Corresponding Author

Key Words: familial hypercholesterolemia, LDL receptor, lipoprotein(a), PCSK9, primary human hepatocytes
Since its discovery in 1963 by Kare Berg (1), multiple observational studies have implicated lipoprotein (a) [Lp(a)] in cardiovascular risk. Lp(a) consists of a cholesterol-rich lipid particle encircled by a modified apolipoprotein B and occasionally studded with other apolipoproteins. The Lp(a) particle contains an apolipoprotein B that has undergone covalent linkage to the defining apolipoprotein, apo(a). Unlike low-density lipoprotein (LDL) cholesterol, which follows a roughly Gaussian distribution in the population, Lp(a) levels skew, with most individuals in a lower range and a tail of individuals that display higher Lp(a) concentrations and corresponding elevated cardiovascular risk. Heritability and ethnicity strongly influence Lp(a) plasma concentrations. In particular, African Americans tend to have higher Lp(a) levels than Caucasians.
The discovery that apolipoprotein (a) [apo(a)] has homology with plasminogen, an important enzyme in fibrinolysis, provided a potential connection between Lp(a) and thrombosis (2). As apo(a) appears to lack the enzymatic activity of plasminogen, some postulated that it may serve as a “dominant negative” plasminogen interfering with thrombolysis. The functional consequences of Lp(a) for thrombosis and thrombolysis remain elusive, however. The study of the mechanisms by which Lp(a) may exert its noxious effects remains fragmentary and controversial. A barrier to unraveling this important problem is the species specificity of Lp(a), a protein that is not present in the animals commonly used in studies of experimental atherosclerosis. The heterogeneity of Lp(a) presents another complexity. The apolipoprotein can contain a variable number of repeats of “kringle”-like motifs, notably Kringle 4. The more Kringle 4 repeats, the lower the plasma concentration of the apolipoprotein. This heterogeneity in Lp(a) isoforms and other issues render it a difficult analyte. These challenges to the standardized measurement of Lp(a) concentrations have also hampered clinical studies. The high heritability of Lp(a) concentrations suggests a strong genetic component to an individual’s Lp(a) status. Despite all of these quandaries encountered at the protein level and mysteries regarding the mechanisms of Lp(a)’s adverse effects on cardiovascular outcomes, strong human genetic and epidemiological data support a causal role of Lp(a) in arteriosclerotic cardiovascular disease 3, 4, 5, and furnish one of the best substantiated genetic links to aortic stenosis that have emerged from genome-wide association studies (6).
Despite this conjunction of observational epidemiology and genetic data, the therapeutic targeting of Lp(a) has proven frustrating. The usual menu of drugs that target lipid metabolism have little effect on Lp(a), save for niacin. Unfortunately, nicotinic acid’s benefit for CAD patients has recently come into question on the basis of results of recent outcome trials 7, 8.
The antibodies that neutralize proprotein convertase subtilisin/kexin type 9 (PCSK9), currently under investigation as LDL-lowering agents, offer a glimmer of hope in this otherwise bleak preventive perspective. Studies that have monitored lipoprotein profiles in patients treated with these agents reproducibly and consistently show reductions in Lp(a) 9, 10. We eagerly await the results of the large outcome trials that are currently underway with 3 agents of this class. Perhaps these studies will enroll enough individuals with higher categories of Lp(a) to permit subgroup analyses of sufficient power to test whether the anti-PCSK9 antibodies can improve outcomes in individuals with augmented risk due to higher concentrations of Lp(a). We might then at last with these new line of agents have an evidence-based therapy to offer to our patients and families with elevated risk of premature arteriosclerotic cardiovascular disease. Antisense oligonucleotide strategies (ASO) for inhibiting apo(a) synthesis also show promise as a novel therapeutic approach to lower plasma Lp(a) levels (11). Yet the anti-PCSK9 antibodies and antisense oligonucleotide strategy agents require parenteral administration and entail considerable expense. Hence, understanding the mechanisms by which anti-PCSK9 antibodies reduce Lp(a) might provide insight that could lead to a more specific approach to lowering levels of this highly atherogenic lipoprotein.
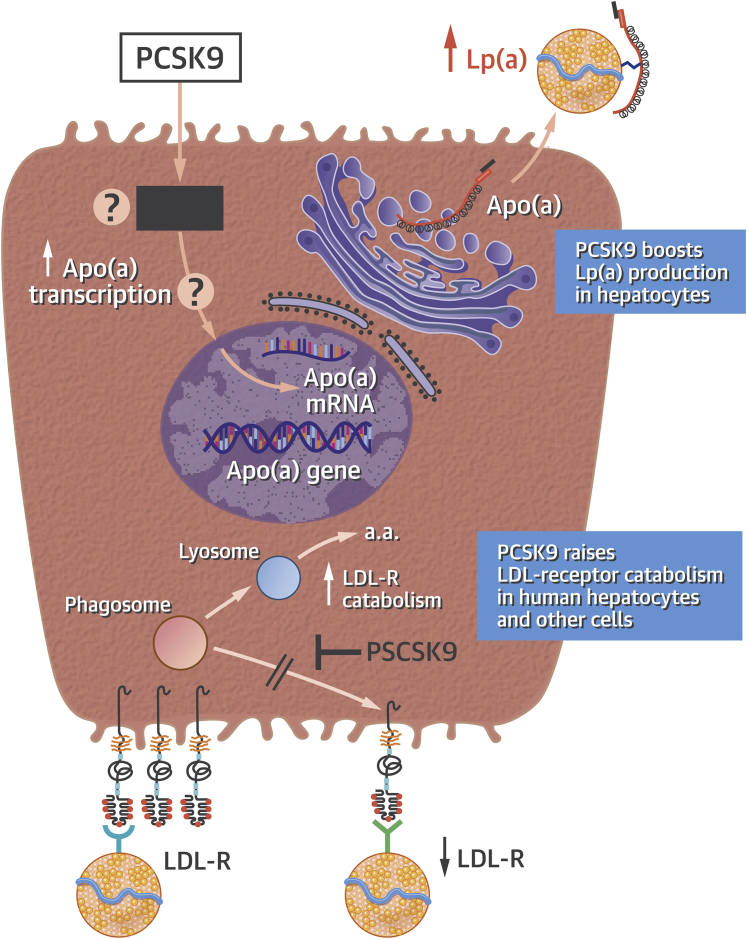
The study by Villard et al. (12) in this issue of JACC: Basic to Translational Science offers a step in this direction. The investigators studied skin fibroblasts from normal individuals and from patients with heterozygous or homozygous familial hypercholesterolemia, a disease caused by defective function of the LDL receptor. They also studied primary cultures of human hepatocytes. Their experiments showed that uptake of Lp(a) by these various cell types did not depend on the LDL receptor (Figure 1). The results further demonstrated that neither PCSK9 nor the anti-PCSK9 antibody alirocumab influenced the internalization of Lp(a) by these cells. As expected, statin treatment elevated LDL receptor expression and increased the internalization of LDL, whereas the addition of exogenous recombinant PCSK9 reduced LDL receptor expression and inhibited uptake of LDL. These observations provided a “control” for the negative studies that evaluated Lp(a) uptake. The findings of this study indicate that anti-PCSK9 antibodies do not alter the catabolism of Lp(a) as a mechanism to explain their reduction in this particle.
Figure 1.
PCSK9 Modulates LDL and Lp(a) Concentrations by Distinct Mechanisms
This diagram depicts a human hepatocyte. (Bottom) The low-density lipoprotein receptor (LDL-R) cycle portrays the well-known effect of proprotein convertase subtilisin/kexin type 9 (PCSK9) in targeting the LDL-R for lysosomal degradation to amino acids (a.a.), increasing its catabolism and decreasing its recycling into the cell surface membrane. The reduced LDL-R numbers on the hepatocyte surface impede low-density lipoprotein (LDL) clearance and hence raise its plasma levels. (Top) The action of PCSK9 to increase apolipoprotein (a) [apo(a)] protein production by the hepatocyte, by a transcriptional or other mechanism that remains undefined (black box). Increased production of apo(a) by the hepatocyte increases the plasma concentration of the atherogenic lipoprotein (a) [Lp(a)] particles. Thus, anti-PCSK9 antibodies lower LDL and Lp(a) concentrations by distinct mechanisms. The in vitro experiments presented by Villard et al. (12) in the accompanying paper indicate that Lp(a) catabolism does not depend on the LDL receptor under the conditions of their experiments. mRNA = messenger ribonucleic acid.
Further studies in liver cells showed that treatment with PCSK9 increased the release of apo(a) from human hepatocytes by 3-fold (Figure 1). Addition of the anti-PCSK9 antibody reversed the substantial increase in Lp(a) secretion provoked by PCSK9. These results offer several novel findings. Lp(a) uptake does not appear to depend on the classical LDL receptor. PCSK9 does not alter the binding or uptake of Lp(a) by “peripheral” cells exemplified by dermal fibroblasts. Finally, the observations in primary human hepatocytes show that PCSK9 directly augments Lp(a) production (Figure 1). The mechanism by which PCSK9 produces this augmented protein production remains unknown, but it should prove tractable to further analysis using the in vitro approach adopted in this study. Yet the phenomenon reported by Villard et al. (12) provides a first step in understanding why anti-PCSK9 antibodies lower levels of Lp(a) in patients.
The finding that anti-PCSK9 can inhibit hepatocyte production of apo(a) indicates that other agents might modulate this process, including small molecules that might be orally available and less costly to produce than biologics or antisense strategies. It is unclear to what extent PCSK9’s elevation in Lp(a) production results from regulation of transcription, translation, or altered intracellular proteolysis. Yet the study by Villard et al. (12) raises the optimistic note that further dissection of the molecular mechanisms by which PCSK9 modulates Lp(a) production will not only increase our understanding of the effects of this pleiotropic molecule but also provide potential new areas of development for therapies that can modulate Lp(a). Given the dearth of acceptable pharmacological approaches to lowering Lp(a) in our current armamentarium, the advent of the anti-PCSK9 antibodies and the new insight that they lower Lp(a) plasma concentrations by inhibiting hepatic production rather than by augmenting catabolism, as they do in the case of LDL, has both mechanistic and therapeutic implications for the future.
Footnotes
Dr. Libby has served as an unpaid consultant for Amgen, Sanofi-Regeneron, and Ionis Pharmaceuticals.
References
- 1.Berg K. A new serum type system in man—the Lp system. Acta Pathol Microbiol Scand. 1963;59:369–382. doi: 10.1111/j.1699-0463.1963.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 2.McLean J.W., Tomlinson J.E., Kuang W.J. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 3.Clarke R., Peden J.F., Hopewell J.C. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 4.Kamstrup P.R., Tybjaerg-Hansen A., Nordestgaard B.G. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61:1146–1156. doi: 10.1016/j.jacc.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Nordestgaard B.G., Chapman M.J., Ray K. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thanassoulis G., Campbell C.Y., Owens D.S. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden W.E., Probstfield J.L., Anderson T. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.HPS2-THRIVE Collaborative Group HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudet D., Kereiakes D.J., McKenney J.M. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials) Am J Cardiol. 2014;114:711–715. doi: 10.1016/j.amjcard.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 10.Raal F.J., Giugliano R.P., Sabatine M.S. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Tsimikas S., Hall J.L. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Villard E.F., Thedrez A., Blankenstein J. PCSK9 modulates the secretion but not the cellular uptake of Lipoprotein (a) ex vivo: an effect blunted by alirocumab. J Am Coll Cardiol Basic Trans Science. 2016;1:419–427. doi: 10.1016/j.jacbts.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]