Abstract
Tumor cells evade immune surveillance through direct or indirect interactions with various types of immune cell, with much recent attention being focused on modifying immune cell responses as the basis for the development of new cancer treatments. Signal regulatory protein α (SIRPα) and CD47 are both transmembrane proteins that interact with each other and constitute a cell‐cell communication system. SIRPα is particularly abundant in myeloid cells such as macrophages and dendritic cells, whereas CD47 is expressed ubiquitously and its expression level is elevated in cancer cells. Recent studies have shown that blockade of CD47‐SIRPα interaction enhances the phagocytic activity of phagocytes such as macrophages toward tumor cells in vitro as well as resulting in the efficient eradication of tumor cells in a variety of xenograft or syngeneic mouse models of cancer. Moreover, CD47 blockade has been shown to promote the stimulation of tumor‐specific cytotoxic T cells by macrophages or dendritic cells. Biological agents, such as Abs and recombinant proteins, that target human CD47 or SIRPα have been developed and are being tested in preclinical models of human cancer or in clinical trials with cancer patients. Preclinical studies have also suggested that CD47 or SIRPα blockade may have a synergistic antitumor effect in combination with immune checkpoint inhibitors that target the adaptive immune system. Targeting of the CD47‐SIRPα signaling system is thus a promising strategy for cancer treatment based on modulation of both innate and acquired immune responses to tumor cells.
Keywords: cancer immunotherapy, CD47, macrophage, phagocytosis, signal regulatory protein α (SIRPα)
Abbreviations
- ADCC
antibody‐dependent cellular cytotoxicity
- ADCP
antibody‐dependent cellular phagocytosis
- CTLA‐4
cytotoxic T lymphocyte‐associated antigen‐4
- DC
dendritic cell
- HER2
human epidermal growth factor receptor 2
- NK
natural killer
- PD‐1
programmed cell death‐1
- PD‐L1
programmed cell death‐ligand 1
- RBC
red blood cell
- SH2
Src homology 2
- Shp
SH2 domain‐containing phosphatase
- SIRPα
signal regulatory protein α
1. INTRODUCTION
The tumor microenvironment consists of immune cells and a variety of stromal cell types, including fibroblasts and endothelial cells, as well as soluble and insoluble factors, such as cytokines, chemokines, and extracellular matrix.1, 2 This microenvironment plays an important role in the regulation of tumor progression by promoting tumor cell survival, invasion, and metastasis as well as angiogenesis.1, 2, 3 Cross‐talk between tumor and immune cells in the tumor microenvironment is also thought to contribute to the evasion of tumor cells from immune surveillance. For example, the binding of PD‐1 on cytotoxic T lymphocytes to its ligand PD‐L1 on tumor cells prevents killing of the latter cells by the former.4 Indeed, Abs to PD‐1 and to PD‐L1 are now in clinical use for the treatment of diverse solid tumors, including advanced melanoma, renal cell carcinoma, and non‐small‐cell lung cancer.5, 6 In addition, Abs to the T‐cell molecule CTLA‐4, which is also thought to suppress T‐cell responses on interaction with CD80 or CD86 on antigen‐presenting cells, are given to treat melanoma as well as prostate and lung cancers.5, 7 Molecules that participate in the negative regulation of the antitumor response of immune cells are thus promising targets for cancer therapy, with drugs that target such molecules being known as immune checkpoint inhibitors.8 We and others have recently shown that blocking Abs to SIRPα, which is highly expressed in macrophages and DCs, also has the potential to function as immune checkpoint inhibitors—that target innate immunity, in particular—for cancer treatment.9, 10, 11
2. SIGNAL REGULATORY PROTEIN α AND ITS BIOLOGICAL FUNCTIONS
Signal regulatory protein α (also known as SHPS‐1, p84, BIT, or CD172a) is a transmembrane protein that was originally identified as a highly expressed glycoprotein in the brain and a binding partner or putative substrate for 2 cytoplasmic‐type protein tyrosine phosphatases, SH2 domain‐containing phosphatase 1 (Shp1, also known as PTPN6) and Shp2 (PTPN11).12, 13, 14, 15 Indeed, SIRPα contains 3 Ig‐like domains in its extracellular region and 4 tyrosine residues that are putative phosphorylation sites in its cytoplasmic region (Figure 1A).16, 17 The extracellular region of SIRPα interacts with its ligand, CD47, which was originally identified in association with αvβ3 integrin and is also a member of the Ig superfamily of proteins, with an Ig‐V‐like extracellular domain, 5 putative membrane‐spanning segments, and a short cytoplasmic tail (Figure 1A).18 In their phosphorylated state, the tyrosine phosphorylation sites (in particular, the 2 COOH‐terminal sites) in the cytoplasmic region of SIRPα bind to the SH2 domains of Shp1 and Shp2 and thereby activate these phosphatases (Figure 1A). Tyrosine phosphorylation of the cytoplasmic region of SIRPα is triggered by various growth factors and cytokines as well as by integrin‐mediated cell adhesion to extracellular matrix proteins.14, 19 Ligation of SIRPα by CD47 also promotes tyrosine phosphorylation of its cytoplasmic region.20, 21 SIRPα thus functions as a docking protein for the recruitment and activation of Shp1 and Shp2 at the cell membrane in response to extracellular stimuli,16 with these phosphatases then being thought to play an important role in signaling downstream of SIRPα. SIRPα is especially abundant in neurons and in hematopoietic cells of the myeloid lineage such as macrophages, neutrophils, and DCs,22, 23, 24, 25, 26 whereas CD47 is expressed in most cell types.18
Figure 1.
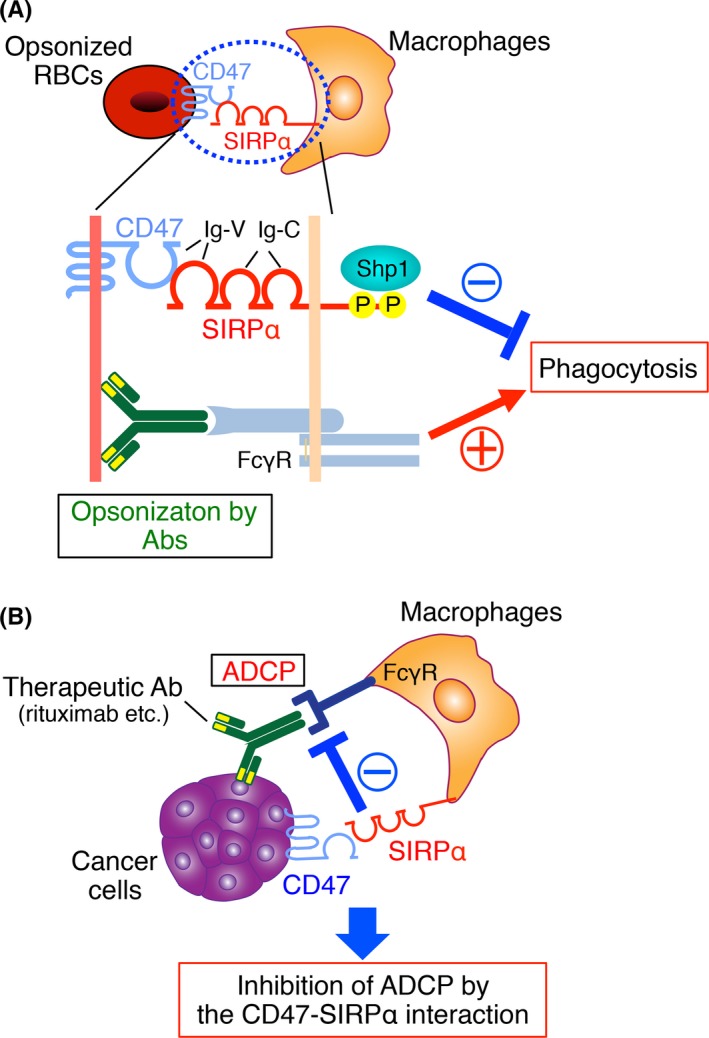
The CD47‐signal regulatory protein α (SIRPα) signaling system and its role in the regulation of phagocytosis by macrophages. A, SIRPα is a transmembrane protein that contains 3 Ig‐like domains (1 V‐like and 2 C1‐like Ig domains) in its NH 2‐terminal extracellular region and 2 key tyrosine phosphorylation sites in its COOH‐terminal cytoplasmic region. The tyrosine‐phosphorylated sites of SIRPα bind and thereby activate the protein tyrosine phosphatases Shp1 and Shp2. The SIRPα ligand CD47 is also a member of the Ig superfamily, with an Ig‐V‐like extracellular domain, 5 membrane‐spanning segments, and a short cytoplasmic tail. The Ig‐V‐like domain of CD47 interacts in trans with the NH 2‐terminal Ig‐V‐like domain of SIRPα and thereby triggers the tyrosine phosphorylation of the latter protein. Ligation of SIRPα on macrophages by CD47 on opsonized red blood cells (RBCs) thus promotes tyrosine phosphorylation of SIRPα and its subsequent association with Shp1, resulting in inhibition of RBCs phagocytosis by the macrophages elicited by the interaction of the Fc region of RBC‐bound Abs with the macrophage Fcγ receptor (FcγR). B, Interaction of CD47 on tumor cells with SIRPα on macrophages attenuates phagocytosis by the macrophages of the tumor cells triggered by opsonization with tumor antigen‐specific therapeutic Abs such as rituximab. ADCP, antibody‐dependent cellular phagocytosis
Signal regulatory protein α mutant mice, which express a mutant form of SIRPα that lacks most of the cytoplasmic region and thus fail to bind Shp1 and Shp2,24 manifested mild anemia associated with a short lifetime of RBCs as a result of increased phagocytotic activity of splenic macrophages against RBCs,21, 24 suggesting the importance of SIRPα for both the lifespan of RBCs and their number in the circulation. In addition, DC‐specific SIRPα knockout mice showed a reduced number of DCs, as well as of fibroblastic reticular cells, a subset of stromal cells, in the spleen.27 Moreover, SIRPα mutant mice, as well as CD47‐deficient mice, were resistant to the development of autoimmune animal models, such as experimental autoimmune encephalomyelitis,28, 29 suggesting that the interaction of SIRPα with CD47 is involved in the development of autoimmune diseases. SIRPα and CD47 are also thought to play a role in the regulation of central nervous system functions. Both SIRPα mutant mice and CD47‐deficient mice, indeed, showed prolonged immobility (depression‐like behavior) in the forced swim test.30 The CD47‐SIRPα signaling system is thus likely to act as a signaling platform for the brain response to stress and the regulation of depression‐like behavior in the forced swim test.
By contrast, mutations associated with human diseases, including hematological disorders, autoimmune diseases and neurological disorders, have not been identified within CD47 and SIRPA genes. Although the N‐terminal IgV‐like domain of human SIRPα, which is responsible for the interaction with CD47, is known to be highly polymorphic,31 any relationship between such polymorphisms of SIRPα and the incidence of diseases has not been reported so far.
3. BINDING OF CD47 TO SIRPα PREVENTS PHAGOCYTOSIS IN MACROPHAGES
Oldenborg and colleagues20, 32 first showed that the rate of clearance of transfused CD47‐deficient RBCs from the bloodstream of WT mice was markedly increased compared with that of transfused WT cells.20, 32 In addition, the phagocytosis of CD47‐deficient RBCs by isolated splenic macrophages from WT mice in vitro was greatly enhanced compared with that of WT RBCs.20 We also subsequently showed that the rate of clearance of transfused WT RBCs from the bloodstream was markedly increased in SIRPα mutant mice. Phagocytosis of antibody‐opsonized WT RBCs by isolated macrophages from the SIRPα mutant mice was also enhanced compared with that seen with macrophages from WT mice.21 Collectively, these observations indicated that the binding of CD47 on RBCs to SIRPα on macrophages prevents the Fcγ receptor (FcγR)‐dependent phagocytosis of the former cells by the latter (Figure 1A). The activity of Shp1, which binds to the tyrosine‐phosphorylated cytoplasmic region of SIRPα, is thought to be important for this prevention of phagocytosis (Figure 1A).32
4. BLOCKADE OF THE CD47‐SIRPα SIGNALING SYSTEM HAS ANTITUMOR EFFECTS
Two major groups of molecularly targeted drugs are currently in clinical use for cancer therapy. One group includes potent inhibitors of a variety of signaling molecules that are essential for the proliferation of tumor cells, such as inhibitors of various tyrosine kinases as well as of the Raf‐MEK and PI3K‐mTOR (mammalian target of rapamycin) signaling pathways.33, 34, 35, 36, 37 The other group consists of mAbs to surface molecules that are highly expressed in particular cancers, such as rituximab (to CD20 in lymphoma), trastuzumab (to HER2 in breast cancer), and cetuximab (to epidermal growth factor receptor [EGFR] in colon cancer). These mAbs are thought to bind to their targets on tumor cells and thereby induce the killing of these cells by complement‐dependent cytotoxicity as well as via ADCP mediated by macrophages and ADCC mediated by NK cells, both of which require the interaction of Fc receptors on the effector cells with the Fc domain of the bound mAbs.38 Many studies have shown that the interaction of CD47 on cancer cells with SIRPα on macrophages serves to inhibit phagocytosis of the former cells by the latter cells (Figure 1B).9, 39, 40, 41 Blockade of the CD47‐SIRPα signaling system was thus shown to enhance the ADCP‐mediated killing of Ab‐opsonized tumor cells by macrophages (Figure 2A) as well as to suppress tumor growth and metastasis in preclinical mouse models of cancer.
Figure 2.
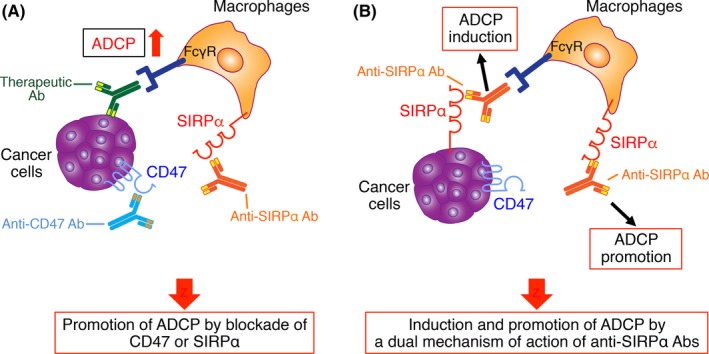
Suppression of tumor growth and metastasis by blockade of CD47‐signal regulatory protein α (SIRPα) interaction and consequent promotion of macrophage‐mediated antibody‐dependent cellular phagocytosis (ADCP). A, Inhibition of the interaction of CD47 on tumor cells with SIRPα on macrophages by biological agents such as Abs to these proteins promotes macrophage‐mediated ADCP of the tumor cells triggered by opsonization with therapeutic Abs to tumor antigens and thereby leads to suppression of tumor growth and metastasis. B, Blocking Abs to SIRPα bind to this protein on both macrophages and certain tumor cells such as melanoma and renal cell carcinoma cells. Such binding results in both direct induction of macrophage‐mediated ADCP of the tumor cells as well as blockade of CD47‐SIRPα signaling that negatively regulates such phagocytosis
4.1. Targeting of CD47
CD47 was shown to be identical to the tumor antigen OV‐3, which interacts with integrins and is markedly upregulated in ovarian carcinoma cells.42 Expression of CD47 was also found to be increased in AML stem cells, non‐Hodgkin lymphoma, and various solid tumors compared with their normal counterparts,40, 43, 44 and such increased expression was associated with poor prognosis in patients with these malignancies.40, 43, 44, 45, 46 Incubation with a blocking Ab to CD47 either alone or in combination with mAbs to tumor antigens in vitro promoted the phagocytosis by macrophages of cancer cells such as AML stem cells, non‐Hodgkin lymphoma cells, colorectal cancer stem cells, and breast cancer cells (Figure 2A).40, 43, 44 Such Ab treatment also attenuated tumor growth and metastasis in a variety of xenograft or syngeneic mouse models of AML, non‐Hodgkin lymphoma, pediatric brain tumors, as well as ovarian, colon, breast, bladder, and small‐cell lung cancers.40, 43, 44, 47, 48, 49 Moreover, an engineered recombinant SIRPα protein, which has a higher affinity for CD47 than does the WT protein and prevents endogenous CD47‐SIRPα interaction, was found to significantly enhance the efficacy of therapeutic Abs such as rituximab, trastuzumab, and alemtuzumab (an Ab to CD52) in mouse xenograft models of human cancer.50
In addition to its effect on ADCP of tumor cells by macrophages, CD47 blockade promotes the activation of tumor‐specific cytotoxic T cells by DCs or macrophages.40, 44, 51, 52, 53 Treatment of tumor‐bearing mice with blocking Abs to CD47 thus promoted the recognition of tumor‐derived DNA through the stimulator of interferon genes (STING) pathway.52 Such recognition increased type I interferon production by DCs and enhanced the cross‐priming of tumor‐specific cytotoxic T cells.53 Tumor‐infiltrating macrophages were also found to participate in the cross‐priming of tumor‐specific cytotoxic T cells.51 Blockade of CD47‐SIRPα interaction is thus thought to promote the phagocytosis of tumor cells by macrophages and DCs, which, in turn, activate tumor‐specific cytotoxic T cells at the site of tumor rejection. In contrast, given that CD47 is expressed on most cell types, off‐target effects of CD47 blockade on normal cells—in particular, erythrocytes—is a potential concern with regard to treatment with drugs that target CD47. Indeed, treatment with Abs to CD47 induced in a dose‐dependent way the development of transient anemia associated with reticulocytosis in cynomolgus monkeys.47
4.2. Targeting of SIRPα
Blockade of SIRPα in combination with Abs to tumor antigens is also a promising strategy for cancer therapy. Indeed, Abs to mouse SIRPα enhanced the rituximab‐induced elimination of human Burkitt's lymphoma Raji cells transplanted into immunodeficient non‐obese diabetic (NOD)/SCID mice,11 whose endogenous SIRPα has a high affinity for human CD47.31 In addition, whereas tumor growth or metastasis did not differ between SIRPα mutant and WT mice injected with syngeneic melanoma cells, treatment with therapeutic Abs specific for a melanoma antigen eliminated tumor cells to a markedly greater extent in the mutant mice than in the WT animals.45 Moreover, an Ab to human SIRPα that inhibits CD47‐SIRPα interaction enhanced killing by human phagocytes of HER2‐positive breast cancer cells opsonized with the HER2‐specific mAb trastuzumab in vitro.45 Abs to mouse SIRPα also enhanced the phagocytic activity of bone marrow‐derived macrophages from NOD mice toward Raji cells opsonized with rituximab.11 Collectively, these findings suggest that blocking Abs to SIRPα has the potential to promote tumor elimination in vivo by enhancement of macrophage‐mediated ADCP of cancer cells opsonized by Abs to tumor antigens (Figure 2A). In addition to Abs to SIRPα, recombinant CD47 proteins that contain the NH2‐terminal Ig‐V‐like domain and block CD47‐SIRPα interaction may also contribute to the killing of tumor cells. Indeed, an NH2‐terminal variant of CD47 with a higher affinity for SIRPα than WT CD47 was found to act synergistically with tumor‐specific mAbs to promote macrophage‐mediated phagocytosis of tumor cells in vitro,54 although its potential antitumor effects in vivo have yet to be evaluated.
Blocking Abs to SIRPα might also have therapeutic effects as single agents in the case of SIRPα‐expressing tumors, with the antitumor effects of such Abs being mediated by a dual mechanism of action: direct induction of ADCP of tumor cells by macrophages and blockade of CD47‐SIRPα signaling that negatively regulates such phagocytosis (Figure 2B). Similar to the increased expression of CD47 apparent in many types of cancer, SIRPα is also more prominently expressed in tumor tissue from patients with renal cell carcinoma or melanoma compared with the surrounding normal tissue.11 Of note, treatment with a blocking Ab to mouse SIRPα resulted in a marked reduction in the tumor burden of immunocompetent mice injected with syngeneic renal cell carcinoma or melanoma cells, both of which highly expressed endogenous SIRPα.11 This antitumor action of the Ab was significantly attenuated by selective depletion of macrophages. Moreover, the expression of SIRPα on tumor cells and the Fc region of the Ab to SIRPα were required for promotion of the phagocytosis of mouse renal cell carcinoma cells by macrophages in vitro, suggesting that opsonization of the tumor cells by the Ab induces ADCP through activation of the Fc receptor on macrophages.
In addition to macrophages, both cytotoxic T cells and NK cells may contribute to the antitumor effects of blocking Abs to SIRPα.11 Such Abs might thus initiate cross‐priming of tumor‐specific cytotoxic T cells by macrophages and DCs. Moreover, the Abs might induce NK cell‐mediated ADCC toward tumor cells directly and contribute to the activation of NK cells by tumor‐infiltrating macrophages or DCs.55, 56, 57 Of interest, treatment with blocking Abs to SIRPα did not induce obvious adverse effects including anemia in mice.11 Treatment of mice with Abs to SIRPα also did not cause any obvious neurotoxicity regardless of the high expression of SIRPα in the neuron.11 Such Abs might thus be worth pursuing for cancer therapy, especially for the treatment of melanoma and renal cell carcinoma.
4.3. Therapeutic drugs targeting CD47 or SIRPα
Several biological agents, including Abs and recombinant proteins, that are able to bind to human CD47 specifically and block the human CD47‐SIRPα interaction have been developed for cancer treatment (Table 1). Hu5F9‐G4 was the first drug to be developed as a humanized mAb to human CD47.47 This agent enhances the phagocytosis of tumor cells by macrophages in vitro and eradicates human hematological malignancies and solid tumors in xenograft mouse models.47, 48, 49 Phase I or I/II clinical trials of Hu5F9‐G4 and another humanized mAb to human CD47, CC‐90002, are now being conducted for both solid tumors and hematological malignancies. Recombinant SIRPα proteins, such as TTI‐621 and ALX148, are also in phase I clinical trials for patients with hematological malignancies or solid tumors (Table 1). TTI‐621 consists of the Ig‐V‐like domain of human SIRPα linked to the Fc region of human IgG1, and it was shown to enhance the phagocytosis of tumor cells by macrophages in vitro and to effectively control tumor growth in xenograft models of aggressive AML or B lymphoma as well as in a syngeneic mouse model of B lymphoma.58, 59 Like TTI‐621, ALX148 has a higher affinity for human CD47 than does WT SIRPα, but it comprises a variant of the Ig‐V‐like domain of human SIRPα fused to an inactive Fc domain. Both of these drugs are currently being tested in clinical trials as monotherapy or in combination either with molecularly targeted agents including Abs to tumor antigens and immune checkpoint inhibitors or with radiotherapy. Various other CD47‐targeting drugs including bispecific Abs that bind to both tumor‐specific antigens and CD47 are also in preclinical development (Table 1).
Table 1.
Therapeutic agents in preclinical or clinical development that target CD47 or SIRPα
| Company | Country | Drug | Description | Phase | Disease | Strategy | Combination agent | ID |
|---|---|---|---|---|---|---|---|---|
| Forty Seven Inc. | USA | Hu5F9‐G4 | Anti‐CD47 Ab (IgG4) | I | Solid tumors, NHL | Mono | NCT02216409 | |
| I | AML, MDS | Mono | NCT02678338 | |||||
| I/II | CRC, solid tumors | Combi | Cetuximab | NCT02953782 | ||||
| I/II | NHL | Combi | Rituximab | NCT02953509 | ||||
| I | AML, MDS | Mono/Combi | Azacitidine | NCT03248479 | ||||
| Celgene | USA | CC‐90002 | Anti‐CD47 Ab (IgG4) | I | Solid tumors, MM, NHL | Mono/Combi | Rituximab | NCT02367196 |
| I | AML, MDS | Mono | NCT02641002 | |||||
| Trillium Therapeutics Inc. | Canada | TTI‐621 | SIRPα‐Fc fusion protein (IgG1) | I | Hematological malignancies, solid tumors | Mono/Combi | Rituximab, nivolumab | NCT02663518 |
| I | Solid tumors | Mono/Combi | PD‐1/PD‐L1 inhibitor, PEG‐IFN‐α2a, T‐Vec, radiation | NCT02890368 | ||||
| Alexo Therapeutics | USA | ALX148 | SIRPα V1‐Fc fusion protein | I | Solid tumors, NHL | Mono/Combi | Pembrolizumab, trastuzumab, rituximab | NCT03013218 |
| Novimmune SA | Switzerland | NI‐1701 | Anti‐CD47/CD19 bispecific Ab | Preclinical | ||||
| NI‐1801 | Anti‐CD47/mesothelin bispecific Ab | Preclinical | ||||||
| Arch Oncology | USA | AO‐104, ‐108, ‐176 | Anti‐CD47 Ab | Preclinical | ||||
| Surface Oncology Inc. | USA | SRF231 | Anti‐CD47 Ab | Preclinical | ||||
| Hummingbird Bioscience | Singapore | HMBD004 | Anti‐CD47/CD33 bispecific Ab | Preclinical | ||||
| OSE Immunotherapeutics | France | OSE‐172 | Anti‐SIRPα Ab | Preclinical |
Combi, combination therapy; CRC, colorectal carcinoma; ID, clinicaltrials.gov identifier; MDS, myelodysplastic syndrome; MM, multiple myeloma; Mono, monotherapy; NHL, non‐Hodgkin lymphoma; PD‐1, programmed cell death‐1; PD‐L1, programmed cell death‐ligand 1; PEG‐IFN‐α2a, pegylated interferon‐α2a; SIRPα, signal regulatory protein α; T‐Vec, talimogene laherparepvec.
5. COMBINATION OF BLOCKING Abs TO CD47 OR TO SIRPα WITH IMMUNE CHECKPOINT INHIBITORS
Treatment with immune checkpoint inhibitors that target PD‐1, PD‐L1, or CTLA‐4 provides substantial clinical benefit in patients with a wide range of malignancies including metastatic melanoma, renal cell carcinoma, and non‐small‐cell lung cancer.5, 6, 7 However, many patients remain unresponsive to these therapies. Combinations of immune checkpoint inhibitors with standard chemotherapeutic drugs, small‐molecule compounds, cancer vaccines, and immune‐stimulatory agents are currently under evaluation in preclinical models and in clinical trials in attempts to increase the efficacy of immune checkpoint inhibition in such unresponsive patients.
Immune checkpoint inhibitors that target PD‐1 or PD‐L1 prevent the interaction of PD‐1 on cytotoxic T cells with PD‐L1 on cancer cells, which generates an inhibitory signal within the T cells. Blocking of this interaction is therefore thought to enhance the killing of tumor cells by cytotoxic T cells.4, 6 Inhibition of both the CD47‐SIRPα and PD‐1‐PD‐L1 axes might therefore be expected have a synergistic antitumor action, given that blockade of CD47 is thought to exert antitumor effects by promoting the phagocytosis of tumor cells and tumor cell‐releasing substances by macrophages or DCs as well as by enhancing the cross‐priming of tumor‐specific cytotoxic T cells by these antigen‐presenting cells (Figure 3).51, 52 Indeed, a nanobody (an antigen‐binding fragment of an Ab heavy chain) that reacts with CD47 and thereby inhibits the CD47‐SIRPα interaction was found to synergize with PD‐L1 antagonism to attenuate the growth of tumors formed in immunocompetent mice by s.c. injected syngeneic melanoma cells.60, 61 The efficacy of combined therapy with Abs to PD‐1 and to CTLA‐4 in a mouse model of esophageal squamous cell cancer was also enhanced by blocking Abs to CD47.62 Moreover, combined blockade of SIRPα and PD‐1 had a synergistic antitumor effect in a syngeneic mouse model of colon cancer.11 The combination of inhibitors of the CD47‐SIRPα interaction with immune checkpoint inhibitors that target the PD‐1‐PD‐L1 axis is thus a potential new approach to immunotherapy for a broad range of cancers.
Figure 3.
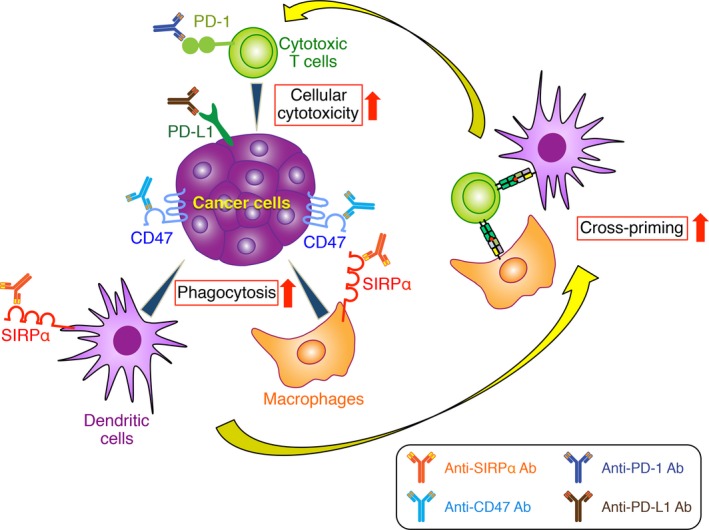
Synergistic antitumor effects of blockade of CD47‐signal regulatory protein α (SIRPα) interaction combined with immune checkpoint inhibitors. Blockade of CD47 or SIRPα with corresponding specific Abs promotes the phagocytosis of tumor cells by macrophages or dendritic cells as well as the consequent cross‐priming of tumor‐specific cytotoxic T cells. Activity of cytotoxic T cells is also enhanced by immune checkpoint inhibitors, such as Abs to programmed cell death‐1 (PD‐1) or programmed cell death‐ligand 1 (PD‐L1) that prevent the interaction of these proteins. Blockade of CD47 or SIRPα together with giving immune checkpoint inhibitors may therefore have synergistic antitumor effects
6. XENOGRAFT TUMOR MODELS FOR PRECLINICAL VALIDATION OF THE ANTITUMOR EFFECTS OF Abs TO HUMAN SIRPα
Animal models that mimic human diseases are important tools with which to investigate the potential therapeutic efficacy and adverse effects of biological agents in patients. However, the antitumor effects of Abs to human SIRPα in preclinical cancer models have remained unclear because such Abs have failed to bind to endogenous SIRPα expressed on macrophages of immunodeficient mice, likely because of differences in the amino acid sequence of the NH2‐terminal Ig‐V‐like domain between human and mouse SIRPα. In this regard, a blocking Ab to human SIRPα was recently shown to promote the antitumor effects of rituximab and of vorsetuzumab (an Ab to CD70) in human tumor‐bearing Rag2 −/− Il2rg −/− immunodeficient mice in which the DNA sequence encoding the extracellular domain of mouse SIRPα was replaced with the corresponding human sequence (hSIRPαKI mice).63, 64 With the use of Rag2 −/− Il2rg −/− mice expressing human SIRPα under the control of human regulatory elements (hSIRPαTg mice),65 we also showed that a blocking Ab to human SIRPα enhanced the inhibitory effect of rituximab on the growth of tumors formed by s.c. injected Raji cells.66 This Ab to human SIRPα also increased the phagocytic activity of macrophages from hSIRPαTg mice, but not that of those from Rag2 −/− Il2rg −/− mice, as measured in vitro with human cancer cells opsonized with Abs to tumor antigens, suggesting that the interaction of human CD47 on human cancer cells with human SIRPα on mouse macrophages generates an inhibitory signal for macrophage ADCP. Moreover, in both of these genetically modified mouse models transplanted with human tumor cells, the antitumor effects of the Abs to human SIRPα were found to be dependent, at least in part, on macrophages.64, 66 These mice may thus serve as models for preclinical validation of Abs to human SIRPα in cancer immunotherapy (Figure 4).
Figure 4.
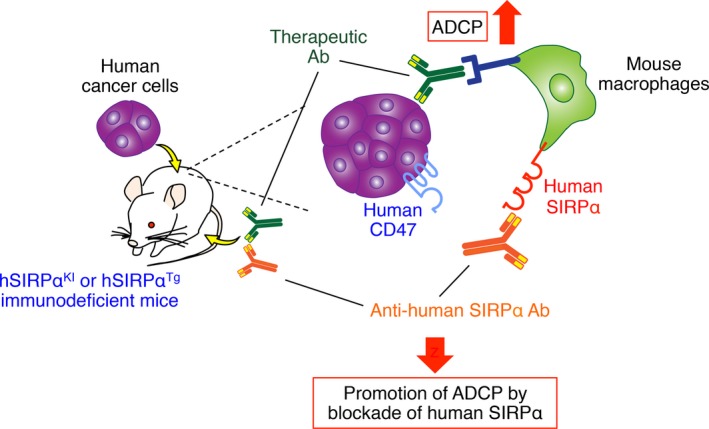
Xenograft tumor models for preclinical validation of the antitumor effects of Abs to human signal regulatory protein α (SIRPα). In xenograft tumor models in which human cancer cells are transplanted into human SIRPα knockin (hSIRPαKI) or human SIRPα transgenic (hSIRPαTg) immunodeficient mice, interaction of human CD47 on human cancer cells with human SIRPα on mouse macrophages inhibits phagocytosis of the former cells by the latter. Prevention of this interaction with Abs to human SIRPα thus enhances antibody‐dependent cellular phagocytosis (ADCP) by macrophages of the cancer cells opsonized with therapeutic Abs to tumor‐specific antigens
7. CONCLUSION
Attention has recently focused on modifying immune responses as a basis for new cancer treatments. Immunotherapy with immune checkpoint inhibitors that target PD‐1, PD‐L1, or CTLA‐4, which enhance the antitumor activity of cytotoxic T cells, has shown clinical activity in a variety of cancer types. The CD47‐SIRPα signaling system serves as an innate immune checkpoint that is thought to help tumor cells evade immune surveillance by preventing their phagocytosis by macrophages and other phagocytes. Numerous studies with preclinical mouse models of cancer have suggested that blockade of CD47 or SIRPα with Abs or recombinant proteins, either alone or in combination with other agents such as Abs to tumor‐specific antigens or immune checkpoint inhibitors, holds promise for the treatment of various types of cancer. However, the mechanisms underlying the initiation and consolidation of immune responses to tumor cells by CD47 or SIRPα blockade, as well as the possible adverse effects of such blockade in vivo, remain to be fully understood. In addition, xenograft models based on immunodeficient mice that lack NK, B, and T cells may not be sufficient for validation of the efficacy of agents that target human CD47 or SIRPα, given that the effects of such agents on immune cells in these mice are limited to myeloid cells such as macrophages and DCs. Immunodeficient mice engrafted with a human immune system by transplantation of human hematopoietic stem cells have been developed as an important new tool for cancer research.67, 68, 69 Future preclinical studies with such humanized and immunodeficient mice, as well as clinical trials, should show the potential of targeting the CD47‐SIRPα axis as a new strategy for immunotherapy of human cancer.
CONFLICTS OF INTEREST
T.M. has received research funding from Daiichi Sankyo Co. Ltd. The other authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The work in the authors’ laboratory was supported by a Grant‐in‐Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS), by the Project for Development of Innovative Research on Cancer Therapeutics (P‐DIRECT) and the Project for Cancer Research and Therapeutic Evolution (P‐CREATE) of the Japan Agency for Medical Research and Development, by research funds from Daiichi Sankyo Co. Ltd, and by Terumo Foundation for Life Sciences and Arts, Uehara Memorial Foundation, Takeda Science Foundation, and Japanese Society of Hematology.
Murata Y, Saito Y, Kotani T, Matozaki T. CD47‐signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci. 2018;109:2349‐2357. 10.1111/cas.13663
REFERENCES
- 1. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309‐322. [DOI] [PubMed] [Google Scholar]
- 2. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2015;39:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD‐1 and their advantages for clinical application. Nat Immunol. 2013;14:1212‐1218. [DOI] [PubMed] [Google Scholar]
- 5. Callahan MK, Postow MA, Wolchok JD. Targeting T cell co‐receptors for cancer therapy. Immunity. 2016;44:1069‐1078. [DOI] [PubMed] [Google Scholar]
- 6. Alsaab HO, Sau S, Alzhrani R, et al. PD‐1 and PD‐L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callahan MK, Wolchok JD. Clinical activity, toxicity, biomarkers, and future development of CTLA‐4 checkpoint antagonists. Semin Oncol. 2015;42:573‐586. [DOI] [PubMed] [Google Scholar]
- 8. Perez‐Gracia JL, Labiano S, Rodriguez‐Ruiz ME, et al. Orchestrating immune check‐point blockade for cancer immunotherapy in combinations. Curr Opin Immunol. 2014;27:89‐97. [DOI] [PubMed] [Google Scholar]
- 9. Matlung HL, Szilagyi K, Barclay NA, et al. The CD47‐SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145‐164. [DOI] [PubMed] [Google Scholar]
- 10. Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100‐109. [DOI] [PubMed] [Google Scholar]
- 11. Yanagita T, Murata Y, Tanaka D, et al. Anti‐SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017;2:e89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noguchi T, Matozaki T, Fujioka Y, et al. Characterization of a 115‐kDa protein that binds to SH‐PTP2, a protein‐tyrosine phosphatase with Src homology 2 domains, in chinese hamster ovary cells. J Biol Chem. 1996;271:27652‐27658. [DOI] [PubMed] [Google Scholar]
- 13. Ohnishi H, Kubota M, Ohtake A, et al. Activation of protein‐tyrosine phosphatase SH‐PTP2 by a tyrosine‐based activation motif of a novel brain molecule. J Biol Chem. 1996;271:25569‐25574. [DOI] [PubMed] [Google Scholar]
- 14. Fujioka Y, Matozaki T, Noguchi T, et al. A novel membrane glycoprotein, SHPS‐1, that binds the SH2‐domain‐containing protein tyrosine phosphatase SHP‐2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887‐6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kharitonenkov A, Chen Z, Sures I, et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181‐186. [DOI] [PubMed] [Google Scholar]
- 16. Matozaki T, Murata Y, Okazawa H, et al. Functions and molecular mechanisms of the CD47‐SIRPα signalling pathway. Trends Cell Biol. 2009;19:72‐80. [DOI] [PubMed] [Google Scholar]
- 17. Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25‐50. [DOI] [PubMed] [Google Scholar]
- 18. Oldenborg PA. CD47: a cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013;2013:614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuda M, Matozaki T, Fukunaga K, et al. Integrin‐mediated tyrosine phosphorylation of SHPS‐1 and its association with SHP‐2. Roles of Fak and Src family kinases. J Biol Chem. 1998;273:13223‐13229. [DOI] [PubMed] [Google Scholar]
- 20. Oldenborg PA, Zheleznyak A, Fang YF, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051‐2054. [DOI] [PubMed] [Google Scholar]
- 21. Okazawa H, Motegi S, Ohyama N, et al. Negative regulation of phagocytosis in macrophages by the CD47‐SHPS‐1 system. J Immunol. 2005;174:2004‐2011. [DOI] [PubMed] [Google Scholar]
- 22. Seiffert M, Cant C, Chen Z, et al. Human signal‐regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633‐3643. [PubMed] [Google Scholar]
- 23. Ohnishi H, Kaneko Y, Okazawa H, et al. Differential localization of Src homology 2 domain‐containing protein tyrosine phosphatase substrate‐1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J Neurosci. 2005;25:2702‐2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishikawa‐Sekigami T, Kaneko Y, Okazawa H, et al. SHPS‐1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341‐348. [DOI] [PubMed] [Google Scholar]
- 25. Okajo J, Kaneko Y, Murata Y, et al. Regulation by Src homology 2 domain‐containing protein tyrosine phosphatase substrate‐1 of α‐galactosylceramide‐induced antimetastatic activity and Th1 and Th2 responses of NKT cells. J Immunol. 2007;178:6164‐6172. [DOI] [PubMed] [Google Scholar]
- 26. Saito Y, Iwamura H, Kaneko T, et al. Regulation by SIRPα of dendritic cell homeostasis in lymphoid tissues. Blood. 2010;116:3517‐3525. [DOI] [PubMed] [Google Scholar]
- 27. Saito Y, Respatika D, Komori S, et al. SIRPα+ dendritic cells regulate homeostasis of fibroblastic reticular cells via TNF receptor ligands in the adult spleen. Proc Natl Acad Sci USA. 2017;114:E10151‐E10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murata Y, Kotani T, Ohnishi H, et al. The CD47‐SIRPα signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155:335‐344. [DOI] [PubMed] [Google Scholar]
- 29. Murata Y, Saito Y, Kaneko T, et al. Autoimmune animal models in the analysis of the CD47‐SIRPα signaling pathway. Methods. 2014;65:254‐259. [DOI] [PubMed] [Google Scholar]
- 30. Ohnishi H, Murata T, Kusakari S, et al. Stress‐evoked tyrosine phosphorylation of signal regulatory protein α regulates behavioral immobility in the forced swim test. J Neurosci. 2010;30:10472‐10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313‐1323. [DOI] [PubMed] [Google Scholar]
- 32. Oldenborg PA, Gresham HD, Lindberg FP. CD47‐signal regulatory protein α (SIRPα) regulates Fcγ and complement receptor‐mediated phagocytosis. J Exp Med. 2001;193:855‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckstein N, Roper L, Haas B, et al. Clinical pharmacology of tyrosine kinase inhibitors becoming generic drugs: Τhe regulatory perspective. J Exp Clin Cancer Res. 2014;33:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holderfield M, Deuker MM, McCormick F, et al. Targeting RAF kinases for cancer therapy: BRAF‐mutated melanoma and beyond. Nat Rev Cancer. 2014;14:455‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu P, Nielsen TE, Clausen MH. FDA‐approved small‐molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422‐439. [DOI] [PubMed] [Google Scholar]
- 36. Caunt CJ, Sale MJ, Smith PD, et al. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat Rev Cancer. 2015;15:577‐592. [DOI] [PubMed] [Google Scholar]
- 37. O'Donnell JS, Massi D, Teng MWL, et al. PI3K‐AKT‐mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91‐103. [DOI] [PubMed] [Google Scholar]
- 38. Braster R, O'Toole T, van Egmond M. Myeloid cells as effector cells for monoclonal antibody therapy of cancer. Methods. 2014;65:28‐37. [DOI] [PubMed] [Google Scholar]
- 39. Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chao MP, Weissman IL, Majeti R. The CD47‐SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown EJ, Frazier WA. Integrin‐associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130‐135. [DOI] [PubMed] [Google Scholar]
- 43. Chao MP, Alizadeh AA, Tang C, et al. Anti‐CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non‐Hodgkin lymphoma. Cell. 2010;142:699‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47‐signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662‐6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao XW, van Beek EM, Schornagel K, et al. CD47‐signal regulatory protein‐α (SIRPα) interactions form a barrier for antibody‐mediated tumor cell destruction. Proc Natl Acad Sci USA. 2011;108:18342‐18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chao MP, Alizadeh AA, Tang C, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu J, Wang L, Zhao F, et al. Pre‐clinical development of a humanized anti‐CD47 antibody with anti‐cancer therapeutic potential. PLoS ONE. 2015;10:e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weiskopf K, Jahchan NS, Schnorr PJ, et al. CD47‐blocking immunotherapies stimulate macrophage‐mediated destruction of small‐cell lung cancer. J Clin Invest. 2016;126:2610‐2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gholamin S, Mitra SS, Feroze AH, et al. Disrupting the CD47‐SIRPα anti‐phagocytic axis by a humanized anti‐CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med. 2017;9:eaaf2968. [DOI] [PubMed] [Google Scholar]
- 50. Weiskopf K, Ring AM, Ho CC, et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tseng D, Volkmer JP, Willingham SB, et al. Anti‐CD47 antibody‐mediated phagocytosis of cancer by macrophages primes an effective antitumor T‐cell response. Proc Natl Acad Sci USA. 2013;110:11103‐11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell‐mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu MM, Pu Y, Han D, et al. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross‐priming through signal regulatory protein α signaling. Immunity. 2017;47:363‐373.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ho CC, Guo N, Sockolosky JT, et al. “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody‐dependent cellular phagocytosis. J Biol Chem. 2015;290:12650‐12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chiba S, Ikushima H, Ueki H, et al. Recognition of tumor cells by Dectin‐1 orchestrates innate immune cells for anti‐tumor responses. Elife. 2014;3:e04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mattiola I, Pesant M, Tentorio PF, et al. Priming of human resting NK cells by autologous M1 macrophages via the engagement of IL‐1β, IFN‐β, and IL‐15 pathways. J Immunol. 2015;195:2818‐2828. [DOI] [PubMed] [Google Scholar]
- 57. Bodduluru LN, Kasala ER, Madhana RM, et al. Natural killer cells: the journey from puzzles in biology to treatment of cancer. Cancer Lett. 2015;357:454‐467. [DOI] [PubMed] [Google Scholar]
- 58. Lin GHY, Chai V, Lee V, et al. TTI‐621 (SIRPαFc), a CD47‐blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS ONE. 2017;12:e0187262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Petrova PS, Viller NN, Wong M, et al. TTI‐621 (SIRPαFc): a CD47‐blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23:1068‐1079. [DOI] [PubMed] [Google Scholar]
- 60. Sockolosky JT, Dougan M, Ingram JR, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA. 2016;113:E2646‐E2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ingram JR, Blomberg OS, Sockolosky JT, et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc Natl Acad Sci USA. 2017;114:10184‐10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tao H, Qian P, Wang F, et al. Targeting CD47 enhances the efficacy of anti‐PD‐1 and CTLA‐4 in an esophageal squamous cell cancer preclinical model. Oncol Res. 2017;25:1579‐1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herndler‐Brandstetter D, Shan L, Yao Y, et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc Natl Acad Sci USA. 2017;114:E9626‐E9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ring NG, Herndler‐Brandstetter D, Weiskopf K, et al. Anti‐SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114:E10578‐E10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strowig T, Rongvaux A, Rathinam C, et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−γc −/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci USA. 2011;108:13218‐13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Murata Y, Tanaka D, Hazama D, et al. Anti‐human SIRPα antibody is a new tool for cancer immunotherapy. Cancer Sci. 2018;109:1300‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rongvaux A, Takizawa H, Strowig T, et al. Human hemato‐lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Theocharides AP, Rongvaux A, Fritsch K, et al. Humanized hemato‐lymphoid system mice. Haematologica. 2016;101:5‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morton JJ, Bird G, Refaeli Y, et al. Humanized mouse xenograft models: narrowing the tumor‐microenvironment gap. Cancer Res. 2016;76:6153‐6158. [DOI] [PMC free article] [PubMed] [Google Scholar]


