Abstract
Using a polypeptide oligomer harboring 16 repeats of the neuritogenic epitope (aa 58–73) of myelin P2 protein separated by spacers, enhancement of the immune response to the P2 protein, an important neuritogenic autoantigen in experimental autoimmune neuritis (EAN), was attempted. In contrast to a previous study with PLP-16-mer antigen-specific response of T cells was attenuated at all doses examined to a variable degree. Treatment of Lewis rats with the P2-16-mer up to 2 months before immunization with P253–78 (vaccination) or after immunization but before appearance of disease (prevention) had a strong tolerizing effect against the induction of EAN on immunization with P253–78. Moreover, rats injected with 200 μg of the P2-16-mer i.v. on day 11 after disease induction, at which time the initial signs of disease had appeared, were almost completely protected against progression of clinical disease, whereas animals treated with the same amount of monomeric control peptide developed severe disease (treatment). Similar results were obtained by i.v. treatment of adoptive-transfer EAN with the P2-16-mer. The lack of clinical signs of disease after 16-mer therapy could be correlated with a reduced proliferative response of P253–78-specific lymph node cells. The frequency of apoptotic T cells in sciatic nerve or in lymph node cells, however, was not increased by the 16-mer treatment, suggesting that induction of anergy or other forms of peripheral tolerance may be responsible for the effect. Thus, the oligomerized P2 peptide antigen was highly effective in all three treatment modalities examined in this specific autoreactive T cell-mediated immune response.
Dimerization or oligomerization of T cell receptor (TCR) chains on binding of major histocompatibility complex (MHC) molecules plays a crucial role during T cell activation. The engagement of multiple TCRs by soluble multivalent peptide/MHC complexes results in stable complexes (1, 2). Also, ligand-driven formation of TCR clusters has been shown to enhance efficacy of T cell activation (3). The extension of peptides bound to class II MHC molecules out of both ends of the peptide binding groove provided the possibility of constructing multivalent polypeptide chains that crosslink class II MHC molecules through repetitive binding sites, possibly by catalyzing the formation of TCR/MHC clusters (4).
Major autoantigens in the peripheral nervous system include the myelin proteins P2 and P0, gangliosides, and glycolipids (5, 6). Myelin P2 protein is an important neuritogenic autoantigen in experimental autoimmune neuritis (EAN), a rodent model for the Guillain-Barré syndrome (GBS) in humans. In Lewis rats, EAN is an acute, demyelinating inflammatory disease of the peripheral nervous system mediated by autoantigen-specific, CD4-positive T cells (summarized in refs. 6 and 7). The neuritogenic epitope of the P2 protein is located within amino acids 53–78 (8, 9), whereas the minimum neuritogenic T cell epitope of P2 was identified as residues 61–70 (10). EAN can be induced actively in Lewis rats by immunization with whole peripheral nerve myelin, with a peptide spanning the neuritogenic epitope (aa 53–78) of the myelin protein P2, or by adoptive transfer of neuritogenic P2-specific T cells (adoptive transfer/AT-EAN) into naive recipients (11–14). In addition, activated T cells of nonneural specificity can open the blood–nerve barrier and form cellular infiltrates (15).
To modulate the P2-directed immune response, a polypeptide oligomer (P2-16-mer) harboring 16 repeats of the neuritogenic epitope (aa 58–73) of the P2 protein separated by peptides as spacers was designed. T cell epitope oligomers of this type have been shown to be particularly effective in the specific suppression of experimental autoimmune encephalomyelitis (EAE) by the induction of “high zone tolerance” (16, 17). In contrast to other broadly immunosuppressive mechanisms, this new therapeutic approach for prevention and therapy of autoimmune diseases aimed more specifically at the antigen-specific elimination of autoreactive T cells.
In the present study, the EAN system was used for evaluation of the in vivo potency of this newly designed P2 peptide oligomer. The ability of the P2-16-mer to suppress induction of EAN in Lewis rats (vaccination or prevention) on immunization with P253–78 by using the 16-mer was assessed, and in addition, therapy of already established disease was examined.
Materials and Methods
Reagents.
All tissue culture supplements were purchased from GIBCO/BRL, except BSA fraction V (Roth, Karlsruhe, Germany), PPD (Tuberculin Purified Protein Derivative; Statens Seruminstitut, Copenhagen), and Con A (Sigma). Bovine P253–78 peptide was synthesized by D. Palm (University of Würzburg), using solid-phase stepwise elongation on an Applied Biosystems model 430. The monomeric bovine P258–73 peptide (the core binding region of P253–78) was produced by standard fluorenylmethoxycarbonyl (Fmoc) chemistry and purchased from the Biopolymers Laboratory at the Harvard Medical School. The multimeric P258–73 16-mer and 4-mer (16 or 4 peptide epitopes separated by spacers of 13 aa) were produced and isolated essentially as described (4, 16). Because of the acidic character of the P258–73 epitope, the elution of the P2-16-mer from the nickel nitrilotriacetic acid (Ni-NTA) resin (with 20 mM EDTA), as well as the HPLC purification, had to be carried out under neutral conditions (5 mM phosphate buffer, pH 7.2). Before lyophilization the oligomer was precipitated with 1% trifluoroacetic acid. Recombinant human (rh) P2 protein and rhP0 protein were expressed and purified as described (18). Bovine P2 was isolated from peripheral nerve myelin according to the protocol of Kitamura et al. (19).
Animals and Therapy Studies.
Four- to eight-week-old female Lewis rats were obtained from Charles River Laboratories. All experiments were conducted according to approved Bavarian state regulations for animal experimentation, including footpad immunization.
For active EAN, rats were inoculated in the hind footpad with 100 μl of an emulsion of equal volumes of saline and complete Freund's adjuvant (CFA) (1 mg/ml of Mycobacterium tuberculosis H37Ra, Difco) containing indicated amounts of peptide P253–78, P2-16-mer, or bovine whole peripheral myelin. AT-EAN was induced by tail vein injection of 1.2 × 107 neuritogenic P2-specific, CD4-positive activated T cells of the neuritogenic T cell clone TKtsA6. This clone exhibited a homogenous usage of TCR Vβ4/Vα11 (20).
Animals were weighed and inspected for clinical signs of disease on a daily basis. Disease severity was assessed clinically, employing a scale ranging from 0 to 10 (21, 22): 0 = normal; 1 = less active, reduced tone of tail; 2 = limp tail, impaired righting; 3 = absent righting; 4 = gait ataxia; 5 = mild paraparesis of the hind limbs; 6 = moderate paraparesis; 7 = severe paraparesis or paraplegia; 8 = tetraparesis; 9 = moribund; 10 = death.
For therapeutic studies, animals were injected in the tail vein with indicated amounts of purified P2 peptide/P2-16-mer or rhP2 protein/1 ml PBS at indicated time points before or after induction of EAN following the schedule given in the individual experiments. Control animals were injected i.v. with PBS.
The method for determination of nerve conduction properties in rats anaesthetized with a neuroleptic/analgesic compound (Hypnorm, Janssen) has been reported (23).
Cell Culture.
P2-specific, neuritogenic, CD4+ rat T cell lines G7, and the T cell clone TKtsA6 were established and maintained by repeated propagation cycles (20, 24), and clone TKtsA6 was generated as described (25). Also, T cell proliferation studies followed standard protocols (20). For lymph node (LN) cell proliferation studies, single cell suspensions of popliteal LN from Lewis rats were prepared. LN cells (2 × 105 per well) were seeded in 96-well round bottom microtiter plates in 100 μl of medium with addition of antigen. Cell culture conditions and measurement of incorporated radioactivity were as described (20).
Histological Analysis and Immunocytochemistry.
AT-EAN was induced by tail vein injection of 1.2 × 107 neuritogenic P2-specific, activated T cells of the clone TKtsA6. Six hours after animals were treated as indicated, they were deeply anesthetized and perfused through the left cardiac ventricle as reported in ref. 18. Further processing of tissue for immunocytochemical studies and histological analyses was carried out as described (18). The percentage of T cells undergoing apoptosis was assessed by using morphological criteria and the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) technique (26).
Statistical Evaluation.
Statistical analysis was performed by the two-way ANOVA test for comparing disease courses or the unpaired t test for cell proliferation studies using the PRISM computer program (Version 3.0, GraphPad, San Diego). P < 0.05, P < 0.01, and P < 0.001 were considered statistically significant.
Results
In Vitro Stimulation of P253–78-Specific T Cells.
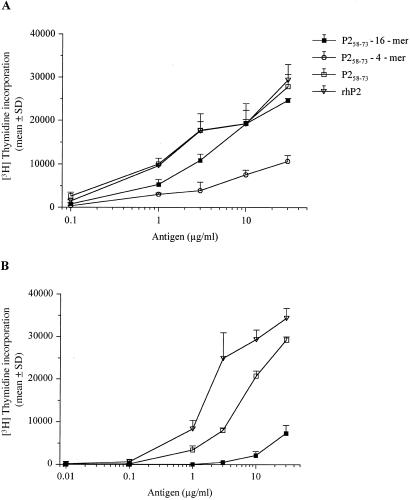
Initially the stimulative capacity of a P2-4-mer and a P2-16-mer (see Materials and Methods) with P258–73-specific T cell clones was compared with the response triggered by the monomeric peptide and recombinant P2 protein (Fig. 1). Two different neuritogenic, P253–78-specific T cell lines (G7 and the clone TKtsA6) were challenged with titrated amounts of antigen. As previously shown in other systems, the 16-mer was significantly more potent than the 4-mer (4, 17). However, in contrast to the previous report (4) the proliferative response toward P2-16-mer, at least with the T cells tested, was in most cell lines weaker than the response triggered by the monomeric peptide. For instance, proliferation of the neuritogenic, P2-specific T cell clone TKtsA6 induced by the P2-16-mer was about 10-fold lower than that to the monomeric control peptide or the rhP2 protein (Fig. 1B). In contrast to clone TktsA6, proliferation of the T cell line G7 induced by the P2-16-mer was similar to the monomeric peptide (Fig. 1A). This finding indicates that P2-specific subpopulations in T cell lines may still exhibit a full proliferative response to P2-16-mer.
Figure 1.
In vitro experiments with P253–78 peptide-specific T cell lines. Resting T cells of stable P2-specific CD4+ rat T cell lines G7 (A) and TKtsA6 (B) were challenged with titrated amounts of the indicated antigens. The specific proliferative response was measured by [3H]thymidine incorporation. Results are expressed as mean cpm + SD of triplicates.
Induction of EAN in Lewis Rats.
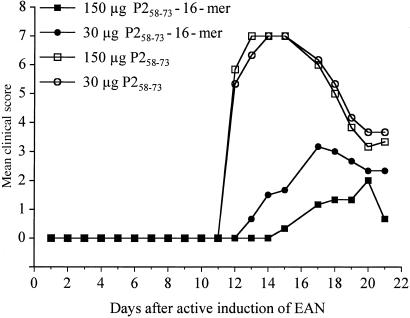
The same trend as for most T cell lines tested was also evident when the P2-16-mer was used for the induction of the disease (Fig. 2). Lewis rats immunized with 30 or 150 μg of P258–73 monomeric peptide in CFA developed a very severe form of EAN, whereas the same amount of 16-mer in CFA triggered a much milder form of the disease. Strikingly, disease severity did not correlate with the amount of oligomer administered to the animals. In fact, with the higher dosage of 150 μg the onset of the disease was delayed by 2 days, the maximal score was slightly lower compared with the immunization with only 30 μg, and less weight loss was observed after P2-16-mer immunization than is usually seen in this disease model.
Figure 2.
Induction of EAN in Lewis rats by monomer or P2-16-mer. EAN was induced in groups of three rats by s.c. injection of an emulsion of saline and CFA containing the indicated amounts of P2-16-mer or monomeric P253–78. Values are given as mean clinical score. P < 0.001 by using ANOVA for both 16-mer groups vs. monomer groups immunized with the same amount of antigen.
Prevention of EAN by the P2-16-mer.
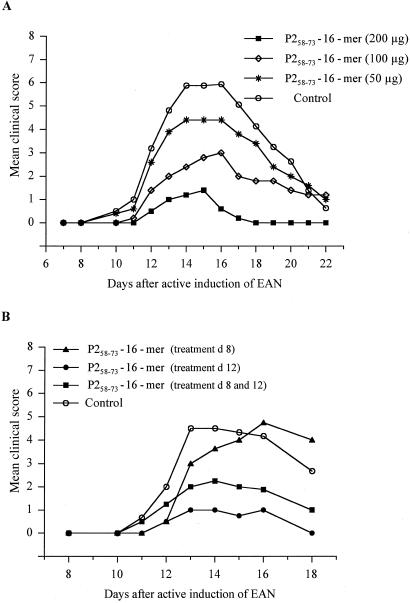
The therapeutic effect of P2-16-mer on the development of clinical signs of EAN was studied by employing a protocol previously used for the treatment of EAE (17). Female Lewis rats were injected intravenously with P2-16-mer on days 8 and 12 after active induction of disease with the neuritogenic P2 peptide (P253–78)—i.e., shortly before and at the onset of EAN. Treatment with 50 or 100 μg of P2-16-mer led only to a partial protection against disease development, whereas injection of 200 μg of 16-mer almost completely protected against disease induction (Fig. 3A). The optimal time point for P2-16-mer injection was also examined (Fig. 3B). Animals that were injected only on day 8 after disease induction developed marked clinical signs of EAN similar to the controls, although the onset of disease was delayed when compared with the control animals. However, two doses at days 8 and 12 markedly reduced the extent of disease. Interestingly, a single injection on day 12 after disease had already begun to appear exerted the same protective effect as the double treatment on days 8 and 12. In contrast to the previous study in the EAE model system, however, an effect of the 16-mer treatment was only observed when the disease was induced with the P2 peptide. The P2-16-mer had virtually no effect when the disease was induced with 2 mg of whole peripheral bovine myelin, which may contain several autoantigens (data not shown).
Figure 3.
Dose-dependence and timing of the induction of tolerance to active, P253–78 peptide-induced EAN by P2-16-mer. (A) Dose-response experiments. Animals were injected in the tail vein with the indicated amounts of P2-16-mer/1 ml PBS on days 8 and 12 after induction of active EAN. Control animals were injected i.v. with PBS. Values are given as mean clinical score in groups of five animals. P < 0.001 for 200 μg group vs. control group; P < 0.001 for 100 μg group vs. control group by ANOVA. (B) Timing experiments. Animals were injected in the tail vein with 200 μg of P2-16-mer/1 ml PBS at the indicated time points after induction of EAN. Control animals were injected i.v. with PBS. Values are given as mean clinical score in groups of four animals each. P < 0.05 for day 8 + 12 group vs. control group; P < 0.01 for day 12 vs. control group by ANOVA.
Treatment of EAN by the P2-16-mer.
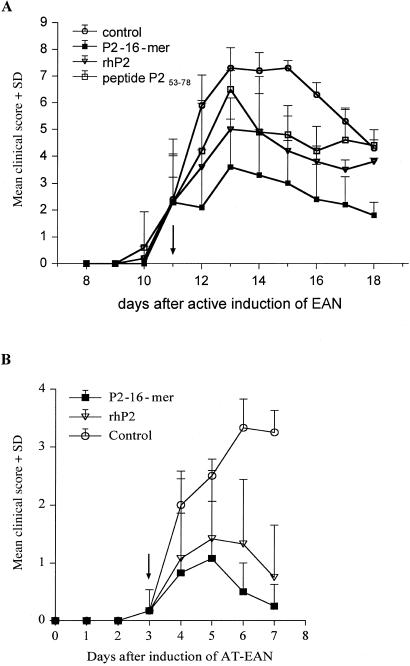
The results shown in Fig. 3B suggested that treatment of already established disease by the P2-16-mer is feasible. Two models were examined: treatment of disease after immunization with P2 peptide in CFA or after adoptive transfer of an established neuritogenic T cell line. In the first model overt disease is becoming evident at day 11, appearing initially as loss of tail tone and slight ataxia (Fig. 4A). Treatment at this time with 200 μg of P2-16-mer halted progression of the disease. Subsequent recovery was slow, as is typical for EAN (5), because considerable demyelination and axonal loss (partly due to inflammation and swelling) have already occurred. Complete recovery occurred in 3–4 weeks. By contrast, untreated animals progressed to quadriparesis from which complete recovery was never obtained, the rats being left with a residual waddling gait (ataxia). Both the protein rhP2 and the peptide P253–78 had an intermediate effect on disease progression and subsequent outcome. Electrophysiological studies were in accord with the clinical disease course (data not shown).
Figure 4.
Treatment of EAN by P2-16-mer. (A) Active EAN. EAN was induced in groups of five rats by s.c. injection of an emulsion of 100 μg of peptide P253–78 and CFA. At day 11 groups received i.v. injection with 200 μg of P2 peptide or 500 μg of rhP2 protein/1 ml PBS as indicated. Control rats were injected i.v. with PBS. Values are given as mean clinical score + SD. P < 0.001 for 16-mer vs. control in ANOVA. (B) AT-EAN. EAN was induced in groups of five rats by i.v. injection of T cell clone TKtsA. At day 3 groups received i.v. injection with 200 μg of P2-16-mer or 500 μg of rhP2 protein/1 ml PBS as indicated. Control rats were injected i.v. with PBS. Values are given as mean clinical score + SD. P < 0.001 for 16-mer vs. control in ANOVA.
In the adoptive transfer model (AT-EAN) disease begins much earlier at 3 days and in the protocol used had a milder course, reaching only a clinical score of 3.5–4 (severe ataxia), although in this model a more severe disease reaching a clinical score of 7–8 can also be produced by using a larger number of cells in AT-EAN. In this experiment the clone TKtsA6 was used for the transfer. Although this clone showed almost no proliferative response to P2-16-mer in vitro (Fig. 1B), treatment starting at day 3 again aborted disease progression, with full recovery by day 7 (Fig. 4B). Protein rhP2 was less effective and recovery was delayed.
Vaccination Against EAN with the P2-16-mer.
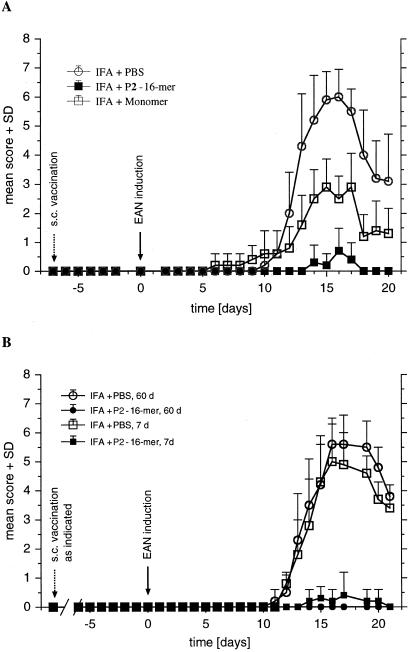
To investigate treatment with the P2-16-mer further, animals were injected with 200 μg of P2-16-mer, P258–73 monomeric peptide, or PBS before induction of EAN by immunization—i.e., vaccination. Two routes of vaccination were used: i.v. injection of P2-16-mer in PBS at days −3 and −7 and s.c. injection of a single dose at the tail base at day −7, followed by s.c. immunization with P253–78 peptide in CFA at day 0. When animals were pretreated s.c. with an emulsion of P2-16-mer in incomplete Freund's adjuvant (IFA) on day −7 before disease induction, nearly complete protection was observed, whereas rats treated similarly with P258–73 monomer developed ataxia and control animals suffered from severe paraparesis (Fig. 5A). With i.v. vaccination, monomeric P258–73 peptide-vaccinated animals exhibited severe clinical signs with paraparesis similar to controls receiving PBS, whereas a very mild disease appeared in rats pretreated with P2-16-mer i.v. (not shown). Subcutaneous administration in IFA may provide for longer availability of the peptide in vivo. To support this notion, in long-term vaccination studies s.c. vaccination with IFA was done at days −60, −30, and −7 before induction of EAN. The vaccinating effect persisted for at least 60 days (Fig. 5B). Results for the group vaccinated 30 days before EAN induction were identical (not shown in Fig. 5B).
Figure 5.
Vaccination with P2-16-mer. (A) Animals were vaccinated by s.c. injection with 200 μg of P2-16-mer/IFA or monomeric peptide P258–73 on day −7 before induction of active EAN (s.c. vaccination). Control animals were injected with PBS/IFA. (B) Animals were vaccinated by s.c. injection with 200 μg of P2-16-mer/IFA or PBS/IFA on days −60 (60 d) or −7 (7 d) before induction of active EAN. Values are given as mean clinical score + SD in groups of five animals. P < 0.001 for 16-mer vs. PBS for A and B by using ANOVA.
Role of Apoptosis in Down-Regulation of P253–78-Specific Immune Response.
Using the AT-EAN model with the T cell clone TKtsA6, the question of whether tolerance toward induction of EAN after 16-mer treatment was mediated by apoptosis of neuritogenic P253–78-specific T cells was examined as described for high-dose antigen therapy (18). Animals were treated twice within 12 h with the indicated peptides at the maximum of disease (days 6/7 after transfer of 1.2 × 107 activated T cells from neuritogenic T cell clone TKtsA6) and killed 6 h after the second injection. An increase in apoptosis rate in peripheral nerve tissue was detected only within the rhP2 protein-treated group (12.4% ± 4.9% vs. 6.2% ± 2.7% in controls) in accord with previous work (18), but no change was observed in the groups treated with the P258–73 monomer or the P2-16-mer. Similar results were obtained when the extent of apoptosis was examined immunohistochemically in fresh frozen lymph node tissue (data not shown).
Soluble P2-16-mer Prestimulated LN Cells Were Not Able to Proliferate After Challenge with Monomeric P2 Peptides.
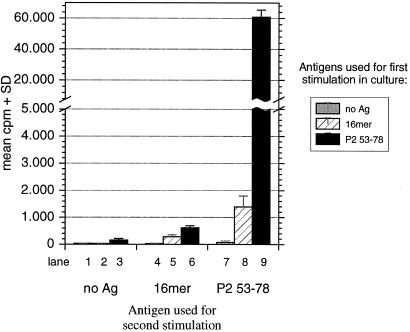
To further analyze the tolerizing mechanisms, the influence of the P2-16-mer on the proliferation of P253–78-primed LN cells was investigated ex vivo (Fig. 6). LN cells first stimulated with the monomeric P2 peptide showed a strong proliferation in response to this peptide when challenged 8 days later (lane 9), but no proliferation was observed when challenged with the P2-16-mer (lane 8). Strikingly, no proliferation in LN cell cultures primed with P253–78 in vivo and first exposed to the P2-16-mer in vitro could be detected. Neither the monomer nor the 16-mer were then able to elicit any response (lanes 5 and 6). A general inhibiting effect of the oligomer on antigen processing and presentation, however, was not seen—i.e., normal responses were found to PPD, a component of CFA used in immunization (data not shown). The recognition of P2 peptides or P2 protein on radiated thymocytes by the TKtsA6 line was not suppressed in the presence of high concentrations of the 16-mer (data not shown). Thus, the suppression was clone-specific and not due to nonspecific or toxic effects.
Figure 6.
P2-16-mer pretreated LN cells did not proliferate after stimulation with P2 peptides. Twelve days after immunization with P253–78 peptide, LN cells from immunized animals were exposed to 10 μg of P253–78 peptide, P2-16-mer, or no antigen (first stimulation). LN cells were propagated by using IL-2-containing medium and were challenged by using 10 μg of the indicated antigens on day 8 (second stimulation). The specific proliferative response was measured by [3H]thymidine incorporation. Note that the y axis is interrupted for the proliferative response of cells stimulated with P253–78 peptide in both cycles. Results are expressed as mean cpm + SD of triplicates.
Discussion
Newer therapeutic approaches for prevention and therapy of autoimmune diseases aim at the specific suppression of autoreactive T cells. This may be achieved by immunomodulation directed at the trimolecular TCR peptide–MHC complex to generate “high zone tolerance” (27), for example, by the induction of anergy (28) or the apoptotic elimination of T cells (18, 29, 30) as occurring during natural disease course (5, 31). In these earlier approaches, however, relatively high amounts of the antigen had to be administered. In the present study, the multimerized P2 peptide was effective at a much lower treatment dose. A single injection with 200 μg of the P2-16-mer administered at day 12 after disease induction with peptide in CFA was remarkably effective in prevention of appearance of disease in Lewis rats (Fig. 3). P2-16-mer-treated animals were almost completely protected against the development of EAN, whereas animals treated with the same amount of the monomeric control peptide still developed relatively severe forms of the disease. In addition, the present study is the first example of the utility of a 16-mer in the treatment of already established disease induced by immunization or adoptive transfer (Fig. 4). Finally, vaccination of animals with P2-16-mer almost completely prevented the latter induction of EAN and this effect lasted for at least 2 months (Fig. 5). In a previous study, the rhP2 protein was also highly effective when used as high-dose antigen therapy for EAN (18, 32). Although similar mean clinical scores were observed in both treatment groups, treatment with the P2-16-mer was superior to treatment with rhP2 protein. In contrast to therapy with rhP2 protein, an enhanced release of the apoptosis-inducing cytokine TNF-α (32) was not detected and severe side effects were not observed, including the liver necrosis that was typical for the P2 protein antigen therapy in EAN (32) or MBP antigen therapy in EAE (ref. 33; data not shown).
When the ex vivo proliferative response after in vivo treatment with P253–78 and after exposure to the P2-16-mer in vitro was characterized, a strikingly reduced proliferation to the neuritogenic P2 peptide was found. The effect was antigen-specific, so that nonspecific effects on antigen processing and presentation are unlikely. However, T cell apoptosis, which plays a critical role in elimination of autoreactive T cells during therapy with rhP2 protein (18) or during high-zone tolerance induction with other oligomerized T cell epitopes (16), could not be detected in the case of the P2-16-mer. The rate of apoptotic T cells in sciatic nerve either in AT-EAN or in actively induced EAN was not increased after P2-16-mer treatment. Also, in a similar experimental setting no increased rate of apoptotic T cells in lymph node cells was found. The stimulative capacity of the P2-16-mer for P253–78-specific cell lines was rather weak. The nonresponsiveness may be due to induction of anergy in this case, although T cell receptor down-regulation was not observed (data not shown). Other forms of tolerance induction, such as induction of regulatory T cells, cannot be excluded. Of note, the nonresponsiveness after treatment with P2-16-mer could not be overcome by addition of IL-2, and the vaccinating effect was much longer than expected in common models of anergy induction that induce nonresponsiveness for less than 3 weeks (34). On the other hand, we were not able to show increased production of IL-10 indicative for regulatory T cell populations. Future experiments are needed to elucidate these complex mechanisms.
Thus, P2-oligomers can be used for the treatment of active EAN by suppressing the response of autoreactive T cells. Moreover, our data provide additional support to the observation that multimerized T cell epitopes are valuable tools for investigation of fundamental mechanisms of tolerance induction. Prevention or therapy of autoimmune diseases in man may also be possible. The major limitation, however, is the lack of definitive identification of the primary autoantigenic protein(s)/peptide(s) in nearly all human autoimmune diseases. For example, in Guillain-Barré syndrome (GBS), for which EAN is an animal model, the peripheral radiculoneuropathy is thought to be triggered by prior infection (Campylobacter jejuni, cytomegalovirus, Haemophilus influenzae, and others) or possibly by vaccination (tetanus toxoid, influenza), but not much information is available concerning autoantigens involved in the immunopathogenesis of GBS (see review in ref. 35). The identification of candidate autoantigens in human diseases remains an important challenge if we were to adapt the paradigms developed in the animal models.
Acknowledgments
We thank Dr. David A. Hafler for critical discussion on fundamental mechanisms of antigen-specific immunotherapies. We appreciate the skillful technical assistance of V. Wörtmann, H. Brünner, S. Jah, and H. Finlay. This study was supported by Bundesministerium für Bildung und Forschung (Germany) 2000 (Interdisziplinäres Zentrum für klinische Forschung Würzburg 01 KS903/C13), Gemeinnützige Hertie Stiftung (GHS 2/307/97), and National Institutes of Health Grants 5R35-CA47554 and N01-AI45198.
Abbreviations
- EAN
experimental autoimmune neuritis
- AT-EAN
adoptive transfer EAN
- EAE
experimental autoimmune encephalomyelitis
- LN
lymph node
- PPD
tuberculin purified protein derivative
- rh
recombinant human
- TCR
T cell receptor
- CFA
complete Freund's adjuvant
References
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Crawford F, Kozono H, White J, Marrack P, Kappler J. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 3.Boniface J J, Rabinowitz J D, Wülfing C, Hampl J, Reich Z, Altman J D, Kantor R M, Beeson C, McConnell H M, Davis M M. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 4.Rötzschke O, Falk K, Strominger J L. Proc Natl Acad Sci USA. 1997;94:14642–14647. doi: 10.1073/pnas.94.26.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold R, Archelos J J, Hartung H P. Brain Pathol. 1999;9:343–360. doi: 10.1111/j.1750-3639.1999.tb00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes R A C. Guillain-Barré Syndrome. London: Springer-Verlag; 1990. [Google Scholar]
- 7.Hartung H-P, Stoll G, Toyka K V. In: Peripheral Neuropathy. Dyck P J, Griffin J W, Low P A, Poduslo J F, editors. Philadelphia: Saunders; 1993. pp. 418–444. [Google Scholar]
- 8.Olee T, Powers J M, Brostoff S W. J Neuroimmunol. 1988;19:167–173. doi: 10.1016/0165-5728(88)90046-x. [DOI] [PubMed] [Google Scholar]
- 9.Rostami A, Gregorian S K. Cell Immunol. 1991;132:433–441. doi: 10.1016/0008-8749(91)90040-i. [DOI] [PubMed] [Google Scholar]
- 10.Olee T, Powell H C, Brostoff S W. J Neuroimmunol. 1990;27:187–190. doi: 10.1016/0165-5728(90)90068-x. [DOI] [PubMed] [Google Scholar]
- 11.Kadlubowski M, Hughes R A C. Nature (London) 1979;277:140–141. doi: 10.1038/277140a0. [DOI] [PubMed] [Google Scholar]
- 12.Brostoff S W, Levit S, Powers J M. Nature (London) 1977;268:752–753. doi: 10.1038/268752a0. [DOI] [PubMed] [Google Scholar]
- 13.Linington C, Izumo S, Suzuki M, Uyemura K, Meyermann R, Wekerle H. J Immunol. 1984;133:1946–1950. [PubMed] [Google Scholar]
- 14.Weishaupt A, Giegerich G, Jung S, Gold R, Enders U, Pette M, Hartung H-P, Toyka K V. J Neuroimmunol. 1995;63:149–156. doi: 10.1016/0165-5728(95)00139-5. [DOI] [PubMed] [Google Scholar]
- 15.Pollard J D, Westland K W, Harvey G K, Jung S, Bonner J, Spies J M, Toyka K V, Hartung H P. Ann Neurol. 1995;37:467–475. doi: 10.1002/ana.410370409. [DOI] [PubMed] [Google Scholar]
- 16.Falk K, Rötzschke O, Strominger J. Eur J Immunol. 2000;30:3012–3020. doi: 10.1002/1521-4141(200010)30:10<3012::AID-IMMU3012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Falk K, Rotzschke O, Santambrogio L, Dorf M E, Brosnan C, Strominger J L. J Exp Med. 2000;191:717–730. doi: 10.1084/jem.191.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weishaupt A, Gold R, Giegerich G, Hartung H-P, Toyka K. Proc Natl Acad Sci USA. 1997;94:1338–1342. doi: 10.1073/pnas.94.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura K, Suzuki M, Uyemura K. Biochim Biophys Acta. 1976;455:806–816. doi: 10.1016/0005-2736(76)90050-x. [DOI] [PubMed] [Google Scholar]
- 20.Stienekemeier M, Herrmann T, Kruse N, Weishaupt A, Weilbach F X, Giegerich G, Theofilopoulos A N, Jung S, Gold R. Brain. 1999;122:523–535. doi: 10.1093/brain/122.3.523. [DOI] [PubMed] [Google Scholar]
- 21.Hartung H-P, Schäfer B, Heiniger K, Stoll G, Toyka K. Brain. 1988;111:1039–1059. doi: 10.1093/brain/111.5.1039. [DOI] [PubMed] [Google Scholar]
- 22.King R H M, Craggs R I, Cross M L P, Thomas P K. Exp Neurol. 1985;87:9–19. doi: 10.1016/0014-4886(85)90129-3. [DOI] [PubMed] [Google Scholar]
- 23.Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka K V, Suter U. Nat Genet. 1995;11:274–280. doi: 10.1038/ng1195-274. [DOI] [PubMed] [Google Scholar]
- 24.Zettl U K, Gold R, Toyka K V, Hartung H-P. J Neuropathol Exp Neurol. 1995;54:540–547. doi: 10.1097/00005072-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Lannes Vieira J, Goudable B, Drexler K, Gehrmann J, Torres-Nagel N, Hünig T, Wekerle H. Eur J Immunol. 1995;25:611–616. doi: 10.1002/eji.1830250245. [DOI] [PubMed] [Google Scholar]
- 26.Schmied M, Breitschopf H, Gold R, Zischler H, Rothe G, Wekerle H, Lassmann H. Am J Pathol. 1993;143:446–452. [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki G, Kawase Y, Koyasu S, Yahara I, Kobayashi Y, Schwartz R H. J Immunol. 1988;140:1359–1365. [PubMed] [Google Scholar]
- 28.Gaur A, Wiers B, Liu A, Rothbard J, Fathman C G. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 29.Critchfield J M, Lenardo M J. Clin Immunol Immunopathol. 1995;75:13–19. doi: 10.1006/clin.1995.1046. [DOI] [PubMed] [Google Scholar]
- 30.Critchfield J M, Racke M K, Zuniga P J, Cannella B, Raine C S, Goverman J, Lenardo M J. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 31.Pender M P, Nguyen K B, McCombe P A, Kerr J F. J Neurol Sci. 1991;104:81–87. doi: 10.1016/0022-510x(91)90219-w. [DOI] [PubMed] [Google Scholar]
- 32.Weishaupt A, Gold R, Hartung T, Gaupp S, Wendel A, Bruck W, Toyka K V. J Neuropathol Exp Neurol. 2000;59:368–376. doi: 10.1093/jnen/59.5.368. [DOI] [PubMed] [Google Scholar]
- 33.Weishaupt A, Jander S, Brück W, Stienekemeier M, Hartung T, Toyka K V, Stoll G, Gold R. J Immunol. 2000;165:7157–7163. doi: 10.4049/jimmunol.165.12.7157. [DOI] [PubMed] [Google Scholar]
- 34.Namba K, Ogasawara K, Kitaichi N, Morohashi T, Sasamoto Y, Kotake S, Matsuda H, Iwabuchi K, Iwabuchi C, Ohno S, et al. J Immunol. 2000;165:2962–2969. doi: 10.4049/jimmunol.165.6.2962. [DOI] [PubMed] [Google Scholar]
- 35.Hartung H-P, Willison H, Jung S, Pette M, Toyka K V, Giegerich G. Springer Semin Immunopathol. 1996;18:97–123. doi: 10.1007/BF00792612. [DOI] [PubMed] [Google Scholar]