Summary
More than a decade ago, a formalized fellowship training program in medical device innovation, the first of its kind, was created at Stanford University. Now in its 15th year, the Stanford Biodesign Fellowship Program is a 10-month program whereby postgraduate students with a prior background in medicine, engineering, and/or business form interdisciplinary teams for an experiential process of identifying unmet clinical needs, inventing new solutions, and implementing these ideas (the 3 “I’s”). A key component of this structured process is focused attention on needs finding and characterization, which differs from the traditional “tech-push” model (i.e., technologies looking for problems to solve). Although the Stanford Biodesign process can be applied to a wide variety of clinical areas, cardiovascular medicine is particularly well suited, given the breadth of clinical presentations it touches and its history of innovation to solve important clinical problems. Physicians play a vital role in the process, especially for needs identification and characterization. This paper outlines the Stanford Biodesign process and presents an argument for its repeat applicability, discusses its relevance to physicians and to cardiologists in particular, and provides a case study of the process that resulted in a currently available cardiovascular medical technology that came directly from the Fellowship Program.
Key Words: biodesign, cardiology, innovation, invention, medical device, medical technology, needs-based, Stanford, translational
Abbreviations and Acronyms: EP, electrophysiologist/electrophysiology; IP, intellectual property
Graphical abstract
Innovation and entrepreneurship have been widely celebrated in recent years, reaching as far as mainstream television with multiple current on-air shows (e.g., Silicon Valley, Shark Tank, etc.). In parallel with this increased cultural awareness, universities across the country have developed entrepreneurship training programs, initially focusing on engineering, but more recently expanding to the life sciences. One of the oldest life science programs is Stanford Biodesign, which focuses on training young innovators of biomedical technologies (particularly medical devices) (1). A primary distinction between the Stanford Biodesign process and more traditional approaches to innovation is an upfront focus on identifying and characterizing the clinical need, rather than beginning with a promising technology. The central dogma of the Stanford Biodesign process is that “a well-characterized need is the DNA of a great invention” (2). This needs-based approach to innovation begins in the clinical environment, where practicing clinicians are ideally placed to spearhead the process. Although many companies have germinated from the fellowship program, the true goal is to teach a repeatable approach to health technology innovation, which can then lead to a “multiplier effect,” where graduates can apply this process serially to solve unmet clinical needs.
The program is both field- and technology-agnostic but has deep roots in cardiovascular medicine; the Stanford Byers Center for Biodesign is directed by Dr. Paul Yock, an interventional-trained cardiologist and serial entrepreneur who invented the Rapid Exchange balloon angioplasty/stenting system and intravascular ultrasound 3, 4. In just 15 years of existence, more than 180 engineers, physicians, and business professionals have completed the fellowship training, and nearly a thousand students have taken undergraduate or graduate courses in Biodesign. To date, 41 companies have been launched by these first-time innovators directly from Stanford Biodesign, and many other technologies have been invented by alums of the program after graduation.
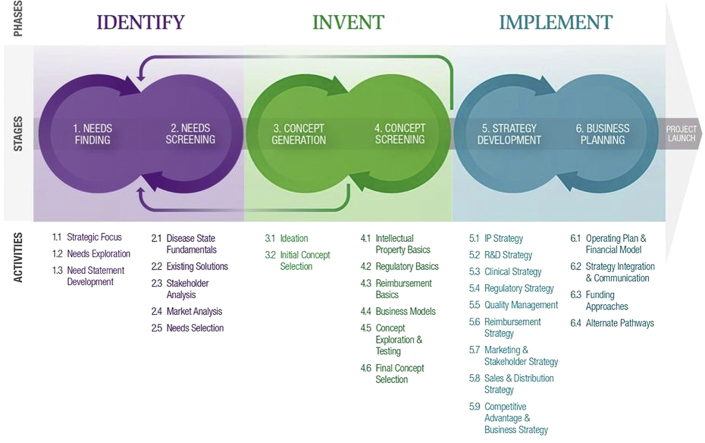
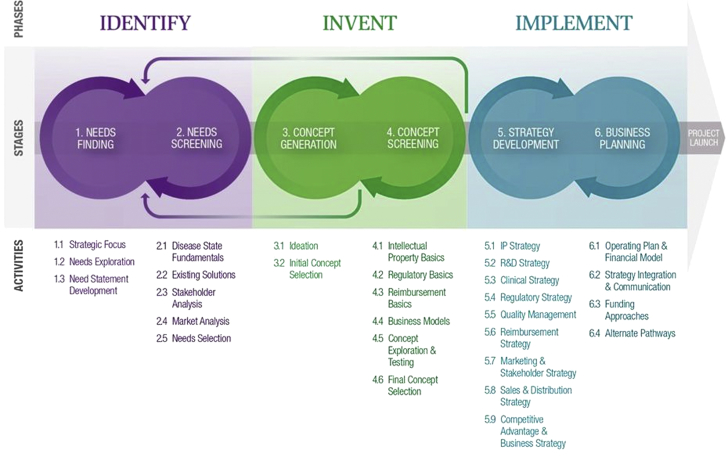
Currently, the fellowship group consists of 3 multidisciplinary teams of 4 fellows who follow the “3 I’s” process each year (identify, invent, and implement) (Figure 1) as they evaluate and solve needs in a particular clinical area. Each phase is described in detail in this paper.
Figure 1.
The 3 Phases of the Stanford Biodesign Process
The 3 phases of the Stanford Biodesign process (3 I’s)—identify, invent, and implement—are outlined, with 2 specific stages performed during each phase. The process is both iterative and cyclical and often requires returning to prior stages and phases as new information becomes available through research. Key activities performed at each stage are detailed below each step in the process.
Reprinted with permission from Yock et al. (4).
Phase 1: Identify—Clinical Immersion, Needs Finding
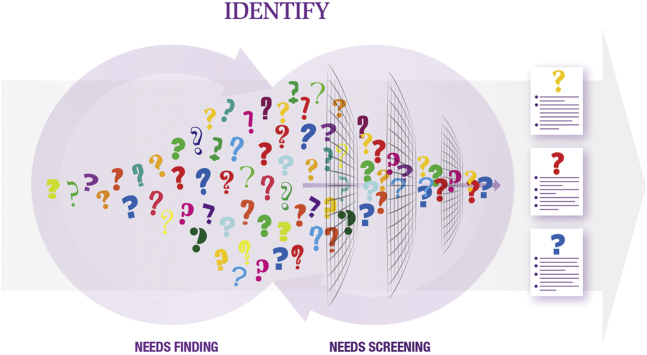
Shortly after the fellowship commences, the teams begin an intensive needs-finding process. Approximately 20% of the fellowship time is devoted to this vital stage, in which each team delves deeply into a specific clinical area by direct immersion in relevant inpatient and outpatient settings. Over the course of several weeks, the fellows document their clinical observations with the goal of creating a list of at least 200 needs.
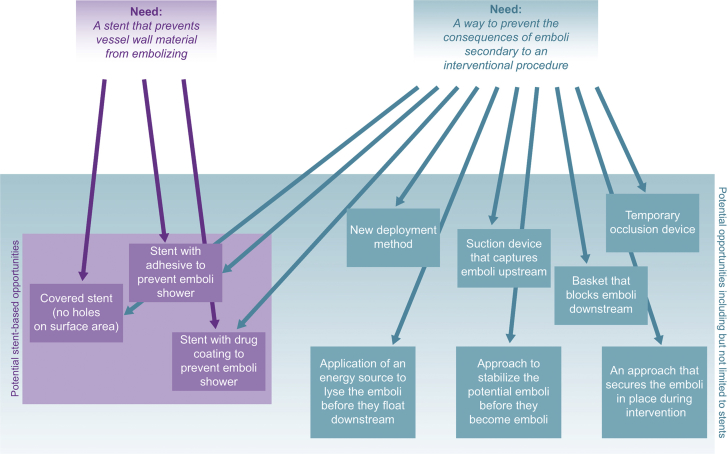
The next step in the process is the development of a need statement. This single sentence is carefully crafted to capture the essence of the need. In effect, it is a mission statement, serving as the driving force behind the team’s efforts to solve the identified need. It encapsulates the clinical problem that has been identified, the specific population afflicted by the problem (which will serve as the focus of possible solutions), and a measureable outcome that can be affected by potential solutions. One particularly important caveat in developing a need statement is that a solution should not be inadvertently embedded within the need statement, as this severely narrows the range of potential solutions the team will consider (see Figure 2 for an illustration of this issue for a cardiovascular-related need). A well-crafted example of a need statement from a team focused on a cardiovascular need (discussed in detail in the case discussion in the following text) is:
Figure 2.
An Example of 2 Possible Need Statements
An example of 2 possible need statements from a fellowship group in the Stanford Biodesign Program. The need statement on the left contains a solution embedded within the statement (a better stent), which limits the potential concepts that can fulfill this need statement. The need statement at right no longer contains an embedded solution and instead places focus on the outcome (the consequences of emboli). Many more potential concepts that fulfill this revised need statement can thus be brainstormed.
Reprinted with permission from Yock et al. (4).
A better way to detect potential rhythm disturbances in nonhospitalized patients with suspected arrhythmias to improve the patient experience and reduce the cost of diagnosis.
The large number of initial needs is then subjected to a rigorous screening process to narrow the list down to a few top needs. These filters include the clinical impact of the need, the degree of understanding of the pathophysiology involved, a consideration of the existing and emerging clinical approaches, and a preliminary assessment of the market potential for a solution to this need (i.e., what might the value of a solution be to the health system?). Again, physicians play a key role at this stage because their understanding of pathophysiology and current treatment methods helps the screening process proceed efficiently. An additional filter follows from an assessment of the stakeholders with an interest in this needs area. The key question for stakeholder analysis is: which parties (including patients, providers, payers, regulators, and others) stand to have an influence—positive or negative—on whether a solution to a particular need will actually make it through to patient care? Once all of the preceding information is compiled, the needs are then ranked and prioritized with the goal of selecting the few most compelling needs to take further in the process.
Once a screened list of contenders for the final needs is created, specific need criteria are developed for each need based on further research and interviews with stakeholders. The need criteria are a relatively small set of key characteristics that a truly successful solution should have to meet that need. Typically, there are 3 to 6 “must-have” criteria and a similar number of “nice-to-have” criteria. These need criteria guide the team during all subsequent steps of the Biodesign process. The background research required to develop a well-crafted need statement and list of criteria has a beneficial effect on innovation: it limits an innovator’s tendency to speed through to the inventing process before developing a deep understanding of the need, and it simultaneously provides a mechanism for objectively assessing the true utility of a proposed solution (Figure 3).
Figure 3.
Phase 1: Identify
In the first phase of the process, the identify phase, needs finding is the primary focus. Many needs are recorded through clinical observations and are then screened and filtered down to those with the greatest opportunity and most interest.
Reprinted with permission from Yock et al. (4).
Phase 2: Invent—Concept Generation and Screening, Creativity
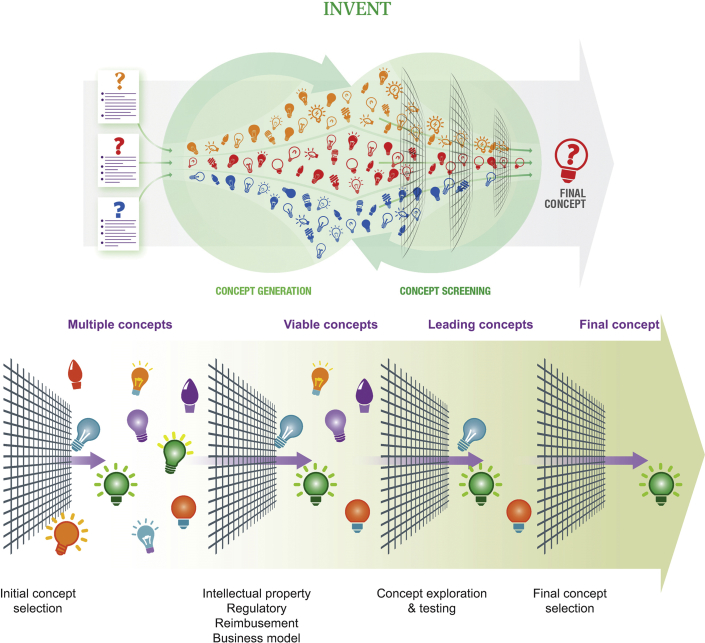
Once several promising needs have been identified, clear need statements created, and accurate need criteria have been developed, teams embark on the inventing phase. Here, each team brainstorms a number of concepts that could potentially meet the need criteria. A robust brainstorming session of an hour’s duration may create 50 to 60 (or more) concepts. On closer consideration, however, a select few of these concepts will truly meet the need criteria, and a few subsequent brainstorming sessions are usually required to generate a strong list of concepts that meet the criteria, while also providing interesting and potentially novel solutions. To aid organization of the brainstormed concepts, grouping into similar categories (e.g., chemical means, biological, mechanical, electrical, etc.) often proves fruitful. This allows similar and overlapping ideas to either be linked or eliminated, and it identifies sparse areas that need to be explored further.
At this point, a second screening process is applied with the goal of filtering concepts. The filters in this case include intellectual property (IP), likely regulatory pathway, reimbursement potential, technical feasibility, and viability of the business model needed to bring the solution to patients. The considerations involved in these filters are often complex, and it may require several weeks or more to conduct sufficient research to find clarity in each category for a particular solution. Customarily, teams will rate the degree of difficulty each filter represents: IP may have a clear path (a “green light”), for example, but the regulatory pathway may be worrisome enough from a standpoint of time and risk to merit a caution (“yellow light”) ranking. This careful filtering process generally provides the team with a short list of top concepts, reducing the tally of 10 to 15 promising solutions emanating from brainstorming to a list of the top 2 or 3 (Figure 4).
Figure 4.
Phase 2: Invent
During the second phase of the process (invent), concepts are created after multiple brainstorming sessions. These concepts are then subjected to a rigorous screening process that leads to final concept selection.
Reprinted with permission from Yock et al. (4).
Next, serious prototyping of the top concepts can begin. Efficient and effective prototyping must be question driven, and multiple iterations of prototypes are typically created for each concept. In some cases, even these early prototypes will be sufficiently advanced to provide the basis for pre-clinical testing, either on the benchtop or in an animal model. This early-stage testing, coupled with further research, will in most cases point to 1 or 2 concepts as clear frontrunners.
Phase 3: Implement—Commercialization Potential, Strategy Development
The top 1 or 2 concepts have now undergone rigorous background research and have survived a rigorous filtering process. In-depth analysis of each concept is undertaken to formulate a plan to proceed. This includes a comprehensive understanding of the IP landscape, a plan for a credible reimbursement pathway, as well as an understanding of the engineering feasibility, resources, and personnel needed to undergo further research and development. A detailed plan for device testing is then devised, including pre-clinical and clinical trials, as well as a quality management protocol. A viable business model must then be created, which includes understanding sales and distribution, financial modeling, funding strategies (i.e., venture capital, corporate funding, government grants, and so on), and marketing and stakeholder strategy (creating a value proposition). The competitive advantage of the proposed concept over the existing competition is a pillar of a successful product launch, and alternative commercialization plans must also be considered.
Case Study: A Single-Use, 14-Day Cardiac Event Monitor
Of the more than 40 companies that have come out of Stanford Biodesign at least 5 have stemmed from physicians with formal training in cardiovascular medicine (5). One is Dr. Uday N. Kumar, a cardiac electrophysiologist (EP) who currently serves on the faculty in Biodesign. As a Biodesign fellow, he and his team focused on the EP field and identified more than 200 needs. One need in particular—the detection of potential arrhythmias in nonhospitalized patients—rose to the top of the filtering process (see the need statement in the preceding text). The team brainstormed a number of solutions and screened these against the categories described earlier in this paper.
After filtering of a number of concepts, the top solution was a long-term (up to 14 days), water-resistant, disposable patch-based monitor to identify cardiac arrhythmias. Shortly after the fellowship concluded, Dr. Kumar formally incorporated a company and licensed the technology from Stanford University to develop the concept as part of a complete solution, including a cloud-based algorithm and robust supporting service. The device is currently commercially available (Figure 5). To date, the device has been used on nearly 500,000 patients, and several publications have documented the clinical and economic utility of the approach (6).
Figure 5.
A Novel Single-Use, 14-Day Cardiac Event Monitor
This device is an example of a successful medical device initially conceived by a fellowship group in the Stanford Biodesign Program. It is currently commercially available in the United States and Europe.
The Stanford Biodesign process played an instrumental role in the development of the new cardiac event monitor, especially during the identify phase of the Biodesign process. First, the intense focus on tracing needs to their root as part of needs finding led the team to understand that a misalignment of care existed. The team identified that although cardiac rhythm monitors are typically prescribed by cardiologists and electrophysiologists (who are also the treatment providers), patients with possible arrhythmias typically first present to primary care providers or the emergency department, where high-quality, specialized testing for arrhythmias is usually not available. Second, based on a detailed understanding of existing solutions, the team identified that current diagnostic testing for cardiac arrhythmias had a multitude of problems, including many nondiagnostic results and poor patient adherence. Third, by performing a rigorous stakeholder analysis, it was clear that even though primary care providers and emergency physicians would need a very simple and easy-to-deploy device to initiate testing, at the same time, cardiologists and cardiac EPs would need very detailed and accurate data to devise a proper treatment plan. With these 3 insights, a final solution emerged that provided a way to reduce the number of nondiagnostic and unnecessary repeat tests, empowered frontline providers with an easy-to-use device to initiate testing, allowed for patients to easily adhere with the test as prescribed, and generated a robust report for specialists to make an accurate treatment plan. Thus, a prolonged effort in the identify phase of the Biodesign process resulted in a well-vetted need that significantly improved the likelihood for success.
Summary
Cardiovascular medicine is a rapidly changing, innovation-centric field with deep roots in medical technology that is perfectly suited for application of a disciplined innovation curriculum. The Stanford Biodesign process described here provides a stepwise approach to creating new biomedical technologies that begins with deep diligence into clinical needs. Physicians, and cardiologists in particular, encounter countless clinical needs each day; the Biodesign process provides a standardized protocol to turn this need identification into action. Utilizing this process, needs eventually lead to solutions that not only advance the field but also directly benefit patients.
Footnotes
Dr. Kumar is the founder, a current consultant, and major stockholder of iRhythm Technologies, Inc.; the founder, president, CEO, and major stockholder of Element Science, Inc.; and a board member of G-Tech Medical, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Wall J., Wynne E., Krummel T. Biodesign process and culture to enable pediatric medical technology innovation. Semin Peadiatr Surg. 2015;24:102–106. doi: 10.1053/j.sempedsurg.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Yock P.G., Brinton T.J., Zenios S.A. Teaching biomedical innovation as a discipline. Sci Transl Med. 2011;3:92cm18. doi: 10.1126/scitranslmed.3002222. [DOI] [PubMed] [Google Scholar]
- 3.Yock P.G., Linker D.T., Angelsen B.A. Two-dimensional intravascular ultrasound: technical development and initial clinical experience. J Am Soc Echocardiogr. 1989;2:296–304. doi: 10.1016/s0894-7317(89)80090-2. [DOI] [PubMed] [Google Scholar]
- 4.Yock P.G., Zenios S., Makower J. 2nd edition. Cambridge University Press; Cambridge, UK: 2015. Biodesign: The Process of Innovating Medical Technologies. [Google Scholar]
- 5.Brinton T.J., Kurihara C.Q., Camarillo D.B. Outcomes from a postgraduate biomedical technology innovation training program: the first 12 years of Stanford Biodesign. Ann Biomed Eng. 2013;41:1803–1810. doi: 10.1007/s10439-013-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turakhia M.P., Hoang D.D., Zimetbaum P. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112:520–524. doi: 10.1016/j.amjcard.2013.04.017. [DOI] [PubMed] [Google Scholar]