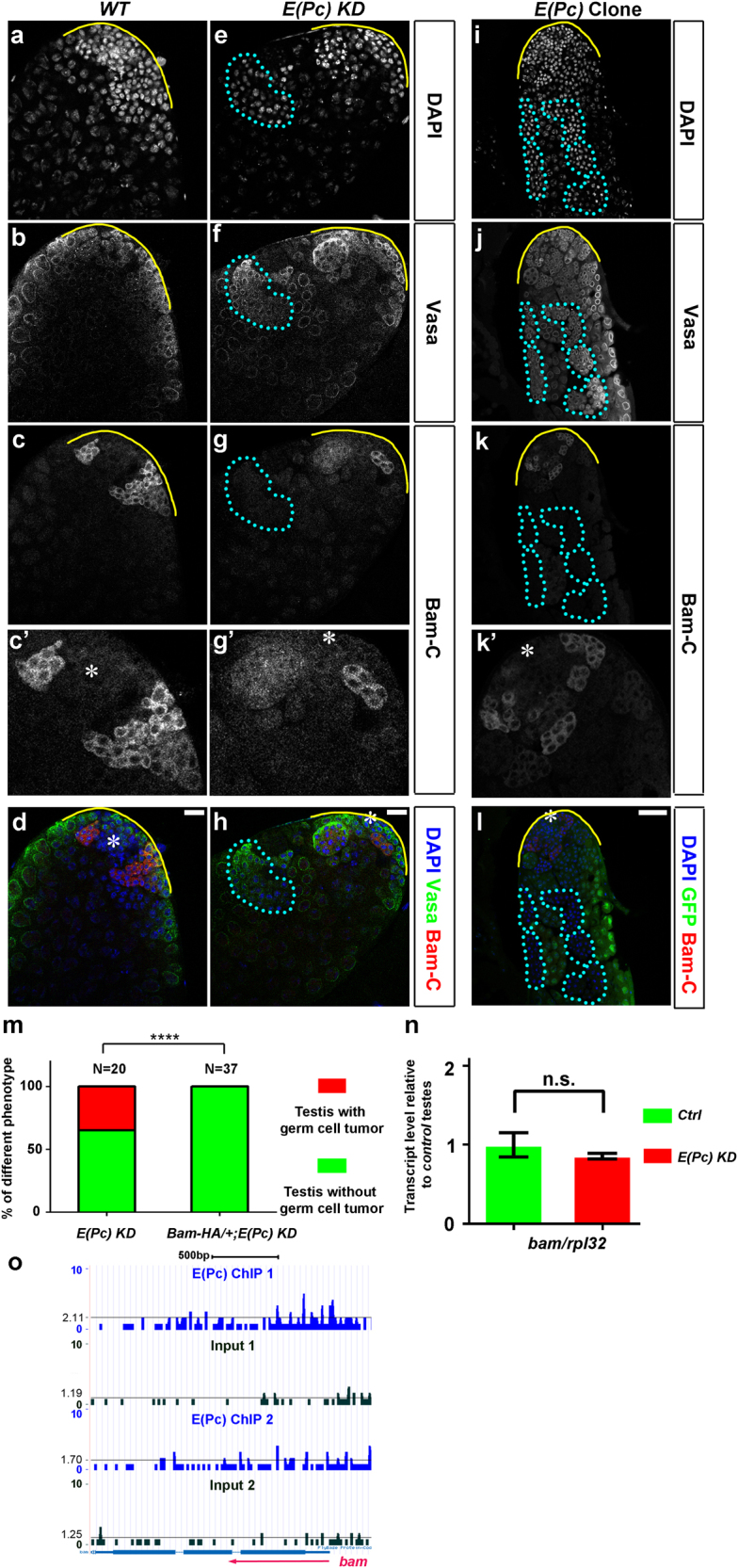
E(Pc) in germ cells is required for the expression of differentiation factor Bam. Apical tip of the wild-type testes a-d and tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes e–h immunostained with DAPI, Vasa and Bam. Higher magnification of Bam-C staining at the apical tip shown in c’, g’ and k’. Asterisk: hub. Scale bar: 20 μm. i–l Apical tip of testes immunostained with GFP, DAPI, and Bam. GFP is used to identify E(Pc) mutant clones. Asterisk: hub. Scale bar: 50 μm. m Percentage of testes with excess germ cells. ****P < 0.0001, Chi-square test. n Quantitative RT-PCR of bam mRNA in tub-Gal80ts, nos-Gal4 (Ctrl) and tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes. Mean ± SD, based on three independent biological replicates. Transcript level in Ctrl testes set to 1. Student’s t-test. o A genome browser snapshot of the bam gene region. Compared with input, no enrichment of E(Pc) at genomic region of bam. The black lines indicate the average read density at chromosome 3R. Two independent replicates are shown here.
Reduced Bam protein could cause spermatogonial overproliferation in both E(Pc) KD (Fig. 1d) and E(Pc) mutant clones (Fig. 1m, Supplementary Fig. 2d). To test this, increased Bam expression using a bam-HA transgene [6] at the E(Pc) loss-of-function background resulted in complete suppression of the spermatogonial tumor phenotype (N = 37, Fig. 4m). This suggests that Bam is an essential downstream factor of E(Pc) in restricting spermatogonial overproliferation. However, bam mRNA in E(Pc) KD testes was unchanged (Fig. 4n) and no enrichment of E(Pc) at bam genomic locus could be identified (Fig. 4o), indicating that E(Pc) regulates bam expression post-transcriptionally.
E(Pc) regulates germ cell survival independent of either JAK-STAT or BMP pathway
Although reducing CycB level (Fig. 2j) or overexpressing bam (Fig. 4m) could suppress the spermatogonial overproliferation phenotype in E(Pc) KD testes, germ cell loss was still detectable in all CycB2/+, nos > E(Pc) shRNA (N = 23) and bam-HA, nos > E(Pc) shRNA (N = 21) testes, suggesting that E(Pc) regulates germline survival using different mechanism(s). Germ cell loss could be caused by GSC self-renewal defect, impaired GSC division and/or increased cell death.
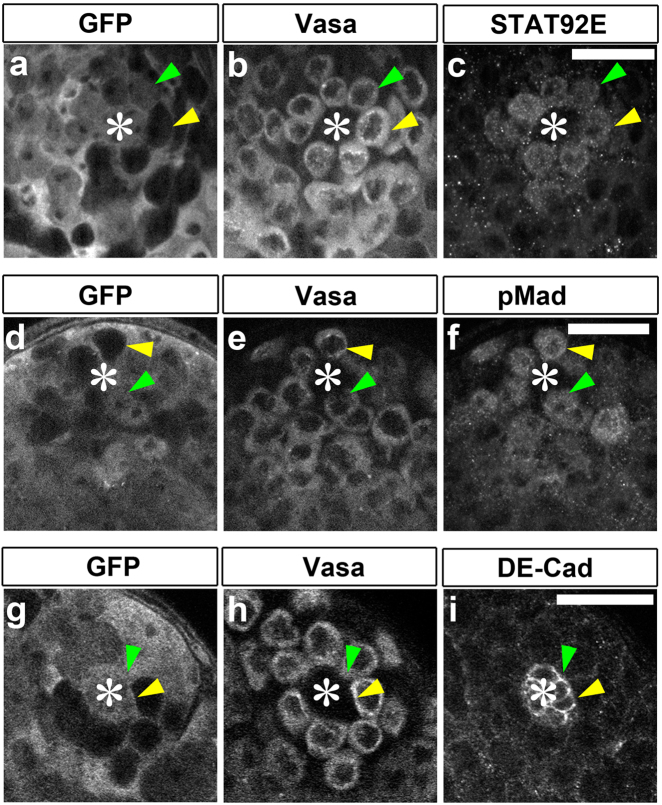
To examine whether E(Pc) is required for GSC self-renewal, several GSC maintenance factors were compared between E(Pc) mutant and Ctrl GSCs, including the Janus kinase-signal transducer and activator of transcription (JAK-STAT) [32–35] and bone morphogenetic protein (BMP) [5, 36] signaling pathway components, as well as the adherens junctions at the hub cell–GSC interface [34, 37]. We found no evidence on changed expression or localization of STAT92E, phosphor-Mad (pMad), and Drosophila E-cadherin (DE-Cad) between E(Pc) and Ctrl GSCs (compare the GSCs indicated by yellow vs. green arrowheads in Fig. 5).
Fig. 5.
E(Pc) mutant GSC clones have unchanged JAK-STAT and BMP signaling activity, as well as normal DE-Cad level. a–i Apical tip of testes immunostained with GFP a, d, g to identify E(Pc) mutant clones (yellow arrowhead) and Ctrl cells (green arrowhead). Vasa b, e, h is used to identify germ cells. Immunostaining with STAT92E c, pMad f and DE-Cad i in E(Pc) mutant GSC clones and Ctrl GSCs shows no difference. Hub: asterisk. Scale bar: 20 μm.
Next, to examine whether E(Pc) mutant GSCs have impaired division rate, EdU incorporation assay and phosphor-histone H3 (pH3) staining were performed to measure the percentage of S-phase and mitotic GSCs, respectively. The percentages of both EdU-positive GSCs [E(Pc) KD testes: 20.3% (N = 113) vs. Ctrl testes: 20.5% (N = 122)] and pH3-positive GSCs [E(Pc) KD testes: 2.02% (N = 248) vs. Ctrl testes: 2.01% (N = 199 GSCs)] were comparable, suggesting normal cell cycle progression of E(Pc) mutant GSCs.
E(Pc) mutant germ cells accumulate DNA DSBs and have increased cell death
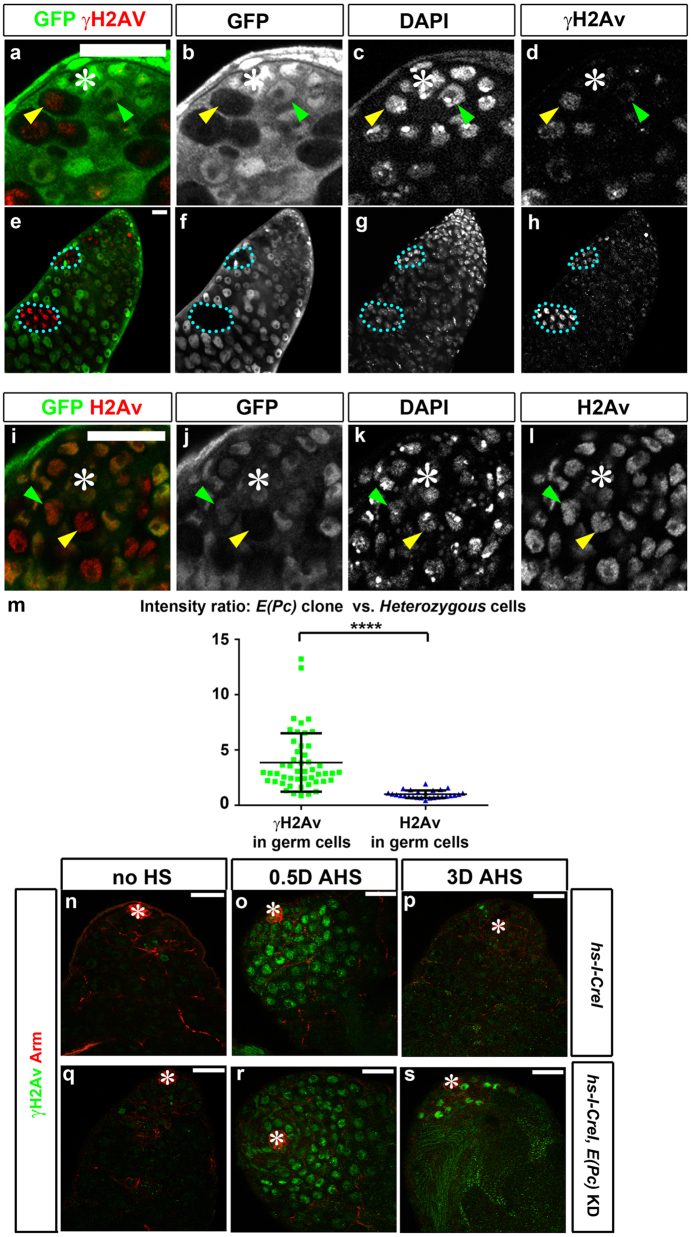
We next examined whether E(Pc) mutant GSCs have increased cell death using anti-phosphorylated H2Av (γH2Av) for immunostaining. Increased γH2Av was detected in E(Pc) mutant (N = 18) compared with Ctrl GSCs (Figs. 6a–d, compare the GSCs indicated by yellow vs. green arrowheads in Figs. 6d, m), and in E(Pc) spermatogonial clones (N = 31, Figs. 6e–h). Furthermore, increased γH2Av signal did not arise from increased H2Av (Figs. 6i–l, compare the GSCs indicated by yellow vs. green arrowheads in Figs. 6l, m). Therefore, E(Pc) mutant germ cells failed to exchange γH2Av with unphosphorylated H2Av, likely leading to accumulation of DNA DSBs.
Fig. 6.
E(Pc) germline clones exhibit increased γH2Av. a–l Apical tip of testes stained with GFP b, f, j to identify GFP-negative E(Pc) mutant clones, DAPI c, g, k, γH2Av d, h and H2Av l. m Quantification of intensity of γH2Av and H2Av signals between GFP-negative E(Pc) mutant clones and GFP-positive control germ cells. γH2Avclone/γH2Avctrl = 4.05 ± 2.58. H2Avclone/H2Avctrl = 1.00 ± 0.33 (mean ± SD). Two-tailed t-test. ****P < 0.0001. n–p Apical tip of hs-I-CreI testes before heat shock (no HS) n, a half day after heat shock (0.5D AHS) o and 3 days after heat shock (3D AHS) p stained with hub and cyst cell marker Arm and DNA DSB marker γH2Av. q–s Apical tip of hs-I-CreI, tub-Gal80ts, nos > E(Pc) shRNA testes before heat shock q, 0.5D AHS r, and 3D AHS s stained with Arm and γH2Av. Asterisk: hub. Scale bar: 20 μm
To further investigate this possibility, endonuclease I-CreI was expressed using heat shock (hs) promoter (hs-I-CreI) to introduce ectopic DNA DSBs at 18s ribosomal gene repeats [38]. Meanwhile, E(Pc) KD was temporally controlled using the tub-Gal80ts, nos > E(Pc) shRNA strain (as in Figs. 1, 2, 4).
Without heat shock, γH2Av signal was detected in very few germline cysts (<5/testis) in both hs-I-CreI (Ctrl, Fig. 6n) and hs-I-CreI, tub-Gal80ts, nos > E(Pc) shRNA [E(Pc) KD] testes under permissive condition (Fig. 6q). By contrast, robust γH2Av signal was detected in all germ cells 12 h after inducing I-CreI expression in both Ctrl (Fig. 6o) and E(Pc) KD (Fig. 6r) testes. After a 3-day recovery, DNA damage was efficiently repaired in most Ctrl (Fig. 6p) but not in E(Pc) KD (Fig. 6s) testes. Under this condition, only 16% of Ctrl testes (N = 24) were found to be devoid of germ cells, whereas 52% of E(Pc) KD testes (N = 27) had no germ cells. These results suggest that the germline KD of E(Pc) caused failure in DNA damage repair and germ cell loss.
Moreover, neither tub-Gal80ts, nos-Gal4 nor tub-Gal80ts, nos > E(Pc) shRNA control testes had detectable γH2Av using a similar regime (Supplementary Fig. 6). For tub-Gal80ts, nos > E(Pc) shRNA testes, the lack of detectable γH2Av likely resulted from partial retention of E(Pc) activity with a short time (≤3 days) under the restrictive condition. These results suggest that the differences in γH2Av signals (Figs. 6o–p vs. r–s) likely resulted from the failure to repair I-CreI-induced DNA damage in E(Pc) KD germ cells.
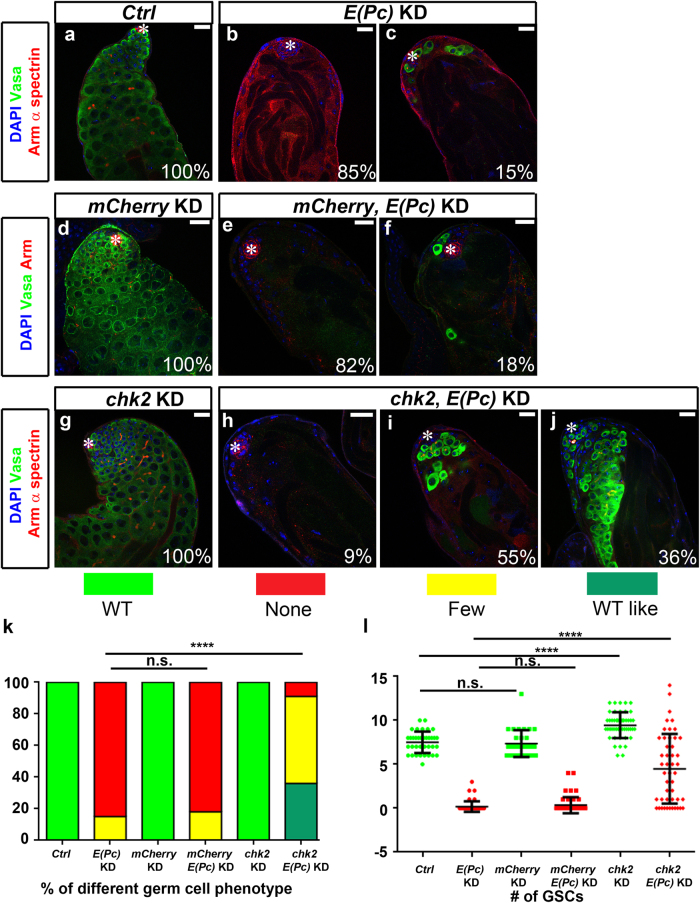
We next explored potential mechanisms underlying increased cell death in E(Pc) loss-of-function germ cells. In response to DNA DSBs, Chk2, a checkpoint kinase, is activated by ataxia-telangiectasia mutated kinase to regulate cell cycle arrest, DNA repair and apoptosis [39]. Recent studies revealed that DNA damage induces Drosophila female GSC loss, which could be rescued by reducing Chk2 [40]. Therefore, we tested the potential roles of Chk2 in regulating germ cell loss in E(Pc) KD testes. To enhance the KD effect, both Ctrl and E(Pc) KD males were grown at 29 °C, under which condition 100% of Ctrl testes showed normal morphology (Fig. 7a), whereas 85% of E(Pc) KD testes (N = 48) were completely devoid of germ cells (Fig. 7b) and 15% had fewer than 40 germ cells (Fig. 7c). Significant rescue of the germ cell loss (P < 10−4) was observed when chk2, but not the control mCherry, was compromised in E(Pc) KD testes (Figs. 7d–k). Quantification showed increased GSC number in chk2, E(Pc) double KD testes compared with E(Pc) single KD testes, but since chk2 KD itself led to GSC increase, this readout could be additive (Fig. 7l). Moreover, in these experiments because both E(Pc) and Chk2 were inactivated throughout Drosophila development, the overall readout could arise from either establishment or maintenance effects, or both.
Fig. 7.
CHK2 mediates germ cell loss induced by E(Pc) knockdown. a–j Control (Ctrl) [nos-Gal4/+], E(Pc) KD [nos > E(Pc) shRNA], mCherry KD [nos > mCherry shRNA], mCherry, E(Pc) KD [nos > mCherry shRNA, E(Pc) shRNA], chk2 KD [nos > chk2 shRNA], and chk2, E(Pc) KD [nos > chk2 shRNA, E(Pc) shRNA] testes stained with DAPI, germ cell marker Vasa, hub and cyst cell marker Arm, and fusome marker α-spectrin. Asterisk: hub. Scale bar: 20 μm. k Quantification of germ cell phenotype. ****P < 0.0001. Chi-square test. l Quantification of GSCs number (average ± SD). Mann–Whitney U-test. ****P < 0.0001.
Functional conservation between Drosophila E(Pc) and mammalian homolog
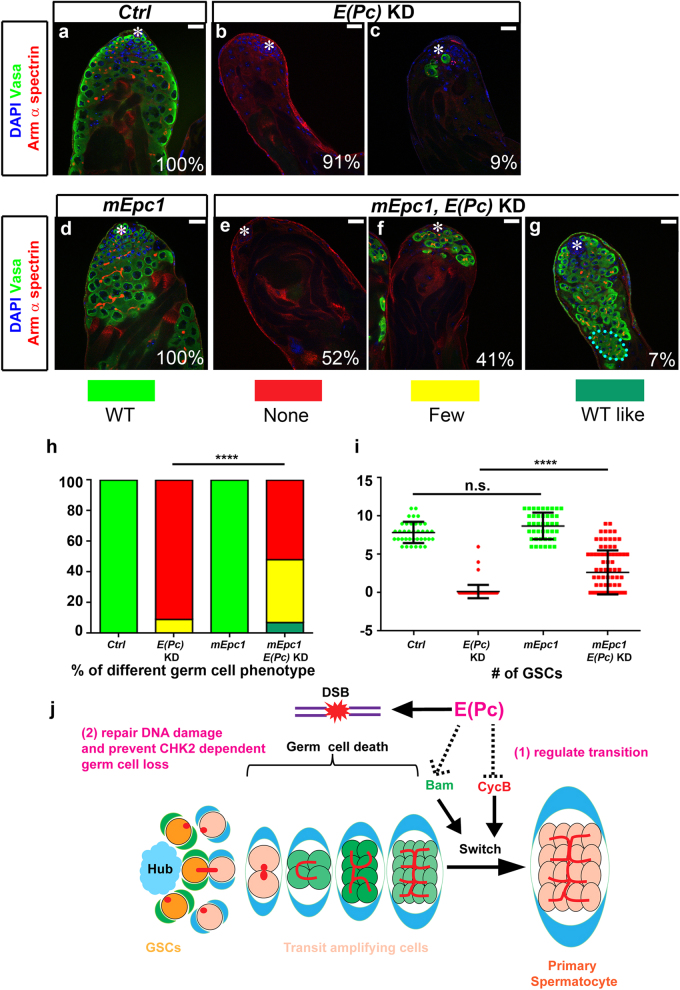
The E(Pc) gene is conserved from yeast to mammals, with two mouse homologs, Epc1 and Epc2. Epc1, compared with Epc2, shares more identity and similarity to the Drosophila E(Pc) [20]. When mouse Epc1 was expressed in germ cells with compromised E(Pc), significant (P < 10−4) rescue of the germ cell loss phenotype was observed (Figs. 8a–i), suggesting functional conservation between Drosophila E(Pc) and mouse Epc1.
Fig. 8.
Expression of mEpc1, mouse homolog of E(Pc), partially complements the germ cell loss phenotype in E(Pc) KD testes. a–g Testes from control (Ctrl) [nos-Gal4/+], E(Pc) KD [nos > E(Pc) shRNA], germline expression of mEpc1 [nos > mEpc1] and germline expression of mEpc1 at E(Pc) KD background [nos > mEpc1, E(Pc) shRNA] males stained with DAPI, germ cell marker Vasa, hub and cyst marker Arm, and fusome marker α-spectrin. Hub: asterisk. Scale bar: 20 μm. h Quantification of germ cell loss phenotype. ****P < 0.0001. Chi-square test. i Quantification of GSCs number (average ± SD). Mann–Whitney U-test. ****P < 0.0001. j A model depicting E(Pc) regulation of Drosophila male germ cell differentiation and maintenance. During spermatogenesis, the switch from mitosis-to-meiosis requires reduced cell cycle regulator CycB and accumulation of the differentiation factor Bam. E(Pc) represses CycB transcription and positively regulates Bam protein accumulation to promote the mitosis-to-meiosis transition (1). In addition, efficient DNA DSB repair to avoid spermatogonial cell death also requires E(Pc) (2).
Discussion
We herein report the critical roles of a chromatin factor E(Pc) in regulating GSC differentiation by targeting two critical genes. First, E(Pc) binds to the genomic region of CycB gene in S2 cells and negatively regulates CycB transcription in the germline, possibly through promoting H4 acetylation at CycB locus. Second, E(Pc) is required for proper accumulation of Bam protein in spermatogonia to promote the mitosis-to-meiosis transition. Moreover, E(Pc) is needed for effective DNA DSB repair in germ cells (Fig. 8j). Together, E(Pc) has pleiotropic roles in the male germline and such functions of E(Pc) could be cell type and stage specific.
Previous studies have shown transcriptional and post-transcriptional regulation of CycB in meiotic germ cells [23, 24, 41, 42]. Here, our studies have identified E(Pc) as an upstream regulator of CycB through modulating H4 acetylation. Histone acetylation is normally linked to gene activation. However, in E(Pc) mutant germ cells, decreased H4 acetylation was accompanied by increased Cyc B expression, indicating a potential repressive role of H4 acetylation, as reported previously. For example, when E(Pc) or Tip60 is knocked down in cyst cells of Drosophila testes, both zfh-1 and yan are upregulated [21]. Moreover, H3K56ac was found to repress transcription of newly replicated DNA in budding yeast [43]. Noticeably, a recent study revealed that expression of the dominant negative dTip60E431Q or KD of E(Pc) reduced CycB expression in Drosophila wing disc, suggesting that the regulation of dTip60 and E(Pc) on CycB could be cell type and/or developmental stage dependent [44].
Bam is both necessary and sufficient for male germline differentiation and exhibits a highly orchestrated spatiotemporal expression pattern, which is regulated by transcriptional, post-transcriptional and protein stability mechanisms [2, 6, 7, 42, 45]. Here, we found that E(Pc) regulates Bam protein accumulation via an unknown post-transcriptional mechanism(s). A recent study in Drosophila female GSC lineage demonstrates that Bam is a component of a deubiquitinase complex and that Bam-dependent deubiquitination stabilizes cyclin A (CycA) [46], suggesting a connection between Bam and cell cycle genes.
Germ cells have a unique quality control mode for survival [10–12]. Our studies suggest that E(Pc) could participate in such a control. In Drosophila testis, GSCs, gonialblasts, and spermatogonia undergo DNA replication and mitosis. Replication stress, such as replication fork collapse caused by unrepaired DNA lesion, could cause DNA DSBs. Indeed, testes from flies fed with the replicative stress-inducing agent hydroxyurea have intensive γH2Av signals in mitotic germ cells [47]. Under normal conditions, lack of γH2Av signal in the germline results from robust DNA repair. Under both physiological conditions and induction of ectopic DNA damage with endonuclease expression, compromising E(Pc) in germ cells led to accumulated DNA damage, demonstrated by robust γH2Av and severe germ cell loss. Reminiscent of the roles of E(Pc) in the male germline, female germline with compromised E(Pc) produce no mature eggs and have severe cell death [48], suggesting similar requirement of E(Pc) for both male and female germ cell survival.
Abnormal activity of the human E(Pc) homolog EPC1 leads to T-cell leukemia/lymphoma [49]. Moreover, analysis of 99 pancreatic tumors has identified significantly higher mutation rate at the Epc1 locus, suggesting a potential tumor-suppressor role of EPC1 in pancreas [50]. However, the in vivo roles of E(Pc) remain unclear. Here, our studies provide insights into the molecular and cellular mechanisms of E(Pc) in a well-established adult stem cell system, which will help understanding its normal functions and relationship with diseases.
Materials and methods
Fly strains and husbandry
Fly stocks were raised under standard yeast/molasses medium at 25 °C unless stated otherwise. The following flies were used: E(Pc)1 (Bloomington Drosophila Stock Center, BL3056), E(Pc)w3 (BL9396), UAS-E(Pc) gDNA-GFP and UAS-E(Pc) cDNA-GFP [21], UAS-E(Pc) shRNA (BL35271), UAS-dTip60E431Q (from Felice Elefant, Drexel University, Philadelphia, Pennsylvania, USA), UAS-chk2 shRNA (BL35152), UAS-mCherry shRNA (from Mark Van Doren, Johns Hopkins University, Baltimore, Maryland, USA), hs-I-CreI [51], nos-Gal4/Cyo [32], nos-Gal4/Cyo;tub-Gal80ts/TM6B (from Yukiko Yamashita, University of Michigan, Ann Arbor, Michigan, USA), bam-HA [6], FRT42B (BL1956), CycB2/Cyo (BL6630).
To study E(Pc) and Tip60 function in germ cells, UAS-E(Pc) shRNA, and UAS-dTip60E431Q were crossed with nos-Gal4/Cyo at 25 °C separately. To study intermediate germ cell phenotype induced by E(Pc) KD, flies with the following genotype were generated: nos-Gal4/Cyo;tub-Gal80ts/UAS-E(Pc)shRNA. One-day-old males were shifted to 29 °C to induce expression of E(Pc)shRNA by inactivating tub-Gal80ts.
To explore if loss of CycB or increased Bam could rescue the overproliferative spermatogonial tumor phenotype induced by E(Pc) KD in germ cells, the following flies were generated: nos-Gal4/CycB2; UAS-E(Pc)shRNA/tub-Gal80ts, nos-Gal4/bam-HA; UAS-E(Pc)shRNA/tub-Gal80ts, nos-Gal4/Cyo;tub-Gal80ts/UAS-E(Pc)shRNA. One-day-old males from 18 °C were collected and shifted to 29 °C.
To investigate if expression of mEPc1 could rescue the E(Pc) KD germ cell loss phenotype, the following fly strains were generated: nos-Gal4/+, nos-Gal4/+; UAS-E(Pc)shRNA/+, nos-Gal4/UASp-mEpc1 complementary DNA (cDNA), nos-Gal4/UASp-mEpc1 cDNA; UAS-E(Pc)shRNA/+. One-day-old males were shifted to 29 °C to enhance E(Pc)shRNA KD effects, followed by dissection at 5 days.
To investigate the roles of Chk2 in E(Pc) KD testes, the following flies were generated: nos-Gal4/+, nos-Gal4/+; UAS-E(Pc) shRNA/+, nos-Gal4/+; UAS-Chk2 shRNA/+, nos-Gal4/+; UAS-Chk2 shRNA/UAS-E(Pc) shRNA, nos-Gal4/+; UAS-mCherry shRNA/+, nos-Gal4/+; UAS-mCherry shRNA/ UAS-E(Pc) shRNA. One-day-old males were shifted to 29 °C to enhance E(Pc)shRNA KD effects, followed by dissection at 5 days.
To induce DNA damage, 1-day-old hs-I-CreI males were heat shocked at 37 °C for 30 min and then transferred to 29 °C for half a day or 3 days before dissection. To investigate the roles of E(Pc) in DNA damage repair, the following flies were generated: nos-Gal4/tub-Gal80ts; UAS-E(Pc) shRNA/hs-I-CreI, nos-Gal4/tub-Gal80ts; hs-I-CreI/+, nos-Gal4/tub-Gal80ts; UAS-E(Pc) shRNA/+, nos-Gal4/tub-Gal80ts.
Clonal analysis
To generate E(Pc) clones, either E(Pc)w3 or E(Pc)1 allele was recombined with FRT42B to generate FRT42B, E(Pc)w3/Cyo or FRT42B, E(Pc)1/Cyo flies. Adult flies with the following genotypes were raised at 25 °C until pupal stage: hs-FLP; FRT42B, Ubi-GFP/FRT42B, E(Pc)w3 or hs-FLP; FRT42B, Ubi-GFP/FRT42B, E(Pc)1 and control flies hs-FLP; FRT42B, Ubi-GFP/FRT42B. Then they were heat shocked for 2 h each on 2 consecutive days. One-day after heat shock, adult flies were collected and aged until dissection.
Generation of transgenic fly lines
For transgenic fly UASp-mEpc1 cDNA, Epc1 was amplified using the mouse sperm cDNA library. The Epc1 cDNA was amplified as a KpnI and XbaI flanked fragment with Epc1 F1 and R1 primers. Epc1 was then ligated into pGEM-T-easy vector (Promega) followed by sequencing. The full-length Epc1 cDNA was retrieved with KpnI and XbaI and ligated into UASp vector cut with the same two enzymes. Transgenic fly lines were generated by Bestgene Inc. (Chino Hills, CA).
Primers:
Epc1 F1: 5ʹ-CGGGGTACCATGGAACACCATCTTCAGCG-3ʹ;
Epc1 R1: 5ʹ-TGCTCTAGATTACGTCACCTCCATTGCTACTGTG-3ʹ.
Immunofluorescence
Testes were dissected and immunostained as previously described [52]. Primary antibodies were as follows: Vasa (rabbit, 1:200, Santa Cruz, sc-30,210), Vasa (rat, 1:50, DSHB), Fas III (mouse, 1:100, DSHB, 7G10), Armadillo (mouse, 1:200, DSHB, N2 7A1), α-spectrin (mouse, 1:50, DSHB, 3A9), GFP (chicken, 1:1000, Abcam, ab13970), STAT92E (rabbit, 1:800, from Denise Montell, UC Santa Barbara), pMAD (rabbit, 1:100, Abcam, ab52903), DE-Cad (rat, 1:20, DSHB, DCAD2), anti-Ser10-phosphorylated Histone H3 (mouse, 1:1000, Millipore, #05–806), H3K27me3 (rabbit, 1:2000, Millipore, #07–449), H3K4me3 (rabbit, 1:2000, Millipore, #07–473), H2Av (rabbit, 1:500, active motif, 39,716), γ-H2Av [53, 54] (pH2AvDS137, rabbit, 1:1000, Rockland, 600–401–914), H4 tetra-acetylation (rabbit, 1:500, from Keji Zhao, NHLBI, NIH), H4 (rabbit, 1:200, Abcam, ab10158), Bam-C (mouse, 1:20, DSHB, bam), CycB (mouse, 1:20, DSHB, F2F4). For CycB and Bam-C staining, testes were dissected in 1 × phosphate-buffered saline and transferred onto slides. Slides were frozen in liquid nitrogen, followed by fixation in chilled methanol for 5 min and acetone for 2 min. Then testes were incubated with primary antibodies as mentioned.
Secondary antibodies were all Alexa Fluor series (1:200, Molecular Probes). Images were taken with Zeiss LSM 510 META or LSM 700 using LSM software. Images were processed using Adobe Photoshop. Lysotracker (Invitrogen, L-7528) was used to label cells undergoing cell death, following the manufacturer’s protocol. EdU incorporation was performed with the Click-iT EdU Alexa Fluor 488 Imaging Kit (Invitrogen C10083). Dissected testes were incubated with EdU solution for 30 min, followed by fixation and immunostaining steps, as described.
ChIP-seq and data analysis
To perform S2 cell ChIP, transfection with 1 μg Actin-Gal4 and 1 μg UAS-E(Pc) cDNA-GFP plasmids was done following the manufacturer’s protocol with Effectene Transfection Reagent (#301425, Qiagen). S2 cells were split the day before the transfection experiment. On the day of transfection, 4 × 106 cells per 25 cm2 flask were seeded in 4 ml growth medium containing serum and antibiotics. After transfection, S2 cells were incubated at room temperature for 40–48 h to obtain maximal level of gene expression. S2 cell fixation and subsequent ChIP were performed with Active Motif’s ChIP-IT High Sensitivity Kit, following the manufacturer’s instruction. Sonication of S2 cells was done with the Bioruptor UCD-200 sonicator (Diagenode), using the following settings: 0.5-min ON and 1-min OFF for a total of 15 min. Fragment size was around 500–600 bp. ChIP-grade GFP antibody (Abcam, ab290) [21, 55–59] was used to pull down sheared chromatin.
Libraries were generated using reagents provided in the Illumina TruSeq ChIP Sample Preparation Kit (IP-202-1012). The Illumina compatible libraries were sequenced with Hi-seq sequencer (Hi-Seq, Illumina). Then 75-bp pair-end read sequencing was performed. FASTQ raw data files were filtered with the Fastqc quality control software (www.bioinformatics.babraham.ac.uk/projects/fastqc/). The BOWTIE program (version 0.12.7) was utilized to align reads to Drosophila genome (dm3), with the running parameters (bowtie -p 8 -t -a–phred33 -quals -n 2 -e 70 -l 48 -m 1–best–strata). Pair-end reads were treated as separate single reads. At each chromosome position, only one read was retained to get non-redundant read count data. SAM (Sequence Alignment/Map)-formatted alignment files will be uploaded onto NIH GEO database upon proper acceptance. Enrichment of reads across the genome was analyzed by MACS2 for peak calling, which was performed with paired experiment and control genome input under default parameter settings. UCSC genome browser customized visualization tools were also applied in the analysis. SAM tools software suite was utilized to convert between related read formats.
ChIP quantitative PCR (qPCR)
As previously described, a similar protocol was used for ChIP with H4 tetra-acetylation antibody (A gift from Keji Zhao, NHLBI, NIH) on 100 pairs of wild-type testes. The sonication setting was 0.5-min ON and 1-min OFF repetitively for a total of 30 min. qPCR was performed as previously described. Two independent biological replicates were used. Each PCR reaction was performed in duplicate, and average Ct values were used.
Primers used for CycB qPCR were as follows:
P5 forward: 5ʹ-AGTCCCTTGAGCAGTTTTCG-3ʹ,
P5 reverse: 5ʹ-CTCCAGTTTAGTTCGGTTCCTAG-3ʹ;
P4 forward: 5ʹ-AGAAAGAGTGCCGTTTGTCC-3ʹ,
P4 reverse: 5ʹ-ATTGCGCCAAAATGTGAGC-3ʹ;
P3 forward: 5ʹ-TCGGAGAATTGACAATCCCG-3ʹ,
P3 reverse: 5ʹ-AGACGCTTGGCTATCACTTG-3ʹ;
P2 forward: 5ʹ-ATATGCTAATGAGCCAGAGCG-3ʹ,
P2 reverse: 5ʹ-GCCATCGCCTCAGAATTTTG-3ʹ;
P1 forward: 5ʹ-CTTCCTTATGGATCGACACTCG-3ʹ,
P1 reverse: 5ʹ-TGCGTTTAAATGACAAAAGACTGG-3ʹ.
Quantitative RT-PCR
The cDNA preparation of testes and RT-PCR were performed and analyzed as previously described. Each PCR reaction was performed in triplicate, and Ct values were averaged. Three biological replicates were used.
Primers:
bam forward: 5ʹ-ACTCAGCGCATGGAGAGATTGCTA-3ʹ,
bam reverse: 5ʹ-AGTAGCGGTGCTCCAGATCCATTT-3ʹ;
CycB forward: 5ʹ-CTGTTCGTTTCGTGTTCGTTAAA-3ʹ,
CycB reverse: 5ʹ-CAAGGGACTCCAGCAGATTAC-3ʹ;
rpl32 forward: 5ʹ-CATGCTGCCCACCGGATTCAAGAAG-3ʹ,
rpl32 reverse: 5ʹ-CTCGTTCTCTTGAGAACGCAGGCGA-3ʹ.
Quantification of immunofluorescent signal intensity
Fluorescence intensity between the GFP-positive control cells and GFP-negative E(Pc) mutant cells in the same testes was quantified using ImageJ software. Data were analyzed and presented using GraphPad Prism software.
Data availability
GEO accession number for ChIP-seq data is GSE98484.
Electronic supplementary material
Acknowledgements
We thank Drs. M. Van Doren, D. Drummond-Barbosa, R. Johnston, and Chen lab members for critical comments. We thank Dr. F. Elefant for the UAS-dTip60E431Q Drosophila line, Dr. Y. Yamashita for the nos-Gal4/Cyo;tub-Gal80ts/TM6B Drosophila line, Dr. Ting Xie for the hs-I-CreI Drosophila line, TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks used in this study, Keji Zhao for anti-ace H4 antibodies, and the Developmental Studies Hybridoma Bank for antibodies. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. This study was supported by NIH RO1HD065816, the David and Lucile Packard Foundation, Howard Hughes Medical Institute, Bill & Melinda Gates Foundation and the Simons Foundation, as well as Johns Hopkins University start-up (XC).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Zhen Shi, Jing Xie, and Binbin Ma contributed equally to this work.
Edited by E Baehrecke
Electronic supplementary material
The online version of this article (10.1038/s41418-017-0056-5) contains supplementary material, which is available to authorized users.
References
- 1.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT. Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc Natl Acad Sci USA. 2009;106:22311–6. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonczy P, Matunis E, DiNardo S. Bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–71. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- 4.McKearin DM, Spradling AC. Bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–51. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- 5.Schulz C, et al. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics. 2004;167:707–23. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eun SH, et al. MicroRNAs downregulate Bag of marbles to ensure proper terminal differentiation in the Drosophila male germline. Development. 2013;140:23–30. doi: 10.1242/dev.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insco ML, et al. A self-limiting switch based on translational control regulates the transition from proliferation to differentiation in an adult stem cell lineage. Cell Stem Cell. 2012;11:689–700. doi: 10.1016/j.stem.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng L, Chen X. Epigenetic regulation of germ cells-remember or forget? Curr Opin Genet Dev. 2015;31:20–27. doi: 10.1016/j.gde.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan Q, et al. Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq. Cell Res. 2010;20:763–83. doi: 10.1038/cr.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yacobi-Sharon K, Namdar Y, Arama E. Alternative germ cell death pathway in Drosophila involves HtrA2/Omi, lysosomes, and a caspase-9 counterpart. Dev Cell. 2013;25:29–42. doi: 10.1016/j.devcel.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Yamashita YM. The regulated elimination of transit-amplifying cells preserves tissue homeostasis during protein starvation in Drosophila testis. Development. 2015;142:1756–66. doi: 10.1242/dev.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan S, Hetie P, Matunis EL. Niche signaling promotes stem cell survival in the Drosophila testis via the JAK-STAT target DIAP1. Dev Biol. 2015;404:27–39. doi: 10.1016/j.ydbio.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailly A, Gartner A. Germ cell apoptosis and DNA damage responses. Adv Exp Med Biol. 2013;757:249–76. doi: 10.1007/978-1-4614-4015-4_9. [DOI] [PubMed] [Google Scholar]
- 14.Bonner WM, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikura T, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–73. doi: 10.1016/S0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 16.Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 2006;16:433–42. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, et al. The chromodomain-containing histone acetyltransferase TIP60 acts as a code reader, recognizing the epigenetic codes for initiating transcription. Biosci Biotechnol Biochem. 2015;79:532–8. doi: 10.1080/09168451.2014.993914. [DOI] [PubMed] [Google Scholar]
- 18.Kusch T, et al. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 2004;306:2084–7. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair DA, et al. Enhancer of polycomb is a suppressor of position-effect variegation in Drosophila melanogaster. Genetics. 1998;148:211–20. doi: 10.1093/genetics/148.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stankunas K, et al. The enhancer of polycomb gene of Drosophila encodes a chromatin protein conserved in yeast and mammals. Development. 1998;125:4055–66. doi: 10.1242/dev.125.20.4055. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Shi Z, Chen X. Enhancer of polycomb coordinates multiple signaling pathways to promote both cyst and germline stem cell differentiation in the Drosophila adult testis. PLoS Genet. 2017;13:e1006571. doi: 10.1371/journal.pgen.1006571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin A, Gally C, Netter S, Anxolabehere D, Ronsseray S. Telomeric associated sequences of Drosophila recruit polycomb-group proteins in vivo and can induce pairing-sensitive repression. Genetics. 2003;164:195–208. doi: 10.1093/genetics/164.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White-Cooper H, Schafer MA, Alphey LS, Fuller MT. Transcriptional and post-transcriptional control mechanisms coordinate the onset of spermatid differentiation with meiosis I in Drosophila. Development. 1998;125:125–34. doi: 10.1242/dev.125.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Baker CC, Gim BS, Fuller MT. Cell type-specific translational repression of cyclin B during meiosis in males. Development. 2015;142:3394–402. doi: 10.1242/dev.122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs HW, Knoblich JA, Lehner CF. Drosophila cyclin B3 is required for female fertility and is dispensable for mitosis like cyclin B. Genes Dev. 1998;12:3741–51. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soto MC, Chou TB, Bender W. Comparison of germline mosaics of genes in the polycomb group of Drosophila melanogaster. Genetics. 1995;140:231–43. doi: 10.1093/genetics/140.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudreault AA, et al. Yeast enhancer of polycomb defines global Esa1-dependent acetylation of chromatin. Genes Dev. 2003;17:1415–28. doi: 10.1101/gad.1056603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorbeck M, Pirooznia K, Sarthi J, Zhu X, Elefant F. Microarray analysis uncovers a role for Tip60 in nervous system function and general metabolism. PLoS ONE. 2011;6:e18412. doi: 10.1371/journal.pone.0018412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tea JS, Luo L. The chromatin remodeling factor Bap55 functions through the TIP60 complex to regulate olfactory projection neuron dendrite targeting. Neural Dev. 2011;6:5. doi: 10.1186/1749-8104-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Kirmizis A, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–5. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 33.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–9. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 34.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–11. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarayrah L, Li Y, Gan Q, Chen X. Epigenetic regulator Lid maintains germline stem cells through regulating JAK-STAT signaling pathway activity. Biol Open. 2015;4:1518–27. doi: 10.1242/bio.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–75. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 37.Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS ONE. 2010;5:e12473. doi: 10.1371/journal.pone.0012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggert KA, Golic KG. Highly efficient sex chromosome interchanges produced by I-CreI expression in Drosophila. Genetics. 2005;171:1103–14. doi: 10.1534/genetics.104.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stracker TH, Usui T, Petrini JH. Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (Amst) 2009;8:1047–54. doi: 10.1016/j.dnarep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, et al. DNA damage-induced CHK2 activation compromises germline stem cell self-renewal and lineage differentiation. Development. 2016;143:4312–23. doi: 10.1242/dev.141069. [DOI] [PubMed] [Google Scholar]
- 41.Baker CC, Fuller MT. Translational control of meiotic cell cycle progression and spermatid differentiation in male germ cells by a novel eIF4G homolog. Development. 2007;134:2863–9. doi: 10.1242/dev.003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monk AC, et al. HOW is required for stem cell maintenance in the Drosophila testis and for the onset of transit-amplifying divisions. Cell Stem Cell. 2010;6:348–60. doi: 10.1016/j.stem.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Voichek Y, Bar-Ziv R, Barkai N. Expression homeostasis during DNA replication. Science. 2016;351:1087–90. doi: 10.1126/science.aad1162. [DOI] [PubMed] [Google Scholar]
- 44.Flegel K, Grushko O, Bolin K, Griggs E, Buttitta L. Roles for the histone modifying and exchange complex NuA4 in cell cycle progression in Drosophila melanogaster. Genetics. 2016;203:1265–81. doi: 10.1534/genetics.116.188581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monk AC, Siddall NA, Fraser B, McLaughlin EA, Hime GR. Differential roles of HOW in male and female Drosophila germline differentiation. PLoS One. 2011;6:e28508. doi: 10.1371/journal.pone.0028508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji S, et al. Bam-dependent deubiquitinase complex can disrupt germ-line stem cell maintenance by targeting cyclin A. Proc Natl Acad Sci USA. 2017;114:6316–21. doi: 10.1073/pnas.1619188114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landais S, D’Alterio C, Jones DL. Persistent replicative stress alters polycomb phenotypes and tissue homeostasis in Drosophila melanogaster. Cell Rep. 2014;7:859–70. doi: 10.1016/j.celrep.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan D, et al. A regulatory network of Drosophila germline stem cell self-renewal. Dev Cell. 2014;28:459–73. doi: 10.1016/j.devcel.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakahata S, et al. Alteration of enhancer of polycomb 1 at 10p11.2 is one of the genetic events leading to development of adult T-cell leukemia/lymphoma. Genes Chromosomes Cancer. 2009;48:768–76. doi: 10.1002/gcc.20681. [DOI] [PubMed] [Google Scholar]
- 50.Biankin AV, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rong YS, et al. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–81. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eun SH, Shi Z, Cui K, Zhao K, Chen X. A non-cell autonomous role of E(z) to prevent germ cells from turning on a somatic cell marker. Science. 2014;343:1513–6. doi: 10.1126/science.1246514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu KL, Yamashita YM. Germ cell connectivity enhances cell death in response to DNA damage in the Drosophila testis. Elife. 2017;6:e27960. doi: 10.7554/eLife.27960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madigan JP, Chotkowski HL, Glaser RL. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 2002;30:3698–705. doi: 10.1093/nar/gkf496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar SV, Wigge PA. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–47. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Asadzadeh J, Neligan N, Kramer SG, Labrador JP. Tinman regulates NetrinB in the cardioblasts of the Drosophila dorsal vessel. PLoS ONE. 2016;11:e0148526. doi: 10.1371/journal.pone.0148526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bras-Pereira C, et al. Dachshund potentiates Hedgehog signaling during Drosophila retinogenesis. PLoS Genet. 2016;12:e1006204. doi: 10.1371/journal.pgen.1006204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castel SE, et al. Dicer promotes transcription termination at sites of replication stress to maintain genome stability. Cell. 2014;159:572–83. doi: 10.1016/j.cell.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saredi G, et al. H4K20me0 marks post-replicative chromatin and recruits the TONSL-MMS22L DNA repair complex. Nature. 2016;534:714–8. doi: 10.1038/nature18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
GEO accession number for ChIP-seq data is GSE98484.