Phagocytosis is a well understood process whereby pathogens and cell debris are removed from within the body, and it is an important homeostatic mechanism in health and disease. What is less commonly discussed is the process of “efferocytosis.” This term, derived from the Latin prefix effero-, meaning “to take away, to carry to the grave, or to bury” (1), is a more nuanced mechanism of phagocytosis, which focuses on the clearance of apoptotic cells by neutrophils and macrophages. Efferocytosis has been studied in cancer and in a more limited capacity in atherosclerosis. In a recent study in Nature, Kojima et al. (2) described the role of CD47 in the development of atherosclerosis and proposed that anti-CD47 antibodies may represent a novel therapeutic strategy for treating coronary artery disease by promoting efferocytosis.
In this paper (2), CD47 was found to be increased in the atherosclerotic regions of human coronary and carotid arteries. Of note, the increase in CD47 was localized to the necrotic core of the plaques and was up-regulated to a greater extent in people with a history of stroke or transient ischemic attack, compared with samples from patients with stable asymptomatic carotid lesions. On the basis of the intriguing human and in vitro data, the authors studied the role of CD47 in apolipoprotein E−/− mice treated with angiotensin II infusion—a small animal model prone to the development of atherosclerosis. When these animals were treated with inhibitory antibodies directed against CD47, Kojima et al. (2) found a marked reduction in atherosclerosis compared with those treated with immunoglobulin G control. In other models of chronic atherosclerosis, and in models more reflective of the clinical disease state observed in humans, the authors found that anti-CD47 antibodies had similar effects. The authors also found that there was a marked decrease in residual apoptotic bodies in the atherosclerotic plaque of animals treated with anti-CD47 antibodies, consistent with impaired efferocytosis in atherosclerosis. Additionally, colocalization studies and electron microscopy studies demonstrated that the number of free apoptotic bodies (i.e., not associated with an intraplaque macrophage) was decreased after anti-CD47 antibody treatment, thereby implicating failed efferocytosis in the pathogenesis of CD47-mediated atherosclerosis. Furthermore, the atherosclerotic lesions in the animals treated with anti-CD47 antibodies had smaller necrotic cores and less apoptotic debris.
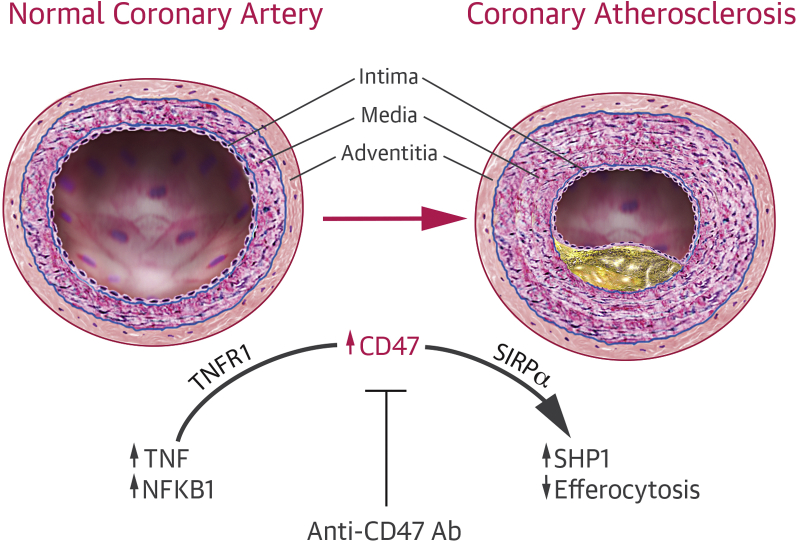
In a series of mechanistic studies, Kojima et al. (2) showed that CD47 interrupts efferocytosis by binding to the antiphagocytic receptor signal regulatory protein (SIRP)-α (Figure 1). Activation of SIRPα typically results in phosphorylation of SHP1, thereby blocking phagocytosis. The downstream effect of anti-CD47 antibodies includes a decrease in phosphorylation of SHP1 inside the atherogenic plaque, thereby reactivating efferocytosis. Using co-expression studies and a bioinformatics approach, the authors showed that CD47 was up-regulated by tumor necrosis factor (TNF) acting on the type 1 TNF receptor (TNFR1). TNF was already known to be increased in atherosclerosis, and the findings by Kojima et al. (2) suggest a potentially important mechanism through which TNF may contribute to the atheromatous plaque. Interestingly, vascular smooth muscle cells treated with recombinant TNF had increased CD47 levels and were less likely to be susceptible to phagocytosis by macrophages, even when exposed to pro-apoptotic stimuli, such as oxidized low-density lipoprotein or staurosporine. Moreover, the effect on atherosclerosis and efferocytosis was even more pronounced when anti-CD47 antibodies were administered in combination with either etanercept or infliximab.
Figure 1.
The Role of CD47 and TNF in the Development of Atherosclerosis
NFKB1 = nuclear factor kappa-light-chain-enhancer of activated B cells subunit 1; SHP1 = Src homology region 2 domain-containing phosphatase-1; TNF = tumor necrosis factor.
Translational Perspective
The translational application of this work is potentially very significant. Within the field of coronary artery disease, no currently available therapeutic agents are approved to specifically target the immunological pathways involved in atherosclerosis, although canakinumab (a human monoclonal antibody targeted at interleukin-1 beta) and methotrexate are currently being evaluated in clinical trials in patients with atherosclerosis (NCT01327846 and NCT01594333, respectively). It is therefore exciting that agents that target CD47 levels might reduce the burden of coronary artery disease. Enthusiasm must be tempered at this time, because of the current absence of demonstrable clinical benefit in the use of TNF antagonists, namely infliximab, adalimumab, and etanercept (NCT01356758), in protecting against coronary artery disease in psoriatic arthritis, although these studies are ongoing. There are also several nonrandomized, population-based studies as well as pre-clinical data that propose a protective effect of immunomodulatory therapy. However, this form of therapy is not currently incorporated into clinical guidelines as a treatment option for atherosclerosis or ischemic heart disease (3).
The link between increased CD47 expression and decreased efferocytosis is important in cancer, and has led to a phase I clinical trial using anti-CD47 in patients with relapsed acute myeloid leukemia (NCT02678338). Moreover, there are additional possibilities for utilizing anti-CD47 antibodies in other disease processes where apoptosis is reduced and cell proliferation is pronounced, such as pulmonary arterial hypertension. Indeed, increased CD47 in pulmonary endothelial cells has already been implicated in the pathogenesis of this occlusive vasculopathy (4). CD47 has also been identified as playing a causative role in systolic heart failure, possibly by altering excitation-contraction coupling (5).
The findings of Kojima et al. (2) open up an exciting avenue in vascular biology that may also lead to new treatment options for treating atheromatous plaque, which still remains an important clinical need.
Footnotes
Dr. Ryan has reported that he has no relationships relevant to the contents of this paper to disclose.
References
- 1.deCathelineau A.M., Henson P.M. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 2.Kojima Y., Volkmer J.P., McKenna K. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fihn S.D., Blankenship J.C., Alexander K.P. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Bauer P.M., Bauer E.M., Rogers N.M. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93:682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifi-Sanjani M., Shoushtari A.H., Quiroz M. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc. 2014;3:e000670. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]