Abstract
PD-1 is an immunoreceptor that belongs to the immunoglobulin (Ig) superfamily and contains two tyrosine residues in the cytoplasmic region. Studies on PD-1-deficient mice have shown that PD-1 plays critical roles in establishment and/or maintenance of peripheral tolerance, but the mode of action is totally unknown. To study the molecular mechanism for negative regulation of lymphocytes through the PD-1 receptor, we generated chimeric molecules composed of the IgG Fc receptor type IIB (FcγRIIB) extracellular region and the PD-1 cytoplasmic region and expressed them in a B lymphoma cell line, IIA1.6. Coligation of the cytoplasmic region of PD-1 with the B cell receptor (BCR) in IIA1.6 transformants inhibited BCR-mediated growth retardation, Ca2+ mobilization, and tyrosine phosphorylation of effector molecules, including Igβ, Syk, phospholipase C-γ2 (PLCγ2), and ERK1/2, whereas phosphorylation of Lyn and Dok was not affected. Mutagenesis studies indicated that these inhibitory effects do not require the N-terminal tyrosine in the immunoreceptor tyrosine-based inhibitory motif-like sequence, but do require the other tyrosine residue in the C-terminal tail. This tyrosine was phosphorylated and recruited src homology 2-domain-containing tyrosine phosphatase 2 (SHP-2) on coligation of PD-1 with BCR. These results show that PD-1 can inhibit BCR signaling by recruiting SHP-2 to its phosphotyrosine and dephosphorylating key signal transducers of BCR signaling.
The immunoreceptor PD-1 belongs to the Ig superfamily (1). PD-1 contains two tyrosine residues in its cytoplasmic region, the N-terminal of which is embedded in a sequence defined as the immunoreceptor tyrosine-based inhibitory motif (ITIM), I/L/VXYXXL/V (1–4). The expression of PD-1 can be detected at certain developmental stages of thymocytes (i.e., double-negative to double-positive stages) and on the activated peripheral T and B lymphocytes (5, 6).
PD-1-deficient mice exhibit splenomegaly, selective augmentation of IgG3 Ab response to a T-independent type II antigen, and enhanced proliferative responses of B cells and myeloid cells by anti-IgM and granulocyte colony-stimulating factor stimulation, respectively (ref. 7 and unpublished data). Thus, PD-1 appears to inhibit immune responses in vivo, and abrogation of this inhibition would result in development of autoimmune diseases. Indeed, PD-1-deficient C57BL/6 mice spontaneously develop typical lupus-like glomerulonephritis and destructive arthritis (8). In addition, we have recently reported that PD-1-deficient BALB/c mice die of autoantibody-mediated dilated cardiomyopathy (9). Because PD-L1, a ligand of PD-1, is highly expressed on heart (10), and the antigen recognized by the autoantibody is strictly restricted to heart, we assume that the direct interaction between the heart tissue and B cells by means of PD-1/PD-L1 is responsible for the prevention of this deadly disease. So the negative effect of PD-1 on B cell receptor (BCR) signaling is strongly suggested, although no direct evidence of the inhibitory effect has been obtained.
Because the sequence surrounding the N-terminal tyrosine fulfills the requirement of ITIM, identified in the cytoplasmic region of immunoinhibitory receptors such as IgG Fc receptor type IIB (FcγRIIB), killer Ig-like receptor (KIR), CD22, and the paired Ig-like receptor B, it is quite natural to speculate that PD-1 mediates its negative signal by means of this tyrosine residue. On stimulation, tyrosine residues of ITIMs are usually phosphorylated to recruit Src homology 2 (SH2)-containing phosphatases that play critical roles in negative regulation of cellular activities. Among ITIM-bearing immunoinhibitory receptor-deficient mice, only FcγRIIB-deficient mice spontaneously develop autoimmune diseases (11–14). In C57BL/6 background, FcγRIIB-deficient mice develop glomerulonephritis, which is similar to but different from that of PD-1-deficient mice. In the BALB/c background, FcγRIIB-deficient mice are reported to be healthy, whereas PD-1-deficient mice develop autoimmune dilated cardiomyopathy (9, 14), suggesting that PD-1 and FcγRIIB regulate the immune system in a similar but distinct manner.
According to the multistep model for BCR signaling (15), BCR stimulation first activates Lyn, which, in turn, phosphorylates the tyrosine residue in the immunoreceptor tyrosine-based activation motif of the Igα and Igβ, resulting in recruitment of another protein tyrosine kinase, Syk. Activated protein tyrosine kinases also phosphorylate a tyrosine residue in ITIM, which recruits SH2-containing phosphatases to dephosphorylate and deactivate signal transducers. Thus regulation of protein tyrosine phosphorylation is important in both the activation and inhibition of B cells. Among ITIM-bearing immunoinhibitory receptors, FcγRIIB is reported to recruit SH2-domain-containing phosphatidylinositol 5-phosphatase (SHIP) on coligation with BCR, engagement of which leads to deactivation of CD19 and inhibition of signaling downstream of CD19 (16, 17). On the other hand, paired Ig-like receptor B is reported to recruit src homology 2-domain-containing tyrosine phosphatase 1 (SHP-1) and SHP-2, engagement of which leads to dephosphorylation of a wider variety of molecules, including Igα/β, Syk, Btk, and phospholipase C-γ2 (PLCγ2) (18). Most of ITIM-bearing immunoinhibitory receptors such as FcγRIIB, paired Ig-like receptor B, and CD72 are expressed constitutively on B cells, whereas PD-1 is expressed only on activated B cells, suggesting that PD-1 may have some unique and important roles in the regulation of B cell activation, which leads to the prevention of autoimmune diseases.
We report here that PD-1 signaling inhibited growth retardation, protein tyrosine phosphorylation, and Ca2+ mobilization of antigen-stimulated B lymphoma cell line IIA1.6. Unexpectedly, coligation of PD-1 and BCR recruited SHP-2 preferentially to the C-terminal phosphotyrosine in its cytoplasmic tail, resulting in dephosphorylation of effector molecules and down-regulation of downstream molecules.
Materials and Methods
Cells, Expression Constructs, and Antibodies.
Murine B cell line IIA1.6 was maintained in RPMI medium 1640 supplemented with 10% FCS, 50 mM 2-mercaptoethanol, 2 mM l-glutamine, and antibiotics. FcγRIIB and FcKIR constructs were as described (16). The mouse PD-1 cDNA fragment downstream of the EcoRV site was fused with the mouse FcγRIIB cDNA fragment upstream of the ApaI site by blunting ligation to obtain FcPD chimera. One copy of a flag tag was added at the C terminus of all constructs by PCR methods as shown in Fig. 1A. Introduction of mutations at tyrosine residues and truncation of the cytoplasmic region were carried out by PCR methods. The resulting constructs were confirmed by DNA sequencing and subcloned into the pMX-IRES-EGFP vector (EGFP, enhanced green fluorescence protein) (19). BOSC23 cells were transfected by these vectors to obtain retroviruses, which were subsequently used to infect IIA1.6 cells. EGFP-positive infectants were sorted by FACS Vantage (Becton Dickinson) to obtain an almost 100% pure population. Intact Abs and F(ab′)2 fragments of rabbit anti-IgG (H + L) (Zymed), anti-IgG2a, hamster anti-CD3 (PharMingen), anti-SHP-2, anti-SHIP, anti-Lyn, anti-Syk, anti-PLCγ, anti-Dok, anti-RasGAP, anti-ERK1, anti-ERK2 (Santa Cruz Biotechnology), anti-flag (Sigma), anti-phospho-ERK (New England Biolabs), anti-phosphotyrosine (Transduction Laboratories, Lexington, KY), and streptavidin (Vector Laboratories) were purchased. Anti-mouse PD-1 mAb (J43) (5), anti-SHP-1, and anti-Igβ were as described elsewhere (18).
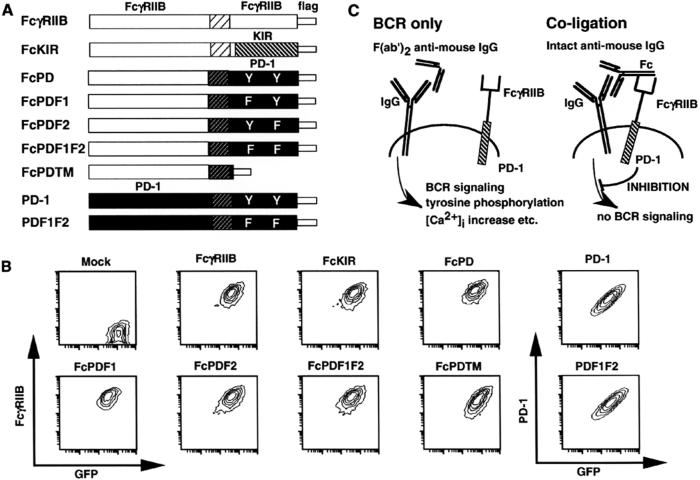
Figure 1.
Strategies to dissect inhibitory mechanisms of PD-1. (A) Fc chimera and PD-1 constructs. The cytoplasmic regions of PD-1, its mutants, and KIR were fused with the extracellular region of FcγRIIB. A flag tag was added to each Fc chimera. (B) Cell surface expression of Fc chimeras and PD-1. Sorted Fc chimera and PD-1 transformants were stained with anti-FcγRIIB and anti-PD-1 mAb, respectively. GFP, green fluorescence protein. (C) Schematic representation of stimulation strategy. Incubation with intact anti-mouse IgG Ab results in coligation of Fc chimera and BCR (Right), whereas a F(ab′)2 fragment of anti-mouse IgG Ab results in BCR-only ligation (Left).
Calcium Measurements.
Cells (2 × 106) were loaded with 4 μM fura-2/AM (Molecular Probes) in Krebs Hepes-buffered saline without calcium at 37°C for 45 min. After the cells were washed twice, they were resuspended with Krebs Hepes-buffered saline supplemented with 1 mM CaCl2. Cytosolic calcium concentrations of 5 × 105 cells were measured with a CAF 110 spectrophotometer (Jasco, Tokyo) as described (20). PD-1-expressing cells were stimulated with 2 μg/ml biotinylated anti-PD-1 mAb and 8 μg/ml biotinylated anti-IgG2a mAb, followed by the addition of 6 μg/ml streptavidin to coligate PD-1 and BCR. For BCR-only ligation, anti-PD-1 mAbs were replaced with 2 μg/ml biotinylated hamster anti-CD3 mAb. Intact Abs or F(ab′)2 fragments of rabbit anti-mouse IgG (10 μg/ml) were used, respectively, for coligation of Fc chimera and BCR or ligation of BCR only.
Proliferation Assay.
IIA1.6 transformants (1 × 104) were incubated in 96-well U-bottomed culture dishes for 48 h with the indicated amount of intact Abs or F(ab′)2 fragments of anti-mouse IgG Abs, pulsed with 0.5 μCi of [methyl-3H]thymidine per well (Amersham Pharmacia) for the last 4 h, and then harvested with Ready Filter (Beckman Instruments, Fullerton, CA) for Skatron (Skatron Instruments, Lier, Norway) followed by scintillation counting. The percentage of 3H incorporation was calculated by dividing incorporation counts per minute (cpm) in the presence of Abs by that in the absence of Abs.
Immunoprecipitation, in Vitro Kination, and Western Blot Analysis.
IIA1.6 transformants (1.5 × 107 cells per ml) were incubated for 2 min at 37°C with 25 μg/ml intact Abs or F(ab′)2 fragments of rabbit anti-IgG Abs. Cells were solubilized in a lysis buffer containing 1% Nonidet P-40, 20 mM Tris⋅HCl (pH 7.4), 100 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM sodium vanadate, and protease inhibitor mixture (Roche Molecular Biochemicals). Precleared cell lysates were incubated with agarose-conjugated anti-flag mAb at 4°C for 1 h. Immunoprecipitates were separated by SDS/PAGE, transferred to poly(vinylidene difluoride) membrane, and detected by appropriate Abs with an enhanced chemiluminescence system (Amersham Pharmacia). An in vito kination assay was performed as described (18).
Flow Cytometric Analysis.
Cells were stained with phycoerythrin-conjugated anti-FcγRIIB mAb (2.4G2) (PharMingen) and analyzed by FACSCalibur (Becton Dickinson).
Results
PD-1 Engagement Inhibits Antigen-Stimulated Growth Retardation of B Cells.
To test the molecular mechanism of PD-1 signaling, we constructed a series of chimeric molecules that consisted of the extracellular region of FcγRIIB and the cytoplasmic region of FcγRIIB, KIR, and PD-1 or its mutants, as illustrated in Fig. 1A. The chimera of the PD-1 mutant with N-terminal, C-terminal, or both tyrosines replaced by phenylalanine was designated FcPDF1, FcPDF2, or FcPDF1F2, respectively. The chimera of a PD-1 mutant with deletion of the total cytoplasmic region is called FcPDTM. These constructs were introduced into FcγRIIB-negative mutant cells of the mouse A20 B cell lymphoma IIA1.6 by retroviral infection, followed by fluorescence-activated cell sorting. Expression levels of these chimeric receptors and cistronic enhanced green fluorescence protein were almost the same among these transformants (Fig. 1B). These transformants were stimulated with intact anti-IgG Ab, resulting in coligation of BCR and chimeric receptors. As a control, F(ab′)2 fragments of anti-IgG Ab, which cannot crosslink BCR and chimeric receptors, were used (BCR only) (Fig. 1C).
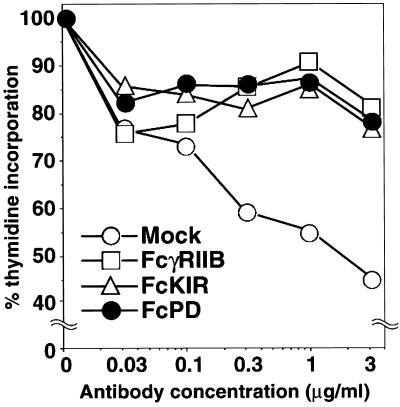
First, PD-1 signaling effects on cell growth were determined by [3H]thymidine incorporation at various concentrations of anti-IgG Ab. Cell growth of mock transformants was retarded by BCR stimulation, whereas coligation of FcPD with BCR inhibited this growth retardation in a manner similar to that of coligation of FcγRIIB or FcKIR with BCR, indicating that PD-1-BCR coligation counteracts BCR signaling (Fig. 2).
Figure 2.
FcPD coligation with BCR inhibited BCR-mediated growth retardation in IIA1.6 cells. Mock, FcγRIIB, FcKIR, and FcPD transformants were stimulated with indicated concentrations of anti-mouse IgG Abs, and cell growth was determined by [3H]thymidine incorporation. Relative growth was calculated by dividing thymidine incorporation of stimulated cells by that of unstimulated cells. Each percentage is the mean of triplicate wells.
Requirement of the C-Terminal Tyrosine Residue of PD-1 for Its Inhibition of BCR-Stimulated Ca2+ Mobilization in B Cells.
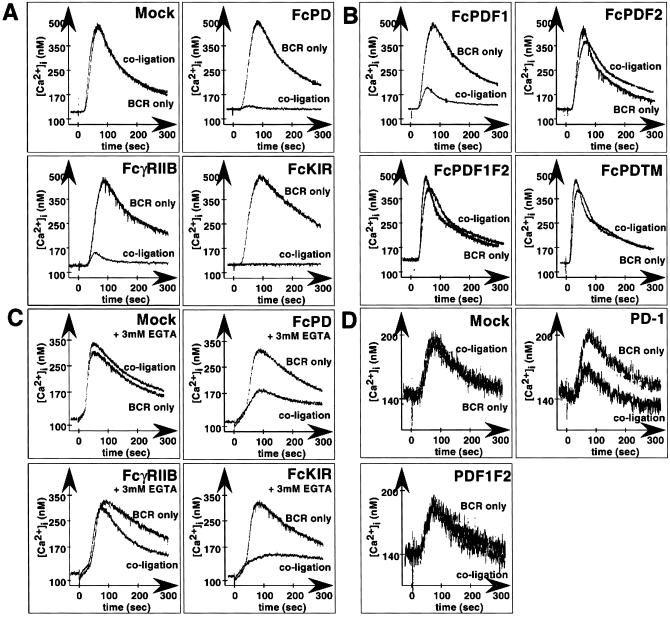
To see that PD-1 inhibits more proximal events of antigen stimulation, we next examined the effect of PD-1 engagement on Ca2+ mobilization, with the use of fura-2 as an indicator. IIA1.6 cells, expressing various chimeric molecules illustrated in Fig. 1A, were stimulated by coligation of BCR and chimeric receptors. Like FcγRIIB and FcKIR (16), FcPD inhibited BCR-mediated Ca2+ mobilization when coligated with BCR (Fig. 3A). FcPDF1 strongly, but not completely, blocked BCR-mediated Ca2+ mobilization, whereas FcPDF2 showed almost no inhibitory effect, and FcPDF1F2 or FcPDTM did not block BCR-mediated Ca2+ mobilization at all, suggesting that C-terminal but not N-terminal tyrosine residue is indispensable for the inhibitory effect of PD-1 on BCR-stimulated Ca2+ mobilization (Fig. 3B). FcPD inhibited Ca2+ mobilization in the presence of EGTA as FcKIR did. In contrast, the inhibitory effect of FcγRIIB is partially attenuated in the presence of EGTA, as reported (16). These results indicate that PD-1 inhibits Ca2+ release from the intracellular pool (Fig. 3C).
Figure 3.
Inhibition of BCR-mediated Ca2+ mobilization by coligation with FcPD and PD-1. Cytosolic calcium concentrations of Fc-chimera transformants were measured with a KAF 110 spectrophotometer. Each transformant was stimulated by coligation of Fc chimera and BCR or ligation of BCR only. (A) FcPD inhibited BCR-mediated Ca2+ mobilization as efficiently as FcγRIIB and FcKIR. (B) FcPDF1 inhibited BCR-mediated Ca2+ mobilization, whereas FcPDF2, FcPDF1F2, and FcPDTM could not. (C) FcPD inhibited BCR-mediated Ca2+ mobilization in the presence of EGTA. (D) Inhibitory effect of full-length PD-1.
To demonstrate that these inhibitory effects are not caused by the artifact of chimeric molecules, the effect of full-length PD-1 itself was examined (Fig. 3D). The transformants of the full-length PD-1 and its tyrosine mutant (PDF1F2) were stimulated by biotinylated anti-IgG2a mAb and anti-PD-1 mAb (J43), followed by crosslinking of BCR and PD-1 with streptavidin. PD-1 transformants showed reduced mobilization of Ca2+ by coligation of BCR and PD-1 as compared with BCR ligation. In mock and PDF1F2 transformants, the coligation did not inhibit Ca2+ mobilization as compared with stimulation by anti-IgG2a mAb alone. These results showed that the assays with the chimeric molecules of FcγRIIB and PD-1 can replace those with full-length PD-1 in a facilitated way.
Association of SHP-2 with Phosphorylated PD-1.
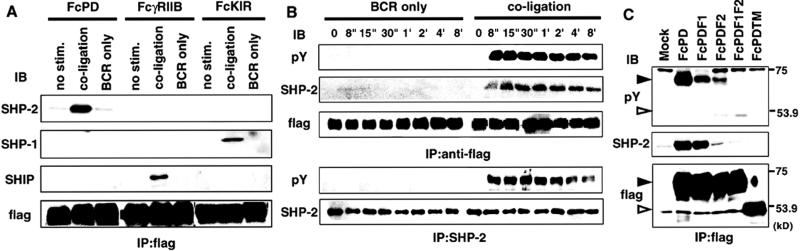
FcPD transformants of IIA1.6 were stimulated for the indicated times, and phosphorylation of tyrosine residues was examined by immunoprecipitation with anti-flag mAb and Western blotting with anti-phosphotyrosine mAb. PD-1 was tyrosine phosphorylated only when coligated to BCR, and this tyrosine phosphorylation could be detected as early as 8 s after stimulation and lasted as long as 8 min (Fig. 4B). Mutation of either of the tyrosine residues reduced tyrosine phosphorylation in FcPD, suggesting that both tyrosine residues are phosphorylated (Fig. 4C). Strong phosphorylation of the tyrosine residues of PD-1 suggests its association with SH2-containing signaling molecules on coligation with BCR. A possible association of SHP-1, SHP-2, or SHIP with PD-1 was tested, because these phosphatases are reported to be recruited by ITIM-bearing immunoreceptors and critical for their inhibitory function. SHP-2 but neither SHP-1 nor SHIP was coimmunoprecipitated with FcPD when BCR and FcPD were coligated (Fig. 4A). Control experiments confirmed association of FcγRIIB and KIR with SHIP and SHP-1, respectively. Association between SHP-2 and FcPD was abrogated when C-terminal tyrosine was mutated to phenylalanine (FcPDF2), suggesting that this association may depend on the C-terminal phosphotyrosine (Fig. 4C). Coligation of PD-1 and BCR induced not only recruitment of SHP-2 but also tyrosine phosphorylation of SHP-2. Both the association with PD-1 and SHP-2 phosphorylation took place in parallel with phosphorylation of PD-1, which was detectable as early as 8 s after BCR stimulation and lasted longer than 8 min (Fig. 4B).
Figure 4.
Recruitment and phosphorylation of SHP-2 to tyrosine-phosphorylated FcPD. (A) IIA1.6 cells expressing FcPD, FcγRIIB, and FcKIR were stimulated, and cell lysates were immunoprecipitated (IP) by anti-flag mAb and analyzed for interaction with SHP-2 by Western blotting. (B) FcPD-expressing IIA1.6 cells were stimulated with intact Abs or F(ab′)2 fragments of anti-mouse IgG for the indicated intervals. Tyrosine phosphorylation of FcPD, association between FcPD and SHP-2, and tyrosine phosphorylation of SHP-2 were determined as described in Materials and Methods. (C) Tyrosine phosphorylation of tyrosine mutants of FcPD and association between tyrosine mutants of FcPD and SHP-2 were determined as above. Closed and open arrowheads indicate full-length and truncated FcPD chimeras, respectively.
PD-1 Signaling Reduces BCR-Mediated Tyrosine Phosphorylation of Various Molecules.
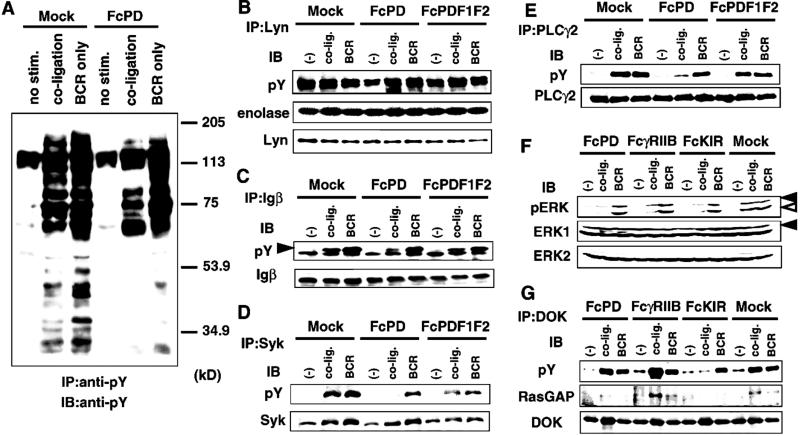
BCR stimulation leads to activation of several protein tyrosine kinases, including Lyn, Syk, and Btk. Because the inhibitory effect of PD-1 is likely to be mediated by the phosphatase activity of SHP-2, effects of FcPD coligation on BCR-mediated tyrosine phosphorylation of various molecules were determined. Tyrosine phosphorylation levels of p150, p95, p85, p75, and p70 were reduced by coligation of FcPD with BCR, whereas phosphorylation of p65, which is most likely FcPD itself, was augmented (Fig. 5A). Lyn, Igβ, Syk, PLCγ2, phosphatidylinositol 3-kinase (PI3K), and vav are well-known positive signal transducers that reside downstream of BCR signaling. We examined tyrosine phosphorylation levels of these molecules and kination activity of Lyn. FcPD coligation with BCR markedly reduced tyrosine phosphorylation levels of Igβ, Syk, PLCγ2, PI3K, and vav. Because this reduction could not be observed by FcPDF1F2 coligation, the involvement of the phosphatase activity of SHP-2 is strongly suggested (Fig. 5 C–E and data not shown). The kinase activity of Lyn against enolase was slightly up-regulated by BCR engagement, and this augmentation was not affected by coligation of FcPD. The phosphorylation level of Lyn was not affected by coligation of FcPD with BCR (Fig. 5B).
Figure 5.
Coligation of PD-1 with BCR inhibited BCR-mediated tyrosine phosphorylation of various molecules. (A) Mock and FcPD transformants were stimulated, and cell lysates were immunoprecipitated (IP) with anti-phosphotyrosine Ab (anti-pY) and examined for phosphotyrosine contents by Western blotting. (B–G) Mock, FcPD, FcPDF1F2, FcγRIIB, or FcKIR transformants were stimulated under indicated conditions. Cell lysates were immunoprecipitated with Abs against Lyn (B), Igβ (C), Syk (D), PLCγ2 (E), and Dok (G); resolved by SDS/PAGE; transferred to membrane; and probed (immunoblotted, IB) with the Abs indicated. The closed arrowhead indicates the tyrosine-phosphorylated Igβ in C. The kinase activity of Lyn on enolase was also measured (B). Cell lysates were probed with anti-pERK1/2 (F). pERK1/2 represents the p44/42 ERK1 and ERK2, which are phosphorylated at Thr-202 and Tyr-204 and thus are activated. Closed and open arrowheads indicate ERK1 and ERK2, respectively, in F.
PD-1 Inhibits BCR-Mediated Activation of Mitogen-Activated Protein Kinase in a Manner Different from That of FcγRIIB or KIR.
PD-1 effects on another signaling pathway leading to cell growth were also examined. BCR-mediated activation of ERK1 and ERK2 was inhibited by coligation of BCR with FcPD, as reported for FcγRIIB (21) and KIR (Fig. 5F). FcγRIIB was shown to suppress BCR-mediated activation of mitogen-activated protein kinase by augmentation of Dok tyrosine phosphorylation in the SHIP/Dok/RasGAP pathway (21). On the other hand, SHP-1, which is recruited to KIR, was reported to suppress phosphorylation of Dok (22). Unlike FcγRIIB and FcKIR, FcPD did not affect the phosphorylation status of Dok, suggesting that PD-1 inhibits activation of mitogen-activated protein kinase by a pathway different from that of FcγRIIB or KIR (Fig. 5G). As reported, activation of shc p52, association between Dok and RasGAP, and association between shc and SHIP were observed only by coligation of BCR with FcγRIIB but not with FcPD (data not shown). SHP-2 was not coimmunoprecipitated with Dok or shc by coligation with FcPD (data not shown).
Discussion
Because PD-1-deficient mice spontaneously develop autoimmune diseases and the sequence surrounding the N-terminal tyrosine residue of PD-1 fulfills the requirement of ITIM, PD-1 has been thought to negatively regulate immune responses (8, 9). Indeed, splenic B cells from PD-1-deficient mice exhibit augmented proliferative responses on BCR stimulation, suggesting that PD-1 may inhibit BCR signaling (7). But recent isolations of PD-1 ligands have made the story complicated (10, 23–26). We have recently reported that the ligands of PD-1 transduce negative signals on T cells (10, 23). But there are other reports that the ligands of PD-1 are costimulatory (24–26). Here we report that PD-1 actually can inhibit BCR signaling and that PD-1 inhibits antigen-stimulated B cell activation according to the following scheme: (i) coligation of PD-1 with BCR results in phosphorylation of both tyrosines in PD-1 (Fig. 4); (ii) SHP-2 is recruited to the C-terminal phosphotyrosine of PD-1 and phosphorylated (Fig. 4); (iii) phosphorylated SHP-2 dephosphorylates proximal signal transducers of BCR such as Syk and Igα/β, which leads to deactivation of downstream molecules, including PI3K, PLCγ2, and ERK (Fig. 5); (iv) deactivation of signal transducers results in inhibition of acute-phase reactions such as Ca2+ mobilization (Fig. 3) as well as long-term effects such as growth retardation (Fig. 2). The inhibition of Ca2+ mobilization should be explained by the reduced phosphorylation of PLCγ2 (Fig. 5E), which converts phosphatidylinositol 4,5-bisphosphate into diacylglycerol and inositol 3,4,5-trisphosphate; each of these products is responsible for protein kinase C activation and intracellular [Ca2+] increase, respectively. The inhibition of growth retardation should be explained by the reduced phosphorylation of mitogen-activated protein kinase (Fig. 5F), which is involved in the proliferation and differentiation of immune cells (27). The costimulatory effect of the PD-1 ligands may come from another receptor that may share the ligands with PD-1.
Although PD-1 engagement leads to dephosphorylation of various molecules, including Igβ, Syk, PLCγ2, and ERK1/2, this does not necessarily mean that PD-1-activated SHP-2 dephosphorylates these molecules directly. SHP-2 is likely to dephosphorylate a few molecules that reside more proximal to the BCR signaling as shown in paired Ig-like receptor B-activated SHP-1 (18). The most probable candidates are Syk and Igα/β, because Syk has been shown to activate all of the molecules mentioned above and their upstream molecules (28), and Igα/β have the immunoreceptor tyrosine-based activation motif in their cytoplasmic regions, to which Syk is recruited.
Between two tyrosine residues in the cytoplasmic region of PD-1, the N-terminal tyrosine residue in the ITIM-like sequence was almost dispensable, and the C-terminal one was necessary for the inhibitory effects of PD-1 on antigen-stimulated Ca2+ mobilization and growth retardation (Fig. 3 and data not shown). It is not known why the N-terminal tyrosine of PD-1 does not associate with either SHP-1 or SHP-2. A possible explanation is the presence of alanine at −1, because an ITIM mutant of KIR that has alanine at −1 is less efficient in the activation of SHP-1 than is the wild type (29). However, the conservation of the PD-1 ITIM in human and mouse (1, 2) and its significant phosphorylation on stimulation (Fig. 4) imply that this ITIM may have important roles other than recruitment of SHP-1 and SHP-2. The amino acid sequence around the C-terminal tyrosine is also well conserved between mouse and human PD-1 (TEYATIVF), and a similar sequence (TEYASI) is found in signal regulatory protein β, which is reported to associate with SHP-1 and SHP-2 (30). These observations suggest that the sequence TEYAS(T)I may be involved in binding of SHP-2 with phosphotyrosine.
PD-1 executes its inhibitory effect only when coligated to BCR (Figs. 3 and 5), suggesting that PD-1 has to get some positive signals to execute its inhibitory function. Immunoprecipitated Lyn but not Syk tyrosine-phosphorylated PD-1 (data not shown), suggesting that on coligation of BCR and PD-1, PD-1 is tyrosine phosphorylated by Lyn, which resides near BCR. Thus Lyn plays critical roles in both the activation and regulation of BCR signaling. Because PD-1 is phosphorylated only by coligation with BCR and expressed only on activated cells (5), PD-1 probably functions in the down-modulation of excessive and prolonged activation and inhibition and/or suppression of inappropriate activation such as autoreactivities by elevating the threshold for restimulation.
Acknowledgments
We thank Drs. S. Miwa and Y. Kawanabe for their technical assistance on calcium measurements and Mses. Y. Tada, T. Toyoshima, and Y. Doi for their technical support. We are grateful to Dr. J. L. Strominger and K. Ikuta for their critical reading of the manuscript. This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan. T.O. is a research fellow of the Japan Society for the Promotion of Science.
Abbreviations
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- FcγRIIB
IgG Fc receptor type IIB
- BCR
B cell receptor
- PLCγ2
phospholipase C-γ2
- SH2
src homology 2
- SHP
SH2-domain-containing protein tyrosine phosphatase
- KIR
killer immunoglobulin-like receptor
- SHIP
SH2-domain-containing phosphatidylinositol 5-phosphatase
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Genomics. 1994;23:704–706. doi: 10.1006/geno.1994.1562. [DOI] [PubMed] [Google Scholar]
- 3.Bolland S, Ravetch J-V. Adv Immunol. 1999;72:149–177. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 4.Unkeless J-C, Jin J. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 5.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura H, Minato N, Nakano T, Honjo T. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, et al. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 10.Freeman G-J, Long A-J, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz L-J, Malenkovich N, Okazaki T, Byrne M-C, et al. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J-V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe T-L, Williams G-T, Davies S-L, Neuberger M-S. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 13.Pan C, Baumgarth N, Parnes J-R. Immunity. 1999;11:495–506. doi: 10.1016/s1074-7613(00)80124-7. [DOI] [PubMed] [Google Scholar]
- 14.Bolland S, Ravetch J-V. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 15.Bolen J-B. Curr Opin Immunol. 1995;7:306–311. doi: 10.1016/0952-7915(95)80103-0. [DOI] [PubMed] [Google Scholar]
- 16.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch J-V. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 17.Hippen K-L, Buhl A-M, D'Ambrosio D, Nakamura K, Persin C, Cambier J-C. Immunity. 1997;7:49–58. doi: 10.1016/s1074-7613(00)80509-9. [DOI] [PubMed] [Google Scholar]
- 18.Maeda A, Scharenberg A-M, Tsukada S, Bolen J-B, Kinet J-P, Kurosaki T. Oncogene. 1999;18:2291–2297. doi: 10.1038/sj.onc.1202552. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura T. Int J Hematol. 1998;67:351–359. doi: 10.1016/s0925-5710(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 20.Iwamuro Y, Miwa S, Minowa T, Enoki T, Zhang X-F, Ishikawa M, Hashimoto N, Masaki T. Br J Pharmacol. 1998;124:1541–1549. doi: 10.1038/sj.bjp.0701984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamir I, Stolpa J-C, Helgason C-D, Nakamura K, Bruhns P, Daeron M, Cambier J-C. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- 22.Berg K-L, Siminovitch K-A, Stanley E-R. J Biol Chem. 1999;274:35855–35865. doi: 10.1074/jbc.274.50.35855. [DOI] [PubMed] [Google Scholar]
- 23.Latchman Y, Wood C-R, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long A-J, Brown J-A, Nunes R, et al. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 24.Tseng S-Y, Otsuji M, Gorski K, Huang X, Slansky J-E, Pai S-I, Shalabi A, Shin T, Pardoll D-M, Tsuchiya H. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H, Zhu G, Tamada K, Chen L. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 26.Tamura H, Dong H, Zhu G, Sica G-L, Flies D-B, Tamada K, Chen L. Blood. 2001;97:1809–1816. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 27.Genot E, Cantrell D-A. Curr Opin Immunol. 2000;12:289–294. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 28.Campbell K-S. Curr Opin Immunol. 1999;11:256–264. doi: 10.1016/s0952-7915(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 29.Burshtyn D-N, Yang W, Yi T, Long E-O. J Biol Chem. 1997;272:13066–13072. doi: 10.1074/jbc.272.20.13066. [DOI] [PubMed] [Google Scholar]
- 30.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. Nature (London) 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]