Summary
Glucagon-like peptide-1-(7-36) amide (GLP-1) is a human incretin hormone responsible for the release of insulin in response to food. Pre-clinical and human physiological studies have demonstrated cardioprotection from ischemia-reperfusion injury. It can reduce infarct size, ischemic left ventricular dysfunction, and myocardial stunning. GLP-1 receptor agonists have also been shown to reduce infarct size in myocardial infarction. The mechanism through which this protection occurs is uncertain but may include hijacking the subcellular pathways of ischemic preconditioning, modulation of myocardial metabolism, and hemodynamic effects including peripheral, pulmonary, and coronary vasodilatation. This review will assess the evidence for each of these mechanisms in turn. Challenges remain in successfully translating cardioprotective interventions from bench-to-bedside. The window of cardioprotection is short and timing of cardioprotection in the appropriate clinical setting is critically important. We will emphasize the need for high-quality, well-designed research to evaluate GLP-1 as a cardioprotective agent for use in real-world practice.
Key Words: GLP-1, glucagon-like peptide-1, ischemia reperfusion injury, ischemic heart disease, percutaneous coronary intervention
Abbreviations and Acronyms: ATP, adenosine triphosphate; ANP, atrial natriuretic peptide; AMI, acute myocardial infarction; DPP, dipeptidyl-peptidase; GLP-1, glucagon-like peptide 1-(7-36) amide; GLP-1R, GLP-1 receptor; GLP-1RA, GLP-1 receptor agonist; IC, ischemic conditioning; IR, ischemia reperfusion; PCI, percutaneous coronary intervention; RISK, reperfusion injury survival kinase; SAFE, survivor-activating factor enhancement; STEMI, ST-segment elevation myocardial infarction
Ischemic heart disease is the most important cause of morbidity and mortality in the developed world. Recent advances in revascularization of coronary arteries through percutaneous coronary intervention (PCI) and coronary artery bypass grafting have had a dramatic improvement in the fate of patients suffering with ischemic heart disease (1). Nonetheless, revascularization does not address damage from ischemia-reperfusion (IR) injury and indeed may even contribute to it. For example, up to fifty percent of the final infarct size of an acute myocardial infarction is attributable to IR injury (2). Additionally, IR injury is responsible for nonlethal effects, for example, myocardial stunning, arrhythmia and late remodeling. Therapies that reduce these detrimental effects of IR injury are desperately needed.
One promising target for cardioprotection is metabolic manipulation by optimizing use of myocardial glucose. The heart is an omnivore and can use a number of substrates for the generation of the adenosine triphosphate (ATP), required to power myocardial work. Glucose is the most efficient generator of ATP per unit of oxygen available (3). Therapies that increase glucose oxidation in preference to free fatty acids could reduce the impact of myocardial ischemia.
Glucagon-like peptide-1-(7-36) amide (GLP-1) is a human incretin hormone produced by the gut in response to food. It is primarily an insulinotropic hormone and has been extensively studied as a novel treatment for type 2 diabetes mellitus. It acts in a glucose-dependent manner, thus reducing the risk of hypoglycemia. However, in contrast to other oral hypoglycemic agents, GLP-1 has other potentially beneficial “off-target” cardiovascular properties including optimization of myocardial metabolism and possibly direct cardioprotection through binding to a cardiac receptor and signaling protection via subcellular ischemic conditioning pathways.
GLP-1 Structure and Function
GLP-1-(7-36) amide is a 30-amino acid cleavage product of pro-glucagon secreted by enteroendocrine L-cells in the gut (4). Oral ingestion of a meal is the primary physiological stimulus to GLP-1 secretion (5). GLP-1 is not secreted in response to an intravenous glucose infusion.
GLP-1 has receptor-dependent and -independent actions. It causes glucose-dependent insulin release through binding the GLP-1 receptor (GLP-1R) on pancreatic beta cells. GLP-1 does not cause hypoglycemia as its insulinotropic effect does not occur at a blood glucose concentrations <70 mg/dl (6). GLP-1 has a number of other physiological effects which serve to lower plasma glucose levels. These include stimulation of insulin gene transcription in the beta cell (7) and reduced gastric emptying (8). Enhancement of peripheral insulin sensitivity remain unproven with conflicting evidence (9).
The GLP-1R is a 463-amino acid, G protein-coupled receptor found on the cell surface membrane of numerous tissues throughout the body. Its presence within the myocardium has remained controversial. Both mouse and primate studies have suggested that GLP-1R remains confined to the atria and possibly just the sinoatrial node 10, 11. The location of the receptor is important in elucidating the mechanism of GLP-1 cardioprotection. Evidence of receptor-independent effects suggest that there may be an alternative receptor or perhaps actions that do not require a receptor 12, 13, discussed further below.
GLP-1 is cleaved by the enzyme dipeptidyl peptidase (DPP)-4 to GLP-1-(9-36) amide with a half-life of approximately 2 min (14). The biological role of this breakdown product is uncertain but it has reduced incretin activity. GLP-1 is further degraded by neutral endopeptidase to GLP-1 fragments whose biological activity is the subject of ongoing research.
A number of pharmaceutical products have been developed to use the incretin effect of GLP-1 while avoiding the difficulties associated with its rapid breakdown to an apparently inactive form. These drugs include DPP-4 inhibitors such as sitagliptin (Januvia, Merck, Kenilworth, New Jersey), saxagliptin (Onglyza, AstraZeneca, Macclesfield, United Kingdom), and vildagliptin (Galvus, Novartis, Basel, Switzerland), all of which increase levels of native GLP-1; and DPP-4-resistant GLP-1 receptor agonists (GLP-1RA) such as exenatide (Byetta, AstraZeneca, United Kingdom) and liraglutide (Victoza, Novo Nordisk, Bagsvaerd, Denmark).
Pre-Clinical Evidence of GLP-1 Cardioprotection
GLP-1 protects cardiomyocytes from cell death. In vitro, GLP-1 has been shown to avert cell death in HL-1 murine adult cardiomyocytes treated with the pro-apoptotic agent staurosporine (15). Both wild-type and GLP-1R homozygous knockout mice experienced cardioprotection from GLP-1-(7-36) and -(9-36), suggesting the protective effect occurred independent of receptor binding (16). GLP-1 protection was similarly associated with reduced activation of pro-apoptotic molecules in rats, and occurred regardless of whether it was given prior to ischemia or in early reperfusion 17, 18. In vivo, GLP-1 infusion given to dogs with pacing-induced cardiomyopathy improved left ventricular function with systemic changes such as reduced heart rate and blood pressure (19). Furthermore, GLP-1 infusion reduced infarct size after IR injury (20).
GLP-1 RAs have also been shown to protect against IR injury. Both albiglutide (Eperzan, GlaxoSmithKline, Stevenage Herts, United Kingdom) and lixisenatide (Lyxumia, Sanofi, Paris, France) reduced final infarct size in rat models 21, 22, and pretreatment with liraglutide protects in mice (23). In a porcine model of IR injury, treatment with exenatide for 3 days reduced infarct size and improved left ventricular function (24). However, initiation of exenatide after onset of ischemia did not result in cardioprotection, suggesting that, at least for GLP-1RAs, there may be a time-dependent element to the cardioprotection (25).
Mechanism of GLP-1-Mediated Cardioprotection
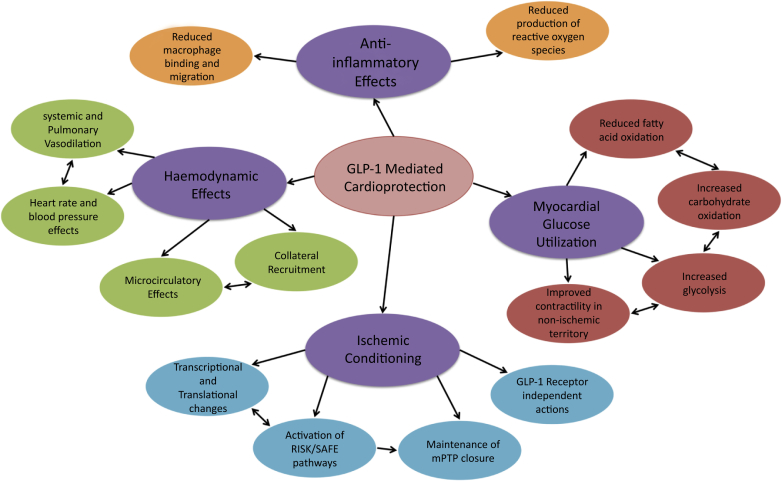
Many of the studies cited above have demonstrated aspects of cardioprotection that provide clues to how GLP-1 protects against IR injury. Several mechanisms have been proposed for GLP-1-mediated cardioprotection. A change in myocardial glucose utilization may result in increased metabolic efficiency and myocardial resistance to ischemia, thus limiting infarction. Vasodilation and reduction in systemic and/or pulmonary vascular resistance can also reduce cardiac work and ATP demand during ischemia. Finally, the pathways of ischemic preconditioning may be activated to increase cellular resistance to IR injury. These pathways may overlap, limiting lethal and nonlethal IR injury to various extents. Figure 1 offers an overview of a number of proposed mechanisms for GLP-1-mediated cardioprotection. Evidence for these mechanisms is reviewed below.
Figure 1.
Possible Mechanism of GLP-1-Mediated Cardioprotection: A Complex Web
GLP-1 cardioprotection appears to be mediated through a number of complex and interrelated cellular mechanisms. Figure 1 shows a number of proposed actions through which GLP-1 may exert its protective effect. Detailed physiological and biochemical studies are needed to tease out the relevant contributions of different actions and, indeed, determine whether some of these are “red-herrings.”
Myocardial glucose utilization
A change in myocardial glucose utilization is consistent with the physiological role of an incretin hormone. Fatty acids are the main fuel for energy production in the heart (26), although the omnivorous myocyte will use a number of different sources for ATP production (3). Glucose is the most oxygen-efficient source of ATP and is the preferred substrate when insulin and glucose levels are high, as in the post-prandial state (27). Glucose is preferentially metabolized in ischemic tissues and this is facilitated by augmentation of glucose uptake through up-regulation of the glucose transporters GLUT1 and GLUT4 to the cell surface membrane (28). Ischemic myocardium relies increasingly on anaerobic respiration through glycolysis, and if ischemia is prolonged intracellular levels of lactate and Ca2+ rise, the pH falls, and ultimately initiates the apoptotic cascade. Increasing substrate availability through increased concentrations of glucose and/or insulin can protect by delaying the initiation of this cascade, the so-called glucose-insulin-potassium (GIK) effect. GIK therapy was among the earliest cardioprotective interventions to be investigated, but its benefit remains uncertain and controversial (29). Some data have shown reduction in infarct size when given during acute myocardial infarction (30). However, these benefits may have been mitigated by clinical problems including risks of hypoglycemia and hyperkalemia. Lack of a clear cardioprotective benefit in subsequent larger studies such as DIGAMI II (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction 2) has limited the translation of the GIK effect into clinical practice (31).
Increased glucose uptake by the myocardium has been shown to be beneficial in IR injury (32). In a canine model of dilated cardiomyopathy, GLP-1 was shown to increase myocardial glucose uptake. This was associated with an improvement in myocardial performance (33). GLP-1 reduced levels of lactate and pyruvate in a porcine model of IR injury, suggesting alteration of myocardial glucose utilization. Of note, infarct size was not affected by GLP-1 in that model (34). Additionally, albiglutide produced an increase in myocardial glucose uptake and a shift from fatty acid to carbohydrate oxidation during IR injury in rats (21). In an ex vivo rat model, chronic treatment with DPP-4 inhibitors reduced infarct size in a glucose-dependent manner (35).
A recent study in rats showed that GLP-1 appeared to induce a switch to carbohydrate oxidation in the area not at risk following IR injury, as well as an increase in anaerobic glycolysis in the area subjected to IR injury (36). An increase in glucose utilization in the area not at risk may allow increased overall contractility of the ventricle during ischemia. Additionally, a rise in glycolysis may represent an increased number of viable cardiomyocytes in the ischemic area.
Conversely, a number of recent human studies have cast doubt on changes in myocardial glucose utilization as the mechanism behind GLP-1-mediated cardioprotection. A study of 20 nondiabetic patients given a 48-h subcutaneous infusion of GLP-1 did not show any significant change in metabolic parameters (37). Furthermore, coronary sinus sampling in humans subjected to brief coronary artery occlusion demonstrated no significant change in myocardial glucose extraction when GLP-1 was infused (38). In healthy volunteers, GLP-1 did not affect overall myocardial glucose utilization in either normo- or hypoglycemic states. The subgroup of volunteers with a high baseline level of glucose utilization had a reduced level in response to GLP-1. In contrast, those with low baseline level were found to exhibit increased glucose utilization (39).
Subtle changes in metabolism may have profound effects on the cardiomyocyte in IR injury but remain difficult to detect in human studies. Careful design of human studies is needed to untangle the metabolic changes induced by GLP-1 and those responsible for cardioprotection.
Conditioning pathways
Ischemic conditioning (IC) is the resistance of tissue to prolonged ischemia, induced by repeated cycles of transient ischemia and reperfusion, either locally or in a remote tissue. It has been demonstrated to be beneficial in both animal models (40) and some human clinical trials 41, 42. IC is mediated through reperfusion injury survival kinase (RISK) and survivor-activating factor enhancement (SAFE) pathways, which are complex but have been well described (43). A number of cell surface G protein-coupled receptors (44) initiate these survival cascades utilizing intracellular signaling molecules including phosphoinositol-3 kinase (PI3K), Akt, p70s6k, p38MAPK, and ERK1/2. These pathways converge upon the mitochondrial K-ATP channel (mK-ATP) 45, 46, the final common step in conditioning pathways that inhibits the opening of the mitochondrial permeability transition pore (mPTP), preventing mitochondrial electrochemical uncoupling, loss of the membrane potential, depletion of ATP, release of cytochrome C, hypercalcemia, and ultimately, cell death 47, 48.
Evidence that GLP-1 may activate IC mechanisms has accumulated (49). GLP-1 is known to activate the PI3K-Akt pathway in the pancreas (50), and GLP-1-mediated cardioprotection in an isolated rat heart model was associated with the RISK pathway. Inhibition of p70s6 kinase, a downstream target of PI3K, inhibited the cardioprotective effect of GLP-1. Although PI3K and Akt are consistently involved in GLP-1 cardioprotection 12, 17, phosphorylation of ERK1/2 occurred in some studies (13) but not others (51). Pigs exposed to IR injury had reduced infarct size when pretreated with exenatide. Levels of phosphorylated Akt and Bcl-2 were elevated after treatment with exenatide, suggesting activation of conditioning pathways (24).
The role of the GLP-1R in the activation of these subcellular signaling pathways is unclear, with inconsistent evidence in animal models. In the glucose-dependent rat model discussed above, the protective effect of DPP-4 inhibition was lost by blocking the GLP-1 receptor (35). However, GLP-1R knockout mice still experienced cardioprotection with GLP-1 (16). Recently, mice with cardiomyocyte-specific disruption of GLP-1R were shown to still experience robust protection from liraglutide (25). Recent evidence suggests that the GLP-1R may not be present in ventricular cardiomyocytes at all (10). Either another as-yet-unidentified receptor is mediating the GLP-1 effect on the RISK pathway of the cardiomyocyte or indirect activation is occurring, with GLP-1 binding a remote GLP-1R on endothelial or smooth muscle cells that triggers paracrine release of cardioprotective agents that act on the adjacent cardiomyocyte.
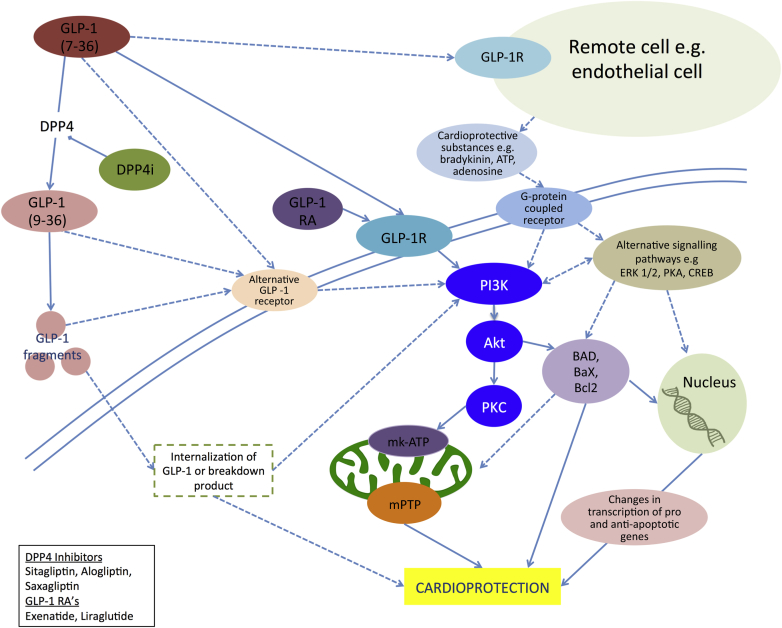
Activation of IC is associated with adenosine signaling pathways 52, 53. Recent work in anesthetized dogs showed that allogliptin-induced cardioprotection was abolished with blockade of adenosine A1 receptor despite continued activation of RISK pathways, suggesting an interaction between adenosine and GLP-1 signaling and cardioprotective “redundancy” (51). A simplified subcellular signaling pathway through which GLP-1 may act to effect cardioprotection is proposed in Figure 2.
Figure 2.
Proposed Schema for GLP-1 Activation of Ischemic Conditioning Pathways
GLP-1 binds to the GLP-1 receptor and activates the PI3K-Akt complex, which is part of the RISK pathway. This complex activates a cascade that opens the mK-ATP channel. Opening of mK-ATP prevents opening of the mPTP during reperfusion, part of the final common pathway of cell death. Activation of PI3-Akt has also been associated with changes in transcription and translation promoting cell survival. Alternative receptors, or activation of pro-survival pathways without binding the GLP-1 receptor, may explain myocardial cardioprotection, despite the purported absence of the GLP-1 receptor in recent studies. GLP-1R = GLP-1 receptor; GLP-1RA = GLP-1 receptor agonist; mPTP = mitochondrial permeability transition pore.
Some actions of GLP-1 have been shown to be dependent upon the mK-ATP channel. This includes concentration-dependent relaxation of rat aorta, which was inhibited by the mK-ATP blocking agent glibenclamide (Diabeta, Sanofi, Guildford, Surrey, United Kingdom) (54). Glibenclamide also inhibited flow-mediated dilatation of human arteries in forearm blood flow studies with GLP-1 (55).
Vasodilatory and hemodynamic effects
Reduction in systemic vascular resistance decreases afterload, and venodilation reduces preload, both reduce cardiac work and increase myocardial resistance to ischemia, as does reduction in heart rate. Coronary vasodilation also reduces ischemia by augmenting blood supply and aiding collateral recruitment to the myocardium.
GLP-1 produces vasodilation and microvascular recruitment in animal models 56, 57. Murine studies show that GLP-1 vasodilates constricted coronary arteries by increasing nitric oxide (NO)-mediated cGMP release in smooth muscle cells. This is associated with reduced IR injury. Vasodilation was present in GLP-1R knockout mice, suggesting it was a receptor-independent effect. Vasodilation occurred with the cleavage product GLP-1-(9-36) amide but not with the GLP-1RA Exendin-4 (16). GLP-1-mediated concentration-dependent relaxation of ex vivo rat aorta was eliminated when K-ATP channels were blocked, linking the hemodynamic effects of GLP-1 to conditioning pathways as discussed above (54). Research involving healthy human volunteers demonstrated that GLP-1 infusion caused recruitment of microvasculature in cardiac muscle assessed with myocardial contrast echocardiography (58).
Studies examining the impact of GLP-1 on heart rate and blood pressure have been limited to GLP-1RA. Liraglutide reduced diastolic and systolic blood pressure in mice by binding the GLP-1R, an effect that was lost in GLP-1R knockout mice and was mediated by atrial natriuretic peptide (ANP) (11). In humans with diabetes mellitus, administration of liraglutide did not result in an increase in ANP, although it was associated with a small and possibly detrimental rise in heart rate (59). A recent meta-analysis including unpublished data from pharmaceutical companies suggested that GLP-1RA are associated with a rise in heart rate (60). Liraglutide also produced an elevated heart rate in an open label trial of patients comparing it to sitagliptin in type 2 diabetics. Sitagliptin was not associated with a heart rate rise suggesting that native GLP-1 may not have the same effect or perhaps implicating the cleavage product of GLP-1 in the hemodynamic effects of GLP-1 (61).
Challenge of understanding the mechanism of GLP-1 cardioprotection
A clear understanding of the mechanism by which GLP-1 elicits cardioprotection has proved elusive. Differences between animal models make studies difficult to compare. It is not clear whether murine models are reflective of larger mammals. The relationship between animal and human physiology, particularly with regard to the location and presence of the GLP-1 receptor, mean that firm conclusions are difficult to draw. Even within published studies there is variability in cardioprotective potency largely attributable to differences in study design.
Physiological studies in humans, although often harder to perform and limited by ethical and practical considerations, may provide clarity over the mechanism by which GLP-1 exerts its cardioprotective effect. Particular importance should be placed on limiting the confounders implicit in human studies (such as medications, diabetic status, comorbidities, and counter-regulatory hormones) to ensure that useful information can be gleaned. Where these factors cannot be controlled, allowance should be made in discussion of results. Areas of useful endeavor may include assessment of the effects of GLP-1 in the human coronary microcirculation and the interaction of RISK/SAFE pathways with GLP-1. GLP-1 may act through multiple pathways of protection, including some not yet described, increasing its potential usefulness but also the complexity of understanding its mechanism.
Clinical Evidence of GLP-1-Mediated Cardioprotection
GLP-1-mediated cardioprotection is conserved in a number of animal species including humans. The evolutionary reason for this cardioprotection is speculative. It may simply be conserved by association with IC-mediated cardioprotection and this may confer a survival advantage by limiting birth-induced fetal ischemia. Similarly, GLP-1-mediated protection may have evolved to protect our prehistoric ancestors when eating, the immobility that ensues may have increased their vulnerability to predators. Whatever the reason, it now provides a target, which may be exploited for clinical benefit, particularly in the setting of cardiac ischemia.
A number of human studies have shown evidence of GLP-1-mediated cardioprotection. These are summarized in Table 1. Protection may limit nonlethal forms of IR injury such as myocardial stunning or post-infarct remodeling. Endpoints in clinical studies have focused more heavily on these forms of IR injury. Future clinical trials during elective or emergent percutaneous coronary revascularization offer an ethically acceptable method of assessing GLP-1 protection from lethal IR-injury.
Table 1.
Human Studies of GLP-1 or GLP-1RA-Mediated Cardioprotection
| First Author (Ref. #) | Year | Cardioprotective Agent | N | Protocol | Result |
|---|---|---|---|---|---|
| Nikolaidis et al. (82) | 2004 | GLP-1 | 21 | 72-hGLP-1 infusion initiated within 4 h of PPCI in patients with severely impaired LV function vs. saline control | Improved global and regional left ventricular function on echocardiography |
| Sokos et al. (68) | 2006 | GLP-1 | 21 | Subcutaneous infusion of GLP-1 given for 5 weeks in patients with NYHA functional class III/IV heart failure vs. saline control | Improved quality of life score, 6-min walk tests and left ventricular ejection fraction |
| Read et al. (64) | 2010 | Sitagliptin | 14 | Patients with coronary artery disease given single dose of sitagliptin / placebo followed by dobutamine stress echocardiogram. Patients acted as their own control. | Sitagliptin improves ejection fraction and regional tissue Doppler indices at peak stress and 30-min recovery |
| Read et al. (66) | 2011 | GLP-1 | 20 | GLP-1/saline infusion initiated after coronary balloon occlusion in patients undergoing PCI with further balloon occlusion at 30 min | GLP-1 infusion protects against stunning and cumulative ischemic dysfunction on pressure-volume loop measurement |
| Read et al. (62) | 2012 | GLP-1 | 14 | Patients with coronary artery disease given infusion of GLP-1 / placebo followed by dobutamine stress echocardiogram. Patients acted as their own control. | GLP-1 infusion improves ejection fraction and regional tissue Doppler indices at peak stress and 30-min recovery |
| Lonborg et al. (70) | 2012 | Exenatide | 172 | GLP-1 infusion initiated 15 min before intervention in PPCI patients and continued for 6 h | Reduced final infarct size on CMR. No change in biomarker rise or overall left ventricular ejection fraction. No difference in clinical outcome. |
| Woo et al. (72) | 2013 | Exenatide | 58 | Twice daily subcutaneous exenatide injection for 72-h compared to placebo in patients receiving PPCI | Reduced infarct size on CMR and reduced biomarker rise (CK-MB and troponin-I) |
| McCormick et al. (65) | 2014 | Sitagliptin | 20 | Diabetic patients with coronary disease underwent dobutamine stress echocardiogram after 4 weeks of sitagliptin | Sitagliptin improves ejection fraction and regional tissue Doppler indices at peak stress and 30-min recovery compared to baseline stress echocardiogram |
| McCormick et al. (38) | 2015 | GLP-1 | 20 | GLP-1/saline infusion initiated prior to coronary balloon occlusion in patients undergoing PCI, monitored with pressure-volume loops | GLP-1 infusion protects against ischemic dysfunction and myocardial stunning when given prior to coronary balloon occlusion |
| McCormick et al. (63) | 2015 | GLP-1 | 10 | GLP-1 infusion given during dobutamine stress echocardiogram, concurrently with hyperglycemic, hyperinsulinemic clamp in type 2 diabetic patients, who acted as their own control | GLP-1 infusion improves ejection fraction and regional tissue Doppler indices at peak stress and 30-min recovery |
| Roos et al. (73) | 2015 | Exenatide | 91 | GLP-1 infusion initiated immediately before PPCI and continued for 72 h | No change in final infarct size, ejection fraction or biomarker rise |
CK-MB = creatine kinase-MB; CMR = cardiac magnetic resonance; GLP-1 = glucagon-like peptide 1-(7-36) amide; GLP-1RA = GLP-1 receptor agonist; LV = left ventricular; PCI = percutaneous coronary intervention; PPCI = primary percutaneous coronary intervention.
GLP-1 has been shown to protect against ischemic left ventricular dysfunction following periods of demand ischemia induced with dobutamine stress echocardiography (62). This effect has recently been shown to be maintained even during a hyperglycemic, hyperinsulinemic clamp, negating the role of insulin in the cardioprotection observed (63). Similar benefit has been derived through the administration of the DPP-4 inhibitor sitagliptin in both diabetic and nondiabetic patients 64, 65.
GLP-1 protects against cumulative myocardial stunning following coronary balloon occlusion during elective PCI, when assessed through the use of a left ventricular conductance catheter 38, 66. The protection is present whether GLP-1 is administered prior to or after supply ischemia. This contrasts with IC, which does not protect the ventricle against stunning in the same manner (67). Protection from myocardial stunning may have particular benefit in those patients with cardiogenic shock where ischemic contractile dysfunction adds to the cascade of hemodynamic failure associated with the condition. A 72-h infusion of GLP-1 initiated following AMI produced improvements in global and regional wall motion on echocardiography (68). Myocardial stunning is reversible, and the mechanisms underlying this protection are almost certainly different to those underlying infarction (69).
Patients treated with exenatide during ST-segment elevation myocardial infarction (STEMI) had reduced infarct size by 15% on cardiac magnetic resonance imaging, although it failed to alter peak troponin levels or left ventricular ejection fraction assessed at 90 days (70). Of note, all the benefit seen in that study occurred in patients with short ischemic times (71). Further study of 58 primary PCI patients treated with exenatide prior to reperfusion showed a reduction in infarct size (on cardiac magnetic resonance imaging and biomarker measurements) as well as improvement in left ventricular systolic function at 6 months’ follow-up (72). In contrast, early results of the EXAMI (Effect of additional treatment with Exenatide in patients with an Acute Myocardial Infarction) trial have shown no benefit (on cardiac magnetic resonance imaging infarct size or biomarkers) from a 72-h exenatide infusion initiated prior to primary PCI for STEMI (73).
GLP-1 has been administered in patients undergoing coronary artery bypass graft. Although it did not result in significant differences in left ventricular dysfunction, as assessed on echocardiography, there was an overall reduction in the need for inotropes and vasoactive infusions compared to controls (74). That study also highlighted a reduction in ventricular arrhythmia, a surrogate for ischemia. A further double-blind trial of GLP-1 infusion during on-pump cardiac surgery resulted in improved glycemic control. No assessment of whether this intervention also had a cardioprotective effect was reported (75).
Large-scale studies of DPP-4 inhibitors (SAVOR–TIMI 53 [Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus-Thrombolysis In Myocardial Infarction 53], TECOS [Trial Evaluating Cardiovascular Outcomes with Sitagliptin], and EXAMINE [Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care]) have demonstrated their cardiovascular safety in diabetic patients. The primary endpoints of those studies were cardiac events following long-term therapy. Despite large numbers, those studies were not designed to assess possible cardioprotection during an acute ischemic event 76, 77, 78. Cardiovascular outcome trials for GLP-RAs have not yet been published, although the EXSCEL (Exenatide Study of Cardiovascular Event-Lowering Trial; NCT01144338) trial is due to finish in 2018. The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; NCT01179048) study recently confirmed a statistically significant reduction in major adverse cardiovascular events when administering liraglutide to type 2 diabetic patients with a high cardiovascular risk (79) indicating a cardioprotective effect, whereas the FIGHT (Functional Impact of GLP-1 for Heart Failure Treatment; NCT01800968) trial presented at the AHA annual meeting in 2015 showed that liraglutide did not negatively affect outcomes when administered to patients with advanced heart failure (80). Whether these trials are informative regarding the role of GLP-1 in cardioprotection will depend upon further analysis of published data. This analysis may determine the subgroup of patients who will derive clinical benefit from GLP-RAs.
Presently there are a number of clinical trials looking to exploit the potential cardioprotective benefits of GLP-1 and GLP-1RA. The GOLD-PCI (GLP-1 Loading During Percutaneous Coronary Intervention; NCT02127996) trial is a study investigating the effect of GLP-1 infusion during elective PCI, whereas COMBAT-MI (Combination Therapy in Myocardial Infarction; NCT02404376) is examining the combination of IC and exenatide in patients with STEMI. Trials such as these will provide further evidence as to whether GLP-1 can move from a promising molecule on the bench to a useful treatment in the catheter laboratory.
Translation to Clinical Practice: Prospects and Challenges
The challenges of translating animal studies or small-scale human physiological studies to large-scale clinical trials of cardioprotection and from there to mainstream clinical practice are significant. The homogenous nature of animal studies, or even highly selected individuals in physiological studies, are often not replicated in larger real world populations where comorbidities and heterogeneity abrogate the therapeutic effect.
At the heart of many problems of translation is a failure to link understanding of mechanism to design of the clinical trial (81). In the case of GLP-1, insights into its place within pathways of cardioprotection will improve understanding of how it might deliver most benefit. For example, if the primary basis of GLP-1 cardioprotection is IC then timing of therapy will be crucial to the success of the intervention —too late and the window for cardioprotection is closed. Alternatively, a change in cardiovascular hemodynamics may point trials towards endpoints around cardiogenic shock.
Appropriate application of cardioprotection to a well-defined, large population at risk of significant IR injury with assessment of appropriate, unambiguous endpoints (infarct size, arrhythmia burden, left ventricular function, heart failure) will help provide clarity as to the therapeutic potential of GLP-1 and its derivatives.
Conclusions
GLP-1 is a promising option for the treatment of IR injury. By accessing multiple cellular pathways it may succeed where other agents have failed. Clearly well-designed human studies of both the mechanisms and benefits of this cardioprotection are needed to evaluate GLP-1 as a cardioprotective agent and the population in which it will be most effective.
Footnotes
Ms. Clarke is an employee of Merck Sharp & Dohme. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Frohlich G.M., Meier P., White S.K., Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Depre C., Vanoverschelde J.L., Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen K., Christensen L.L., Holst J.J., Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann C., Goke R., Richter G., Fehmann H.C., Arnold R., Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy D.J., Yellon D.M. GLP-1 therapy: beyond glucose control. Circ Heart Fail. United States. 2008:147–149. doi: 10.1161/CIRCHEARTFAILURE.108.810887. [DOI] [PubMed] [Google Scholar]
- 7.Skoglund G., Hussain M.A., Holz G.G. Glucagon-like peptide 1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes. 2000;49:1156–1164. doi: 10.2337/diabetes.49.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nauck M.A., Niedereichholz U., Ettler R. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 9.Holst J.J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 10.Pyke C., Heller R.S., Kirk R.K. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 11.Kim M., Platt M.J., Shibasaki T. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. United States. 2013:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 12.Bose A.K., Mocanu M.M., Carr R.D., Yellon D.M. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther. 2007;21:253–256. doi: 10.1007/s10557-007-6030-6. [DOI] [PubMed] [Google Scholar]
- 13.Ban K., Kim K.H., Cho C.K. Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–1531. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 14.Deacon C.F., Nauck M.A., Toft-Nielsen M., Pridal L., Willms B., Holst J.J. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 15.Ravassa S., Zudaire A., Carr R.D., Diez J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am J Physiol Heart Circ Physiol. 2011;300:H1361–H1372. doi: 10.1152/ajpheart.00885.2010. [DOI] [PubMed] [Google Scholar]
- 16.Ban K., Noyan-Ashraf M.H., Hoefer J., Bolz S.S., Drucker D.J., Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 17.Bose A.K., Mocanu M.M., Carr R.D., Brand C.L., Yellon D.M. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 18.Bose A.K., Mocanu M.M., Carr R.D., Yellon D.M. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11. doi: 10.1007/s10557-005-6892-4. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaidis L.A., Elahi D., Hentosz T. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 20.Nikolaidis L.A., Doverspike A., Hentosz T. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–308. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- 21.Bao W., Aravindhan K., Alsaid H. Albiglutide, a long lasting glucagon-like peptide-1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS One. 2011:e23570. doi: 10.1371/journal.pone.0023570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohlfart P., Linz W., Hubschle T. Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies. J Transl Med. 2013;11:84. doi: 10.1186/1479-5876-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noyan-Ashraf M.H., Momen M.A., Ban K. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmers L., Henriques J.P., de Kleijn D.P. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Ussher J.R., Baggio L.L., Campbell J.E. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab. 2014:507–517. doi: 10.1016/j.molmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopaschuk G.D., Saddik M. The relative contribution of glucose and fatty acids to ATP production in hearts reperfused following ischemia. Mol Cell Biochem. 1992;116:111–116. doi: 10.1007/BF01270577. [DOI] [PubMed] [Google Scholar]
- 27.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 28.Sun D., Nguyen N., DeGrado T.R., Schwaiger M., Brosius F.C., 3rd Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- 29.Kloner R.A., Nesto R.W. Glucose-insulin-potassium for acute myocardial infarction: continuing controversy over cardioprotection. Circulation. 2008;117:2523–2533. doi: 10.1161/CIRCULATIONAHA.107.697979. [DOI] [PubMed] [Google Scholar]
- 30.Selker H.P., Beshansky J.R., Sheehan P.R. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. United States. 2012:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myat A., Redwood S.R., Gersh B.J., Yellon D.M., Marber M.S. Diabetes, incretin hormones and cardioprotection. Heart. 2014;100:1550–1561. doi: 10.1136/heartjnl-2012-303242. [DOI] [PubMed] [Google Scholar]
- 32.Luptak I., Yan J., Cui L., Jain M., Liao R., Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 33.Nikolaidis L.A., Elahi D., Shen Y.T., Shannon R.P. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 34.Kavianipour M., Ehlers M.R., Malmberg K. Glucagon-like peptide-1 (7-36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides. 2003:569–578. doi: 10.1016/s0196-9781(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 35.Hausenloy D.J., Whittington H.J., Wynne A.M. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013;12:154. doi: 10.1186/1475-2840-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aravindhan K., Bao W., Harpel M.R., Willette R.N., Lepore J.J., Jucker B.M. Cardioprotection resulting from glucagon-like peptide-1 administration involves shifting metabolic substrate utilization to increase energy efficiency in the rat heart. PLoS One. 2015;10:e0130894. doi: 10.1371/journal.pone.0130894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halbirk M., Norrelund H., Moller N. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010:H1096–H1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 38.McCormick L.M., Hoole S.P., White P.A. Pre-treatment with glucagon-like Peptide-1 protects against ischemic left ventricular dysfunction and stunning without a detected difference in myocardial substrate utilization. J Am Coll Cardiol Intv. 2015;8:292–301. doi: 10.1016/j.jcin.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Gejl M., Lerche S., Mengel A. Influence of GLP-1 on myocardial glucose metabolism in healthy men during normo- or hypoglycemia. PLoS One. 2014;9:e83758. doi: 10.1371/journal.pone.0083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 41.Hoole S.P., Heck P.M., Sharples L. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 42.Davies W.R., Brown A.J., Watson W. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 43.Hausenloy D.J., Tsang A., Yellon D.M. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Kin H., Zatta A.J., Lofye M.T. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Garlid K.D., Paucek P., Yarov-Yarovoy V. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 46.Kristiansen S.B., Henning O., Kharbanda R.K. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol. 2005;288:H1252–H1256. doi: 10.1152/ajpheart.00207.2004. [DOI] [PubMed] [Google Scholar]
- 47.Hausenloy D.J., Ong S.B., Yellon D.M. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189–202. doi: 10.1007/s00395-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 48.Hausenloy D.J., Duchen M.R., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 49.Ravassa S., Zudaire A., Diez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res. 2012;94:316–323. doi: 10.1093/cvr/cvs123. [DOI] [PubMed] [Google Scholar]
- 50.Doyle M.E., Egan J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ihara M., Asanuma H., Yamazaki S. An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308:H1287–H1297. doi: 10.1152/ajpheart.00835.2014. [DOI] [PubMed] [Google Scholar]
- 52.Krieg T., Qin Q., McIntosh E.C., Cohen M.V., Downey J.M. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol Heart Circ Physiol. 2002;283:H2322–H2330. doi: 10.1152/ajpheart.00474.2002. [DOI] [PubMed] [Google Scholar]
- 53.Kitakaze M., Hori M., Takashima S., Sato H., Inoue M., Kamada T. Ischemic preconditioning increases adenosine release and 5'-nucleotidase activity during myocardial ischemia and reperfusion in dogs. Implications for myocardial salvage. Circulation. 1993;87:208–215. doi: 10.1161/01.cir.87.1.208. [DOI] [PubMed] [Google Scholar]
- 54.Green B.D., Hand K.V., Dougan J.E., McDonnell B.M., Cassidy R.S., Grieve D.J. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 2008;478:136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Basu A., Charkoudian N., Schrage W., Rizza R.A., Basu R., Joyner M.J. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 56.Dong Z., Chai W., Wang W. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E222–E228. doi: 10.1152/ajpendo.00473.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golpon H.A., Puechner A., Welte T., Wichert P.V., Feddersen C.O. Vasorelaxant effect of glucagon-like peptide-(7-36)amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 58.Subaran S.C., Sauder M.A., Chai W. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci (Lond) 2014;127:163–170. doi: 10.1042/CS20130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovshin J.A., Barnie A., DeAlmeida A., Logan A., Zinman B., Drucker D.J. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38:132–139. doi: 10.2337/dc14-1958. [DOI] [PubMed] [Google Scholar]
- 60.Robinson L.E., Holt T.A., Rees K., Randeva H.S., O'Hare J.P. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pratley R.E., Nauck M., Bailey T. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 62.Read P.A., Khan F.Z., Dutka D.P. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2012;98:408–413. doi: 10.1136/hrt.2010.219345. [DOI] [PubMed] [Google Scholar]
- 63.McCormick L.M., Heck P.M., Ring L.S. Glucagon-like peptide-1 protects against ischemic left ventricular dysfunction during hyperglycemia in patients with coronary artery disease and type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:102. doi: 10.1186/s12933-015-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Read P.A., Khan F.Z., Heck P.M., Hoole S.P., Dutka D.P. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 65.McCormick L.M., Kydd A.C., Read P.A. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circ Cardiovasc Imaging. 2014:274–281. doi: 10.1161/CIRCIMAGING.113.000785. [DOI] [PubMed] [Google Scholar]
- 66.Read P.A., Hoole S.P., White P.A. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4:266–272. doi: 10.1161/CIRCINTERVENTIONS.110.960476. [DOI] [PubMed] [Google Scholar]
- 67.Hoole S.P., Khan S.N., White P.A. Remote ischaemic pre-conditioning does not attenuate ischaemic left ventricular dysfunction in humans. Eur J Heart Fail. 2009;11:497–505. doi: 10.1093/eurjhf/hfp040. [DOI] [PubMed] [Google Scholar]
- 68.Sokos G.G., Nikolaidis L.A., Mankad S., Elahi D., Shannon R.P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 69.Kloner R.A., Jennings R.B. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 70.Lonborg J., Vejlstrup N., Kelbaek H. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 71.Lonborg J., Kelbaek H., Vejlstrup N. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012;5:288–295. doi: 10.1161/CIRCINTERVENTIONS.112.968388. [DOI] [PubMed] [Google Scholar]
- 72.Woo J.S., Kim W., Ha S.J. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–2260. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 73.Roos S, Timmers L, van Hout G et al. Exenatide Does Not Improve Myocardial Salvage in Patients With An Acute Myocardial Infarction Successfully Treated With Primary Percutaneous Coronary Intervention: The First Results of the EXAMI Trial. Presented at: TCT 2015. October 11-15, 2015; San Francisco, CA.
- 74.Sokos G.G., Bolukoglu H., German J. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 75.Kohl B.A., Hammond M.S., Cucchiara A.J., Ochroch E.A. Intravenous GLP-1 (7-36) amide for prevention of hyperglycemia during cardiac surgery: a randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. 2014;28:618–625. doi: 10.1053/j.jvca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Scirica B.M., Bhatt D.L., Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 77.White W.B., Cannon C.P., Heller S.R. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 78.Green J.B., Bethel M.A., Armstrong P.W. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 79.Novo Nordisk. Victoza® significantly reduces the risk of major adverse cardiovascular events in the LEADER trial. Available at: http://www.novonordisk.com/bin/getPDF.1991879.pdf. Accessed March 2016.
- 80.Margulies K, Anstrom K, Redfield M et al. A randomized trial of liraglutide for high-risk heart failure patients with reduced ejection fraction. Presented at the Proceedings of the American Heart Association Scientific Sessions. November 7-11, 2015; Orlando, FL.
- 81.Giblett J.P., West N.E., Hoole S.P. Cardioprotection for percutaneous coronary intervention—reperfusion quality as well as quantity. Int J Cardiol. 2014;177:786–793. doi: 10.1016/j.ijcard.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 82.Nikolaidis L.A., Mankad S., Sokos G.G. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]