Abstract
Adult T‐cell leukemia/lymphoma (ATL) is caused by Human T‐cell lymphotropic/leukemia virus type 1 (HTLV‐1), and a higher HTLV‐1 provirus load in PBMC is a risk factor for ATL development. Here, we document a significant inverse correlation between the function of HTLV‐1 Tax‐specific CTL (Tax‐CTL), as assessed by ex vivo cytokine production in response to cognate peptide, and the HTLV‐1 provirus load in PBMC in both HTLV‐1 asymptomatic carriers (AC) (Spearman rank correlation coefficient [Rs] = −0.494, P = .037, n = 18) and ATL patients (Rs = −0.774, P = .001, n = 15). There was also a significant correlation between the HTLV‐1 provirus load and the percentage of PD‐1‐positive Tax‐CTL in both HTLV‐1 AC (Rs = 0.574, P = .013) and ATL patients (Rs = 0.676, P = .006). Furthermore, the percentage of PD‐1‐positive Tax‐CTL was inversely correlated with their function in HTLV‐1 AC (Rs = −0.542, P = .020), and ATL patients (Rs = −0.639, P = .010). These findings indicate that the function of Tax‐CTL decreased as their programmed cell death protein 1 (PD‐1) levels increased, parallel to the increased HTLV‐1 provirus load in PBMC. We propose that functional Tax‐CTL are crucial for determining the HTLV‐1 provirus load in PBMC, not only in HTLV‐1 AC, but also in ATL, and that PD‐1 expression levels are reliable markers of Tax‐CTL function. Thus, modulating the immunological equilibrium between Tax‐CTL and HTLV‐1‐infected cells to achieve dominance of functional effectors could represent an ideal strategy for controlling HTLV‐1‐associated disease.
Keywords: adult T‐cell leukemia/lymphoma, CTL, HTLV‐1, programmed cell death protein 1, Tax
1. INTRODUCTION
Adult T‐cell leukemia/lymphoma (ATL) is caused by Human T‐cell lymphotropic/leukemia virus type 1 (HTLV‐1).1, 2, 3 The cumulative risk of HTLV‐1 carriers developing ATL is estimated at approximately 5%. Which HTLV‐1 asymptomatic carriers (AC) will go on to develop ATL has not been unequivocally established, although a higher HTLV‐1 provirus load in PBMC has been reported as a risk factor.4 It is likely that prior to disease development, HTLV‐1‐infected lymphocytes will have been controlled by the host immune response for many years, and that eventually a small number escape immunosurveillance and develop into overt ATL. In this scenario, it is important to understand which antigens on the HTLV‐1‐infected cells are or could be targeted by the host immune response. HTLV‐1‐associated antigens such as Tax or HBZ,5, 6, 7 cancer testis antigens,8 or neoantigens arising as a consequence of tumor‐specific mutations9, 10 are all candidates. Of these candidates, immunogenicity of HBZ is not strong.2, 3 We previously reported that HTLV‐1 transmission from mothers to infants through breast milk in early life might induce tolerance to HBZ and result in insufficient HBZ‐specific T‐cell responses in HTLV‐1 asymptomatic carriers or ATL patients.7 Cancer testis antigen expression profiles in ATL are variable, thus reducing their utility as therapeutic targets as well.8 Neoantigens are, by definition, most likely limited to individual cases.9, 10 Therefore, here we focused on Tax, which is obligatory for transformation of infected cells by HTLV‐1,11 and which is relatively strongly immunogenic.2, 3, 5, 6 We explored the relationship between the function of HTLV‐1 Tax‐specific CTL (Tax‐CTL) and the HTLV‐1 provirus load in PBMC.
2. PATIENTS AND METHODS
2.1. Primary cells from HTLV‐1 AC or ATL patients
PBMC were isolated from 18 HTLV‐1 AC and 15 ATL patients using Ficoll‐Paque centrifugation (Pharmacia, Uppsala, Sweden). Of the 15 ATL patients, 1 with a chronic and 1 with a smoldering subtype were carefully observed using a watch‐and‐wait approach. Among the remaining 13 patients, 9 had been in remission for aggressive ATL after systemic chemotherapy and/or treatment with mogamulizumab12, 13, 14 for more than 6 months before blood draw for the present study. The remaining 4 were in remission after allogeneic hematopoietic stem cell transplantation (HSCT) from an unrelated HTLV‐1‐negative donor more than 2 years earlier. The transplanted patients had been free of any immunosuppressive treatment for more than 6 months prior to the study. All donors provided written informed consent before sampling, according to the Declaration of Helsinki, and the present study was approved by the institutional ethics committee of Nagoya City University Graduate School of Medical Sciences.
2.2. Human leukocyte antigen typing
Human leukocyte antigen (HLA)‐A genotyping was carried out using WAKFlow® HLA‐typing kits (WAKUNAGA Pharmacy Co. Ltd, Hiroshima, Japan). In the present study, all enrolled individuals had at least 1 HLA‐A*02:01, ‐A*02:06, or ‐A*24:02 allele.
2.3. Antibodies, tetramers, and flow cytometry
Phycoerythrin (PE)‐conjugated HLA‐A*02:01/Tax11‐19 and HLA‐A*24:02/Tax301‐309 tetramers, peridinin chlorophyll protein‐conjugated anti‐CD8 monoclonal antibody (mAb) (SK1) (Medical & Biological Laboratories, Co., Ltd, Nagoya, Japan), allophycocyanin (APC) conjugated anti‐PD‐1 mAb (EH12.2H7; BioLegend, Inc., San Diego, CA, USA), FITC‐conjugated anti‐T‐cell immunoglobulin and mucin domain‐containing protein‐3 (anti‐TIM‐3) mAb (344823), FITC‐conjugated anti‐lymphocyte‐activation gene 3 (anti‐LAG‐3) Ab (FAB2319A) (both from R&D Systems Inc., Minneapolis, MN, USA), and APC‐conjugated anti‐cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) mAb (BNI3) (BD Biosciences, San Jose, CA, USA) were used here. For intracellular staining, cells were cocultured with or without cognate peptide (final concentration 100 nmol/L) at 37°C in 5% CO2 for 3 hours, after which brefeldin A (BD Biosciences) was added. The cells were then incubated for an additional 2 hours. Subsequently, they were stained with FITC‐conjugated anti‐interferon (IFN)‐γ (45.15; Beckman Coulter, Fullerton, CA, USA) and APC‐conjugated anti‐tumor necrosis factor (TNF)‐α (MAb11; eBioscience, San Diego, CA, USA) mAbs, using the Intracellular Fixation & Permeabilization Buffer Set (88‐8824‐00; eBioscience). An appropriate isotype control Ab for each Ab was used for drawing the quadrant lines in each case. Cells were analyzed on a FACSCalibur (BD Biosciences) with the aid of FlowJo software (Tree Star, Inc. Ashland, OR, USA). The HTLV‐1 provirus load in PBMC was quantified by SRL, Inc. (Tokyo, Japan).
2.4. Absolute number of Tax‐CTL
Absolute numbers of PD‐1‐positive and ‐negative Tax‐CTL were calculated from the total lymphocyte count (/μL) and the percentages of PD‐1‐positive and ‐negative Tax‐CTL in this population.
2.5. Statistical analysis
Correlations between 2 variables were assessed using the Spearman rank correlation coefficient (Rs). The analyses were carried out with SPSS Statistics 17.0 (IBM Corporation, Armonk, NY, USA). In this study, P < .05 (two‐sided) was considered significant.
3. RESULTS
There was a significant positive correlation between the HTLV‐1 provirus load in PBMC and the percentage of PD‐1‐positive Tax‐CTL in HTLV‐1 AC (Rs = 0.574, P = .013; Figure 1A). In contrast, there were no significant correlations between provirus load in PBMC and TIM‐3‐, LAG‐3‐ or CTLA‐4‐positive Tax‐CTL (Rs = −0.043, P = .864; Rs = 0.368, P = .133; Rs = −0.172, P = .494, respectively). Thus, PD‐1 expression levels on Tax‐CTL increased as the HTLV‐1 provirus load in PBMC increased in HTLV‐1 AC. Consistent with this, there was a significant inverse correlation between the percentage of PD‐1‐positive Tax‐CTL and the percentage of Tax‐CTL producing both IFN‐γ and TNF‐α ex vivo in response to cognate peptide (Rs = −0.542, P = .020) (Figure 1B). Thus, cytokine production by Tax‐CTL decreased as their PD‐1 expression increased. In contrast, there were no significant correlations between the percentages of TIM‐3‐, LAG‐3‐ or CTLA‐4‐positive Tax‐CTL and the frequency of cells producing both IFN‐γ and TNF‐α ex vivo in response to cognate peptide (Rs = −0.038, P = .880; Rs = 0.044, P = .861; Rs = 0.003, P = .991, respectively. Finally, we did find a significant inverse correlation between the HTLV‐1 provirus load in PBMC and the percentages of IFN‐γ‐ and TNF‐α‐producing Tax‐CTL in HTLV‐1 AC (Rs = −0.494, P = .037) (Figure 1C). Thus, cytokine production by Tax‐CTL decreases as the HTLV‐1 provirus load in PBMC increases. Quantification of PD‐1 and TIM‐3, LAG‐3 and CTLA‐4 expression on Tax‐CTL, and cytokine production in response to cognate peptide, is shown in Figure 1D (for HTLV‐1 AC numbers 1‐4).
Figure 1.
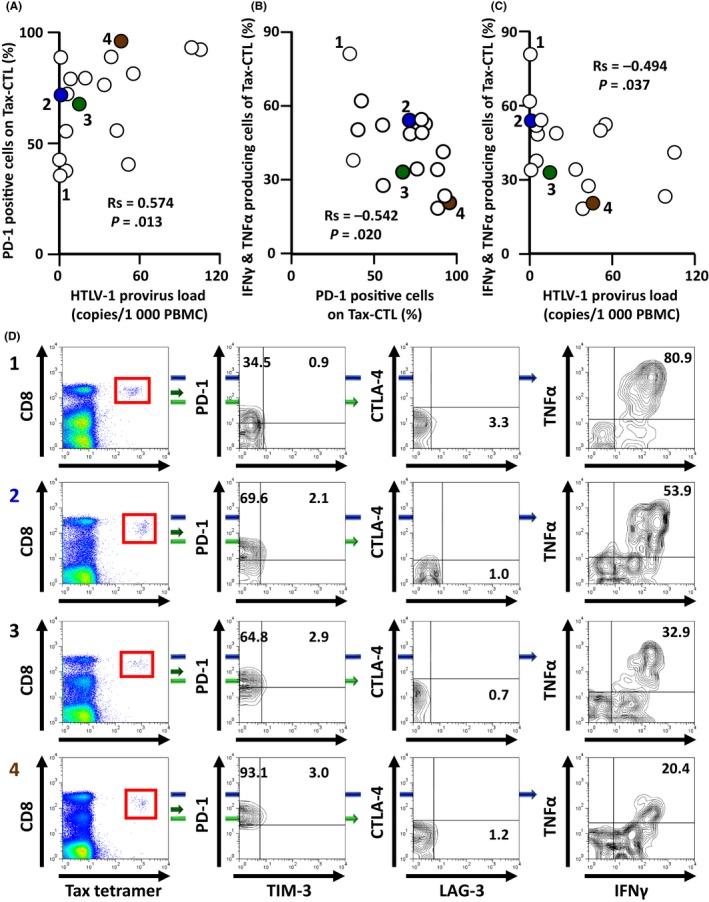
Relationships between programmed cell death protein 1 (PD‐1) positivity of Human T‐cell lymphotropic/leukemia virus type 1 (HTLV‐1) Tax‐specific cytotoxic T cells (Tax‐CTL) and the production of interferon (IFN)‐γ and tumor necrosis factor (TNF)‐α ex vivo in response to cognate peptide in HTLV‐1 asymptomatic carriers (AC). A, Correlation between HTLV‐1 provirus load in PBMC and the percentage of PD‐1‐positive Tax‐CTL in HTLV‐1 AC. There was a significant positive correlation between these two factors (Rs = 0.574, P = .013). B, Correlation between the percentage of PD‐1‐positive Tax‐CTL and the percentage of both IFN‐γ‐ and TNF‐α‐producing Tax‐CTL ex vivo in response to cognate peptide. There was a significant inverse correlation between these 2 factors (Rs = −0.542, P = .020). C, Correlation between the HTLV‐1 provirus load in PBMC and the percentage of both IFN‐γ‐ and TNF‐α‐producing Tax‐CTL ex vivo in response to cognate peptide. There was a significant inverse correlation between these 2 factors (Rs = −0.494, P = .037). D, Lymphocyte populations from HTLV‐1 AC donors 1, 2, 3, and 4 showing CD8 and HTLV‐1 Tax tetramer positivity. CD8 and HTLV‐1 Tax tetramer‐positive cells are gated as shown by the red squares (left panels), and plotted to show T‐cell immunoglobulin and mucin domain‐containing protein‐3 (TIM‐3) and PD‐1 positivity. Percentages of PD‐1‐positive but TIM‐3‐negative, and of PD‐1‐ and TIM‐3‐double‐positive cells are indicated in each panel (second left). CD8 and HTLV‐1 Tax tetramer‐positive cells are plotted to show lymphocyte‐activation gene 3 (LAG‐3) and cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) positivity. Percentage of LAG‐3‐positive but CTLA‐4‐negative cells is indicated (second right). PBMC obtained from HTLV‐1 AC 1, 2, 3, and 4 were cocultured with or without cognate peptide (final concentration 100 nmol/L) at 37°C in 5% CO 2 for 3 h, after which brefeldin A was added. The cells were then incubated for an additional 2 h. Subsequently, IFN‐γ and TNF‐α production from Tax‐CTL was evaluated. Percentage of Tax‐CTL producing both IFN‐γ and TNF‐α in response to cognate peptide is indicated in each panel (right‐hand side). Plots labeled 1, 2, 3, and 4 in (A,B,C), correspond to 1, 2, 3, and 4, in (D), respectively. Plots labeled 2, 3, and 4 in (A,B,C) are colored blue, green, and brown, respectively
Next, we undertook the same analysis with ATL patients. As observed in the HTLV‐1 AC, there was a significant positive correlation between the HTLV‐1 provirus load in PBMC and the percentage of PD‐1‐positive Tax‐CTL (Rs = 0.676, P = .006; Figure 2A), but no correlations with TIM‐3‐, LAG‐3‐ or CTLA‐4‐positivity (Rs = 0.275, P = .321; Rs = −0.154, P = .584; Rs = −0.095, P = .737, respectively. Subsequently, we observed a significant inverse correlation between the percentage of PD‐1‐positive Tax‐CTL, and the percentage of IFN‐γ‐ and TNF‐α‐producing Tax‐CTL ex vivo, after stimulation with cognate peptide (Rs = −0.639, P = .010) (Figure 2B). However, in this instance, we also observed a significant inverse correlation between the percentage of TIM‐3‐positive Tax‐CTL and the percentage of cells producing IFN‐γ and TNF‐α ex vivo after stimulation with cognate peptide (Rs = −0.532, P = .041). Nonetheless, again there were no significant correlations for LAG‐3 (Rs = 0.045, P = .874), or CTLA‐4 (Rs = −0.004, P = .988) expression. Finally, there was a significant inverse correlation between the HTLV‐1 provirus load and the percentage of IFN‐γ‐ and TNF‐α‐producing Tax‐CTL (Rs = −0.774, P = .001) (Figure 2C). Note that the four gray circles in Figure 2A‐C denote ATL patients who had received allogeneic HSCT. The levels of PD‐1 and TIM‐3 or LAG‐3 and CTLA‐4 expression by Tax‐CTL, and their cytokine production in response to cognate peptide, are shown in Figure 2D for ATL patients numbers 1‐4.
Figure 2.
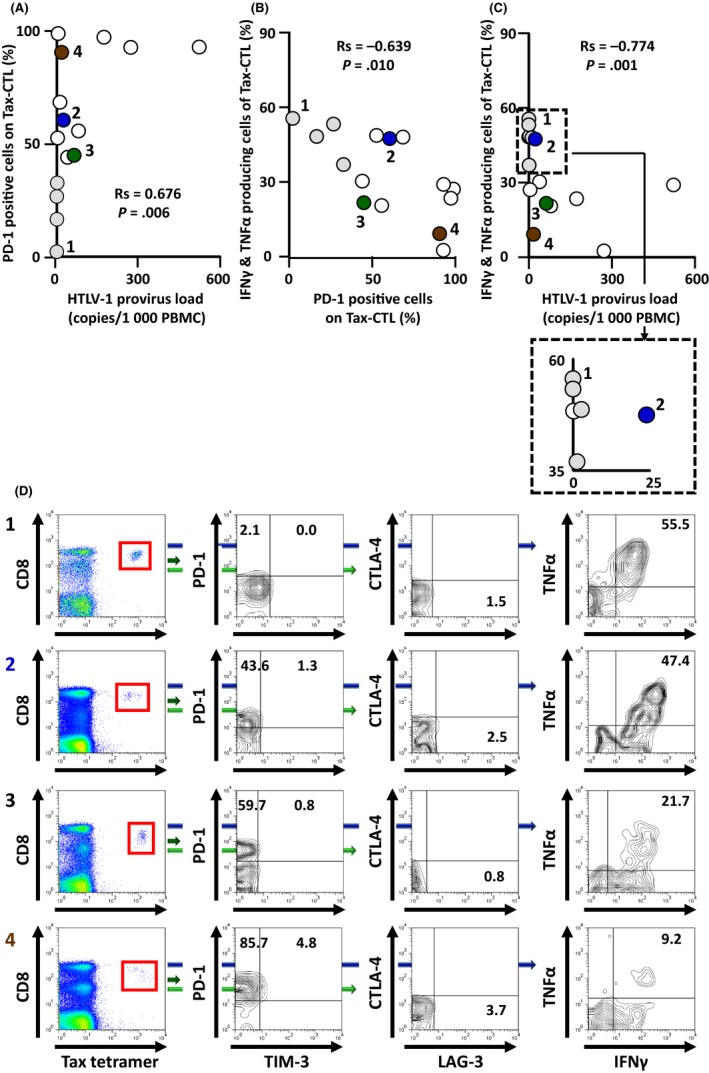
Relationships between programmed cell death protein 1 (PD‐1) positivity of Human T‐cell lymphotropic/leukemia virus type 1 (HTLV‐1) Tax‐specific cytotoxic T cells (Tax‐CTL) and their production of interferon (IFN)‐γ and tumor necrosis factor (TNF)‐α ex vivo in response to cognate peptide in adult T‐cell leukemia/lymphoma (ATL) patients. A, Correlation between HTLV‐1 provirus load in PBMC and the percentage of PD‐1‐positive Tax‐CTL in ATL patients. There was a significant positive correlation between these 2 factors (Rs = 0.676, P = .006). B, Correlation between the percentage of PD‐1‐positive Tax‐CTL and the percentage of Tax‐CTL producing both IFN‐γ and TNF‐α ex vivo in response to the cognate peptide. There was a significant inverse correlation between these 2 factors (Rs = −0.639, P = .010). C, Correlation between HTLV‐1 provirus load in PBMC and the percentage of Tax‐CTL producing both IFN‐γ and TNF‐α ex vivo in response to cognate peptide. There was a significant inverse correlation between these 2 factors (Rs = −0.774, P = .001). The 4 symbols in gray in the plots (A,B,C) indicate ATL patients after allogeneic hematopoietic stem cell transplantation (HSCT). D, Lymphocyte populations of PBMC, ex vivo, from ATL patients 1, 2, 3, and 4 are plotted to show CD8 and HTLV‐1 Tax tetramer positivity. CD8 and HTLV‐1 Tax tetramer‐positive cells are shown gated by the red squares (left panels), and are plotted to show T‐cell immunoglobulin and mucin domain‐containing protein‐3 (TIM‐3) and PD‐1 positivity. Percentages of PD‐1‐positive but TIM‐3‐negative, and of PD‐1‐, TIM‐3‐double‐positive cells are indicated in each panel (second left). CD8 and HTLV‐1 Tax tetramer‐positive cells are plotted to show lymphocyte‐activation gene 3 (LAG‐3) and cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) positivity. Percentage of LAG‐3‐positive but CTLA‐4‐negative cells is indicated second right. PBMC obtained from ATL patients 1, 2, 3, and 4 were cocultured with or without cognate peptide (final concentration 100 nmol/L) at 37°C in 5% CO 2 for 3 h, after which brefeldin A was added. The cells were then incubated for an additional 2 h. Subsequently, IFN‐γ and TNF‐α production from Tax‐CTL was evaluated. Percentage of both IFN‐γ‐ and TNF‐α‐producing Tax‐CTL in response to cognate peptide is indicated in each panel (right panels). Plots labeled 1, 2, 3, and 4 in (A,B,C) correspond to 1, 2, 3, and 4, in (D), respectively. Plots labeled 2, 3, and 4 in (A,B,C) are colored blue, green, and brown, respectively
Because the present observations indicated that the function of Tax‐CTL decreases as their PD‐1 levels increase, parallel to the increased HTLV‐1 provirus load in PBMC, we determined the absolute number of Tax‐CTL in the blood. In HTLV‐1 AC, there were no significant correlations between the HTLV‐1 provirus load in PBMC and the absolute number of PD‐1‐negative Tax‐CTL (Rs = −0.152 P = .548) (Figure 3A) or PD‐1‐positive Tax‐CTL (Rs = 0.126, P = .618) (Figure 3B). In contrast, in ATL patients, there was a significant inverse correlation between the HTLV‐1 provirus load in PBMC and the absolute number of PD‐1‐negative Tax‐CTL (Rs = −0.561, P = .029) (Figure 1C). However, no such correlation was seen for the absolute number of PD‐1‐positive Tax‐CTL (Rs = −0.061, P = .830) (Figure 1D).
Figure 3.
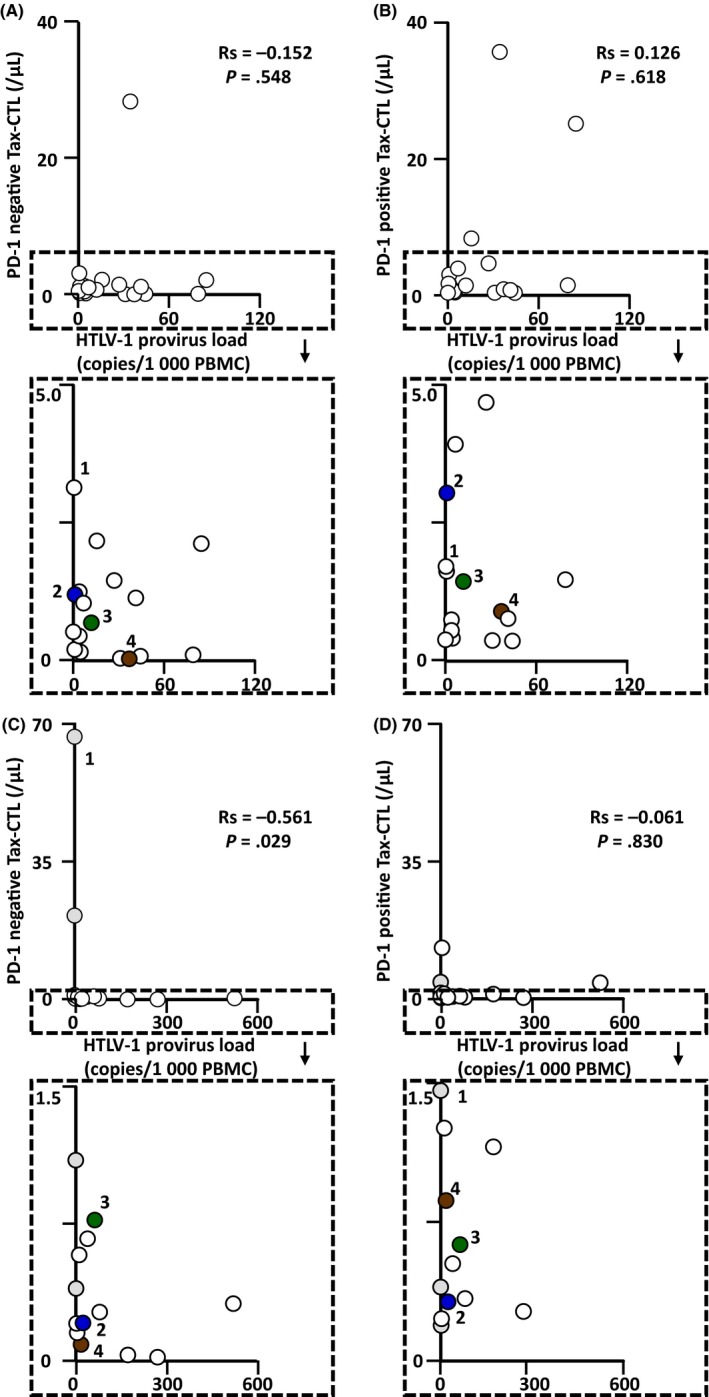
Relationships between the absolute number of Tax‐specific cytotoxic T cells (Tax‐CTL) and the Human T‐cell lymphotropic/leukemia virus type 1 (HTLV‐1) provirus load in PBMC from HTLV‐1 asymptomatic carriers (AC) and in adult T‐cell leukemia/lymphoma (ATL) patients. A, No significant correlation between HTLV‐1 provirus load in PBMC and the absolute number of PD‐1‐negative Tax‐CTL in HTLV‐1 AC (Rs = −0.152, P = .548). B, No significant correlation between HTLV‐1 provirus load in PBMC and the absolute number of PD‐1‐positive Tax‐CTL in HTLV‐1 AC (Rs = 0.126, P = .618). C, Significant inverse correlation between HTLV‐1 provirus load in PBMC and the absolute number of PD‐1‐negative Tax‐CTL in ATL patients (Rs = −0.561, P = .029). D, No significant correlation between HTLV‐1 provirus load in PBMC and the absolute number of PD‐1‐positive Tax‐CTL in ATL patients (Rs = −0.061, P = .830). Plots labeled 1, 2, 3, and 4 in (A,B) correspond to 1, 2, 3, and 4 in Figure 1, respectively. Plots labeled 1, 2, 3, and 4 in (C,D) correspond to 1, 2, 3, and 4 in Figure 2, respectively. The 4 symbols in gray in (C,D) indicate ATL patients after allogeneic hematopoietic stem cell transplantation
4. DISCUSSION
The findings from the present study are consistent with the phenomenon of “T‐cell exhaustion” in both HTLV‐1 AC and ATL patients,15, 16 similar to what has been reported in other chronic viral infections such as hepatitis B virus,17 hepatitis C virus,18 human immunodeficiency virus (HIV) infections,19 and also in cancer.20 The present study indicates that persistent continuous exposure of Tax‐CTL to HTLV‐1‐infected cells attenuates their function, which would allow the latter to proliferate more and potentially lead to the development of overt ATL. In this context, a treatment strategy targeting Tax, such as Tax peptide‐pulsed dendritic cell vaccination,21 would seem to be promising, especially for preventing ATL development in HTLV‐1 AC by means of suppressing the proliferation of HTLV‐1‐infected cells. In addition, the present study indicates that PD‐1 is a reliable marker of Tax‐CTL exhaustion. Thus, a treatment strategy targeting PD‐1 should also be effective for ATL patients. Accordingly, we are now conducting an investigator‐initiated phase II study of nivolumab for relapsed/refractory ATL (UMIN000020601). However, in this respect, it must be noted that rapid progression of ATL after nivolumab treatment has been reported by other investigators.22 The mechanisms responsible for ATL progression on nivolumab have not yet been determined, but, clearly, great caution is required in our own phase II study of nivolumab for ATL. Although TIM‐3, LAG‐3 and CTLA‐4 have also been reported to be markers of exhausted T‐cells,15, 16 the present study showed that these were not reliable markers of Tax‐CTL exhaustion. The reason for this is not clear, but it is conceivable that appropriate markers of exhaustion might vary according to the type of CTL.
Allogeneic HSCT is considered to be the only curative treatment for aggressive ATL.23, 24 In this context, it is notable that the functions of the Tax‐CTL originating from HTLV‐1‐negative donors in the present transplanted patients were generally well retained, and the fraction of PD‐1‐positive cells was low. This is likely to be related to the control of HTLV‐1‐infected cells in these patients after allogeneic HSCT. Although an earlier study analyzed PD‐1 expression in general on lymphocytes in HTLV‐1 AC or in ATL patients comprehensively,25 the present study quantified PD‐1 expression levels on Tax‐CTL. Relating these to the HTLV‐1 provirus load in PBMC and to the functional integrity of Tax‐CTL has not been previously reported. Herein lies the novelty and significance of this work.
In conclusion, the functional integrity of Tax‐CTL is crucial for determining HTLV‐1 provirus load in PBMC not only in HTLV‐1 AC, but also in ATL patients. Exploiting the immunological equilibrium between Tax‐CTL vs HTLV‐1‐infected cells to achieve long‐term dominance of functional Tax‐CTL would represent an ideal strategy for controlling HTLV‐1‐associated disease.
CONFLICTS OF INTEREST
Takashi Ishida obtained research funding from Kyowa Hakko Kirin Co., Ltd, Bayer Pharma AG, and Celgene K.K., and honoraria from Kyowa Hakko Kirin Co., Ltd, and Celgene K.K. Ryuzo Ueda has a consultancy with Mundipharma K.K., Ono Pharmaceutical Co., Ltd, and Terumo Co., Ltd, and receives research funding from Kyowa Hakko Kirin Co., Ltd, Rikaken Co., Ltd, Medical & Biological Laboratories Co., Ltd, and Chugai Pharmaceutical Co., Ltd. Shinsuke Iida received research funding and declares honoraria from Janssen Pharmaceutical K.K., and Celgene Co., Ltd, Novartis Pharma K.K., Bristol‐Myers Squibb, Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Co., Ltd. SI also received research funding from Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd, and Sanofi K.K. The remaining authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
We thank Chiori Fukuyama for her excellent technical assistance, and Naomi Ochiai for her expert secretarial assistance. This work was supported by grant‐in‐aid for scientific research (B) (no. 16H04713 to Takashi Ishida), and grants‐in‐aid from the Japan Agency for Medical Research and Development (nos. 17ck0106287h0001 to Takashi Ishida, 16cm0106301h0001 to Takashi Ishida, and 15ck0106132h0002 to Takashi Ishida, Ryuzu Ueda, and Shinsuke Iida).
Masaki A, Ishida T, Suzuki S, et al. Human T‐cell lymphotropic/leukemia virus type 1 (HTLV‐1) Tax‐specific T‐cell exhaustion in HTLV‐1‐infected individuals. Cancer Sci. 2018;109:2383–2390. 10.1111/cas.13654
Funding information
This work was supported by Grants‐in‐Aid for scientific Research (B) (No. 16H04713 to Takashi Ishida), grants‐in‐aid from the Japan Agency for Medical Research and Development (Nos. 17ck0106287h0001 to Takashi Ishida, 16cm0106301h0001 to Takashi Ishida, and 15ck0106132h0002 to Takashi Ishida, Ryuzu Ueda, and Shinsuke Iida).
REFERENCES
- 1. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984‐87). Br J Haematol. 1991;79:428‐437. [DOI] [PubMed] [Google Scholar]
- 2. Ishitsuka K, Tamura K. Human T‐cell leukaemia virus type I and adult T‐cell leukaemia‐lymphoma. Lancet Oncol. 2014;15:e517‐e526. [DOI] [PubMed] [Google Scholar]
- 3. Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270‐280. [DOI] [PubMed] [Google Scholar]
- 4. Iwanaga M, Watanabe T, Utsunomiya A, et al. Human T‐cell leukemia virus type I (HTLV‐1) proviral load and disease progression in asymptomatic HTLV‐1 carriers: a nationwide prospective study in Japan. Blood. 2010;116:1211‐1219. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki S, Masaki A, Ishida T, et al. Tax is a potential molecular target for immunotherapy of adult T‐cell leukemia/lymphoma. Cancer Sci. 2012;103:1764‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masaki A, Ishida T, Suzuki S, et al. Autologous Tax‐specific CTL therapy in a primary adult T cell leukemia/lymphoma cell‐bearing NOD/Shi‐scid, IL‐2Rγnull mouse model. J Immunol. 2013;191:135‐144. [DOI] [PubMed] [Google Scholar]
- 7. Narita T, Ishida T, Masaki A, et al. HTLV‐1 bZIP factor‐specific CD4 T cell responses in adult T cell leukemia/lymphoma patients after allogeneic hematopoietic stem cell transplantation. J Immunol. 2014;192:940‐947. [DOI] [PubMed] [Google Scholar]
- 8. Nishikawa H, Maeda Y, Ishida T, et al. Cancer/testis antigens are novel targets of immunotherapy for adult T‐cell leukemia/lymphoma. Blood. 2012;119:3097‐3104. [DOI] [PubMed] [Google Scholar]
- 9. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69‐74. [DOI] [PubMed] [Google Scholar]
- 10. Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304‐1315. [DOI] [PubMed] [Google Scholar]
- 11. Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T‐cell leukemia virus type 1. Blood. 1995;86:4243‐4249. [PubMed] [Google Scholar]
- 12. Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clin Cancer Res. 2010;16:1520‐1531. [DOI] [PubMed] [Google Scholar]
- 13. Ishida T, Joh T, Uike N, et al. Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837‐842. [DOI] [PubMed] [Google Scholar]
- 14. Ishida T, Jo T, Takemoto S, et al. Dose‐intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma: a randomized phase II study. Br J Haematol. 2015;169:672‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492‐499. [DOI] [PubMed] [Google Scholar]
- 16. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reignat S, Webster GJ, Brown D, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195:1089‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K. Liver‐infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD‐1 and low levels of CD127 expression. J Virol. 2007;81:2545‐2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV‐specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094‐3101. [PubMed] [Google Scholar]
- 20. Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor‐associated antigens in melanoma patients. Nat Med. 1999;5:677‐685. [DOI] [PubMed] [Google Scholar]
- 21. Suehiro Y, Hasegawa A, Iino T, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide‐pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol. 2015;169:356‐367. [DOI] [PubMed] [Google Scholar]
- 22. Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid progression of adult T‐cell leukemia‐lymphoma after PD‐1 inhibitor therapy. N Engl J Med. 2018;378:1947‐1948. [DOI] [PubMed] [Google Scholar]
- 23. Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T‐cell leukemia‐lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood. 2012;120:1734‐1741. [DOI] [PubMed] [Google Scholar]
- 24. Ishida T, Hishizawa M, Kato K, et al. Impact of graft‐versus‐host disease on allogeneic hematopoietic cell transplantation for adult T cell leukemia‐lymphoma focusing on preconditioning regimens: nationwide retrospective study. Biol Blood Marrow Transplant. 2013;19:1731‐1739. [DOI] [PubMed] [Google Scholar]
- 25. Kozako T, Yoshimitsu M, Fujiwara H, et al. PD‐1/PD‐L1 expression in human T‐cell leukemia virus type 1 carriers and adult T‐cell leukemia/lymphoma patients. Leukemia. 2009;23:375‐382. [DOI] [PubMed] [Google Scholar]


