Neurocysticercosis accounts for approximately 30% of all epilepsy cases in most developing countries. The immunodiagnosis of cysticercosis is complex and strongly influenced by the course of infection, the disease burden, the cyst location, and the immune response of the host.
KEYWORDS: Peru, Taenia solium, Western blot, antibody, antigen, cysticercosis, neurocysticercosis, EITB, ELISA
ABSTRACT
Neurocysticercosis accounts for approximately 30% of all epilepsy cases in most developing countries. The immunodiagnosis of cysticercosis is complex and strongly influenced by the course of infection, the disease burden, the cyst location, and the immune response of the host. The main approach to immunodiagnosis should thus be to evaluate whether the serological results are consistent with the diagnosis suggested by imaging. Antibody detection is performed using lentil lectin-purified parasite antigens in an enzyme-linked immunoelectrotransfer blot format, while antigen detection uses a monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA). Promising new assay configurations have been developed for the detection of both antibody and antigen, including assays based on synthetic or recombinant antigens that may reduce costs and improve assay reproducibility and multiplex bead-based assays that may provide simultaneous quantitative results for several target antigens or antibodies.
INTRODUCTION
Taenia solium, the pork tapeworm, is endemic in most developing countries where pigs are raised. The coexistence of domestic pig raising and poor sanitary conditions enables the establishment of the parasite life cycle, in which pigs get infected with the larval cystic stage (cysticercus) by ingesting infective Taenia eggs excreted in the stools of a human carrying the adult intestinal tapeworm. Humans, in turn, get infected with the adult tapeworm stage by ingesting cysts in poorly cooked pork. Humans may also host the larval stage and acquire cysticercosis by fecal-oral contamination (1). While cysts in most tissues are asymptomatic and rarely noticed, cysts in the nervous system (neurocysticercosis [NCC]) are a major cause of epilepsy and other neurological morbidities in regions of endemicity (2, 3). Cases of NCC are seen in regions where it is not endemic with increasing frequency because of travel and migration. In the United States, more than 1,800 NCC-related hospitalizations are estimated to occur per year. The hospitalization costs for cysticercosis exceed those for malaria and all other neglected tropical diseases combined (4).
CYSTICERCOSIS INFECTION
Very little is known regarding the usual evolution of human cysticercosis infections. In the pig model, the embryos contained in ingested tapeworm eggs are released, cross the intestinal mucosa, migrate through the circulatory system, and develop into cysticerci that reach their definitive size in 3 to 4 months. Cysticerci are typically found in muscle and subcutaneous tissue and less frequently in the nervous system (5). There is no reason to suspect that this initial process is different in humans than in pigs.
Neurocysticercosis: parasite stages, localization, and clinical manifestations.
It is generally accepted that although human cysticercosis affects multiple tissues, the parasite is usually destroyed by the host's immune system, surviving mainly in immunologically privileged sites like the brain or the eye. The infection of the nervous system is more likely to result in prominent symptoms and therefore more likely to be diagnosed than infections of other tissues. Despite this, it is likely that most infections remain undiagnosed for months or years. The evidence from a large series of NCC cases occurring in British soldiers who served in India for a defined period demonstrated that in a significant proportion of cases, neurological symptoms present years after infection (6).
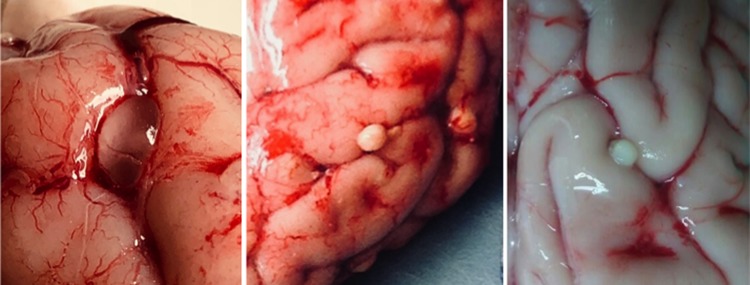
The process by which embryos invade the central nervous system has not been clearly elucidated. However, once infection is established, the evolution of cysticerci in the human nervous system follows a somewhat predictable course. Viable intraparenchymal brain cysts develop into rounded vesicles composed of a thin parasitic membrane filled with a clear cerebrospinal fluid (CSF)-like fluid and containing a retracted tapeworm head (scolex). There is evidence that the parasite employs multiple active immune evasion mechanisms to avoid recognition (7). Pericystic inflammation at this initial stage is minimal or nonexistent. At some point, the host's immune system detects the parasite and launches a cellular response with local perilesional inflammation that gradually leads to the death of the cyst. The fluid inside the cyst becomes turbid and dense, the cyst shrinks, and remnant parasite tissue is eventually cleared or replaced with a residual calcification (Fig. 1).
FIG 1.
Macroscopic views of cysticerci in different stages of involution.
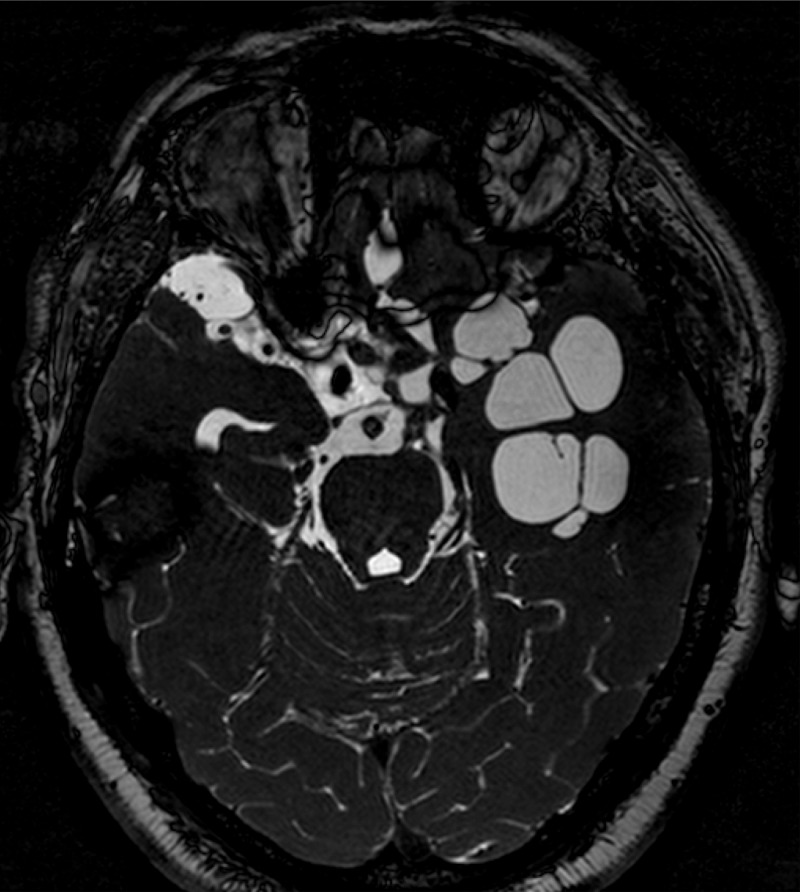
In contrast, cysts that develop in the subarachnoid spaces may not result in rounded vesicles. Without the constraints of surrounding brain parenchyma, the cyst membrane may infiltrate and grow into neighboring spaces and cavities, resulting in large cystic structures or cyst clumps invading wide areas of the subarachnoid space (Fig. 2). This is frequently accompanied by a profuse inflammatory reaction characterized by CSF pleocytosis, an elevated protein concentration, and low glucose. Cysts in the ventricles are usually individual vesicles that frequently do not cause symptoms, although in some cases, cysts may block CSF circulation leading to hydrocephalus.
FIG 2.
Basal subarachnoid neurocysticercosis (magnetic resonance imaging).
The location of the parasites in the human nervous system determines the clinical manifestations of the infection. Parenchymal brain cysts primarily manifest with seizures and epilepsy, though headache, focal signs, and cognitive deficits are not uncommon. Ventricular and subarachnoid cysts present as space-occupying lesions with or without hydrocephalus and with headache and intracranial hypertension as the most frequently associated symptoms.
Evolution of the immunological diagnosis in NCC.
The initial attempts at immunodiagnosis date back to complement fixation described by Weinberg in 1909 and later adapted by Nieto in Mexico in the early 1940s (8). Hemagglutination and radioimmunoassay were used for many years despite suboptimal sensitivity and specificity (9). Soon after the advent of the enzyme-linked immunosorbent assay (ELISA), several teams applied this technique to cysticercosis with good results (10–13), though cross-reactions with other helminth infections (including Echinococcus, Hymenolepis, and Schistosoma, among others) were frequent. In 1989, the introduction of the enzyme-linked immunoelectrotransfer blot using lentil lectin-bound glycoproteins (LLGP-EITB) significantly improved the performance of immunodiagnosis.
All of the above-described assays are based on the detection of antibodies, taking advantage of the multiplier effect of the antibody production system in the host. The detection of antigen was deemed poorly efficient (14, 15) until the use of monoclonal antibodies (MAbs) enabled improved ELISAs. Two assays for veterinary use detecting Taenia saginata cysticercosis in cattle were developed in Europe using MAbs against T. saginata, one using the HP10 MAb from Edinburgh (16) and the other using the B158-B60 antibodies from Antwerp (17). These MAbs are cross-reactive with T. solium in pigs and Taenia ovis in sheep but not with other common cestodes that infect humans, such as Hymenolepis nana or Echinococcus granulosus. These assays were initially reported to be useful to support the diagnosis of NCC using CSF (15) and were subsequently demonstrated to have similar utility for serum (18–21) and urine (22, 23).
ANTIBODY DIAGNOSIS
Assays.
The reference assay for antibody detection is the LLGP-EITB (9). This assay uses a lentil lectin-purified glycoprotein antigen mixture that is separated by gel electrophoresis, transferred to nitrocellulose paper, and then cut into strips. A strip is placed in a well containing the sample (usually serum or CSF) and incubated overnight. Conjugated goat anti-human IgG antibody is then added to reveal antigen-antibody reactions that appear as dark bands on the strip. Reactions to one or more of the seven LLGP antigens are considered positive.
In clinical settings, the diagnostic performance of the LLGP-EITB performed in serum samples is very high, approaching 98% sensitivity and 100% specificity in patients with more than one viable brain cysticercosis cyst (9). In patients with a single viable or degenerating cyst, the sensitivity is lower (60% to 70%) (24). The presence of circulating antibodies detectable by LLGP-EITB in patients with only calcified lesions is extremely variable and likely affected by the burden of the original infection and the time since resolution. Currently, the LLGP-EITB assay is available through the CDC Parasitic Disease Reference Laboratory for clinical diagnosis in U.S. cases.
Antibody detection ELISAs have mostly used semipurified somatic parasite or cyst fluid antigens. Their performance in general is poor, with suboptimal sensitivity and frequent cross-reactions with other common cestode infections such as hymenolepiasis or hydatid disease (25). Some authors suggest that antibody detection in an ELISA is more specific and substantially more sensitive when performed in CSF rather than in sera. In research settings, good test accuracies have been obtained in several platforms of ELISAs (traditional ELISA, FAST-ELISA, and QuickELISA) using recombinant or synthetic antigens (26–28), though these assays have not yet become commercially available.
Antigens.
A systematic list of antigens used for antibody diagnosis in cysticercosis can be found in the article by Rodriguez et al. (24). In short, the first antigen characterized was the dominant antigen B, which was described in Mexico in 1980. As the use of antigen B did not demonstrate much advantage over other antigen sources, and further characterizations of antigenic proteins were carried out. After the LLGP antigens were characterized and applied in the EITB format, this assay became the reference assay for serodiagnosis. Other assays based on the LLGP antigens have been developed (9, 26–31). The LLGPs belong to three families, including the GP50, T24, and 8-kDa families.
The GP50 protein is a glycosylated and glycosylphosphatidylinositol (GPI)-anchored membrane protein. The native protein migrates at 50 kDa, but the predicted molecular weight of the mature protein is 28.9. Expressed in a baculovirus expression system, recombinant GP50 in an EITB assay showed 100% specificity for cysticercosis and 90% sensitivity for cysticercosis-positive serum samples reactive with the GP50 component of LLGP (30).
The T24 protein is an integral membrane protein that belongs to the tetraspanin superfamily. It migrates at a position corresponding to 24 kDa and as a homodimer at 42 kDa. A portion of T24, the large extracellular loop domain, was expressed in an immunologically reactive form in insect cells and also in bacterial cells. When tested in a EITB assay with several well-defined batteries of serum samples (ranging from 149 to 249 NCC cases, as well as from 131 to 401 negative controls), this protein, T24H, has a sensitivity of 94% for detecting cases of cysticercosis with two or more viable cysts and a specificity of 98% (27, 29, 31).
The 8-kDa proteins are the diagnostic proteins found at 14, 18, and 21 kDa lentil lectin-bound fraction from urea-solubilized cysticerci and are also found in the bands at 24 and 39 to 42 kDa of LLGP. The 8-kDa family is likely composed of extracellular secreted proteins that accumulate in the cyst fluid. This family consists of 4 clades of proteins (TsRS1, TsRS2, Ts14, and Ts18), and from each representative clade, a synthetic peptide has been produced and evaluated as a diagnostic antigen.
Samples.
Serum samples are preferred for antibody diagnosis. CSF has the advantage of being in more direct contact with the CNS infection, but its collection requires a lumbar puncture, an invasive and moderately painful procedure that is poorly accepted in some cultures. In general, antibody detection by EITB is similarly sensitive in serum as in CSF, and antibody detection by ELISA seems higher in CSF (32). However, CSF examination may complement the serological diagnosis and also add information such as cell counts and CSF biochemistry. Antibodies can be also found in saliva samples (32).
Antibody profiles by type of NCC.
The presence of specific antibodies does not definitively indicate active cysticercosis infection, since antibodies can result from exposure to the parasite and from infections that did not establish or were resolved at very early stages. In fact, a positive LLGP-EITB result can be found in up to 20% to 25% of some rural populations where the parasite is endemic and all of these scenarios are present (33). Moreover, in settings of endemicity, transient antibody responses have been reported to be relatively common in both humans and pigs, suggesting that exposure is frequent and does not necessarily result in sustained seropositivity (34). However, the strength of the antibody response, and the particular profile of individual LLGP reactions, can provide useful information in the clinical setting. Positive antibody responses in asymptomatic people from populations where the parasite is endemic are usually characterized by weak reactions against GP50 only or to GP50 and GP42-39. In contrast, antibody reactions in clinical cases are much stronger and frequently involve all the LLGP families. We have been able to dilute a serum sample from a patient with subarachnoid NCC 16 times before the EITB became negative (Fig. 3). A recent study described the associations between LLGP-EITB antibody banding patterns and brain imaging findings in 548 NCC cases. Samples with a negative result or with only antibodies to GP50 were associated with nonviable or single viable parenchymal cysticerci, and samples with low-molecular-weight antibodies (8-kDa family) were more likely to be from extraparenchymal NCC or multiple viable intraparenchymal cysts (35).
FIG 3.

Sustained EITB response despite sequential (2-fold) dilutions of a strong positive serum sample.
Longevity of antibody responses in NCC.
One of the drawbacks of antibody detection is that the life span of the antibody response is extremely variable and likely dependent on the immune history of the host, the burden of infection, and other variables. Thus, some individuals with infections involving multiple cysts may have persistently detectable antibodies on LLGP-EITB years after successful antiparasitic treatment, while others may become seronegative after 9 months or so (36). While seroreversion to negative is a marker of cure, waning of the antibody response over less than a year is not the norm in most patients. Antibodies to the 8-kDa antigens are the first to disappear, followed by antibodies to GP24 and GP42-39. Antibodies to GP50 are the most persistent. Patients presenting with only calcified NCC lesions may still have persistently positive reactions; the positive EITB result in these cases does not necessarily imply the presence of undetected viable parasitic cysts, particularly in cases with weak or decreasing antibody responses. On the other hand, strong LLGP-EITB reactions (4 to 7 bands) in a patient with calcified NCC may indicate the presence of viable cysts, though the predictive value is lower than that of antigen detection (35).
ANTIGEN DETECTION
A diagnosis by antigen detection is limited by the amount of circulating antigens that are produced or released from the parasites, unlike the antibody responses that have been amplified by the host's immune system.
Assays.
The introduction of MAb-based antigen detection ELISAs improved the specificity of the tests and enabled their use for the diagnosis of human NCC. These assays use a sandwich ELISA technique with one MAb as the capture antibody and a different MAb as the detection antibody.
Antibodies.
Two assays have been described in the literature. One of them uses HP10 and HP6 antigens, and another assay from a different group utilizes MAbs B158 and B60. All of these MAbs were developed against Taenia saginata but cross-react with Taenia solium, enabling the diagnosis of viable cysticercosis. The HP10 and HP6 monoclonal antibody pairs were developed by injecting antigens from viable cysts into mice and were screened against lentil lectin-adherent glycoproteins from T. saginata cysts (16, 17). The HP10 monoclonal antibody is an IgM class antibody and recognizes an epitope present on a heterogeneous group of phosphorylcholine-bearing, lentil lectin-adherent trichloroacetic acid-soluble glycoproteins present on the surface and in the secretions of the T. saginata cysticerci (16).
The B158 and B60 monoclonal antibodies were developed against the excretory secretory antigens of T. saginata-viable cysticerci. These monoclonal antibodies are of an IgM antibody class and recognize bands at 87 kDa and 100 kDa of somatic extracts of adult T. saginata and also at 65 kDa from excretory-secretory antigens of T. saginata cysticerci (17). The performances of these assays appear to be comparable. One commercial version of the B158 assay (cysticercosis AG ELISA; ApDia, Turnhout, Belgium) is available in the United States.
Samples.
Antigen detection was initially reported using CSF but later also reported to be possible using serum and urine. While there are no published controlled data, antigen levels appear to be higher in CSF than in serum. As mentioned above for antibody detection, serum is the preferred sample.
The same advantages (sample being in more direct contact with the CNS infection) and disadvantages (more invasive and less acceptable) apply regarding CSF versus the other samples types.
Antigen profiles by type of NCC.
Detectable levels of circulating antigen demonstrate the presence of live parasite cysts in the host. Patients with only calcified NCC should therefore be antigen negative, and a positive result in this scenario should make the clinician suspect that viable lesions have been missed by imaging. The results of antigen testing are frequently negative in patients with a degenerating cyst or with one or a few viable parenchymal cysts and consistently positive in patients with several viable parenchymal cysts. The levels of circulating antigens are very high in patients with subarachnoid NCC, to the extent of frequently saturating the assay detection limit.
Longevity of antigen responses in NCC.
Unlike circulating antibodies, antigen levels drop fairly quickly after a successful course of antiparasitic therapy in which all viable parasites are destroyed. The resolution of extensive subarachnoid NCC may, however, take several courses of antiparasitic treatment.
Role of immunological diagnosis in the diagnosis of NCC.
Understanding how immunological assays can best contribute to the diagnosis of NCC is not as intuitive as it may sound. To begin with, brain imaging is a key part of the evaluation of a patient suspected to have NCC and should be used to establish the diagnosis, define the key characteristics of the infection, and determine the medical or surgical treatment approaches. The diagnosis suggested by imaging needs to be considered when evaluating serological results. For example, it is important to consider that the levels of antigens and antibodies vary enormously depending on the stage and number of the parasites present. In general, it is to be expected that most patients with viable infections would be seropositive for both antibodies and antigens. However, patients with a single lesion may test negative for either, and patients with a low infection burden may be antigen negative but positive for antibodies. Patients with only calcified lesions are typically antigen negative, and many of them will also be antibody negative, though a proportion of them will continue having detectable levels of circulating antibodies due to the persistence of the response months or years after the parasites have died. Subarachnoid NCC is commonly associated with very high levels of circulating antigens and antibodies; thus, a negative or weak result should raise questions about the diagnosis.
Role of immunological diagnosis to screen for cysticercosis or NCC infections.
A frequent question is whether immunodiagnostic assays should be used to identify people suspected of having NCC in areas of endemicity where confirmatory brain imaging is not possible. We contend that the utility of this approach is limited, as immunodiagnosis using the tests that are currently available would not modify the clinical management for the vast majority of people with either asymptomatic or symptomatic NCC. In addition, most experts will not prescribe antiparasitic treatment in the absence of brain imaging, because the risks associated with the resulting inflammatory response depend greatly on the number and location of viable cysts present.
At the population level, most individuals with asymptomatic NCC will have only calcified disease. While it is not known what proportion of these will develop epilepsy or other neurological symptoms, clinical management is limited to the administration of antiepileptic drugs or other symptomatic measures regardless of serologic status. A smaller proportion of individuals with NCC will have viable or degenerating cysts. Again, an unknown but likely small proportion will go on to develop symptoms, and the prognosis with or without antiparasitic treatment is favorable. However, an even smaller proportion will have a large CNS cyst burden (many cysts) or early subarachnoid NCC involvement, with substantial risk of disease progression or complications. In our assessment, the only potential contribution of an immunological screening test for NCC would be to identify this small subset, as early intervention could potentially improve the prognosis and reduce long-term costs. These potential benefits have yet to be demonstrated. Clinical management of these patients also requires brain imaging.
The scenario is similar for individuals with symptomatic NCC. The majority will have epilepsy secondary to parenchymal brain cysticercosis and should be managed with antiepileptic drugs regardless of serologic status. There is no indication for antiparasitic treatment in the sizable proportion of clinical cases presenting with calcified NCC only. Although symptomatic individuals with few viable cysts may benefit from antiparasitic drugs, the clinical prognosis with symptomatic management is again favorable, and the blind use of antiparasitic treatment in cases with cysts in delicate locations such as the brainstem or individuals with large cyst burdens may be deleterious or even lethal. Those with severe neurologic manifestations such as intracranial hypertension require referral for brain imaging, and neurosurgery will be indicated independent of whether the symptoms are due to NCC. As mentioned above, the utility of screening may be limited to identifying those individuals with a heavy cyst burden or subarachnoid involvement.
Future trends in immunological diagnosis.
The limited availability of the LLGP-EITB is a serious drawback. The preparation of the antigens used on the test strips requires a complex purification process that is both expensive and difficult to standardize, as well as dependent on the availability of parasite material. Because of this complex and labor-intensive process, the adaptation to a version involving titers does not seem a practical alternative. In recent years, however, representative proteins for all three antigenic protein families have been developed in either recombinant (rGP50 and rT24) or synthetic (sTSRS1, sTS18var1, sTSRS2var1, and sTs14) forms that may reduce costs and improve assay reproducibility. Standardized EITB assays using these new antigens are now available in the research setting (26–31) but require validation in a variety of settings of endemicity to better understand their performance and limitations. These efforts are under way.
Another drawback is that the result is qualitative and requires considerable experience for the correct interpretation of the banding profile. A quantitative, multiplex bead-based assay, such as the Luminex platform, offers the possibility of simultaneously detecting cysticercosis antigens as well as quantifying the antibody response to each specific cysticercosis antigen. A quantitative assay would provide an estimate of the intensity of the antibody response (improving diagnostic accuracy) and would also enable a direct comparison of antibody levels between samples (providing a guide for monitoring therapy or following the evolution of the infection). This platform assay also enables the possibility of combining testing for cysticercosis with other diseases, which could be beneficial for integrated control programs. With respect to antigen detection, new MAbs that are specific to Taenia solium and that have greater binding capacity to improve detection sensitivity are needed.
ACKNOWLEDGMENTS
Other members of the CWGP include Robert H. Gilman, Armando E. Gonzalez, and Victor C. W. Tsang (coordination board); Silvia Rodriguez, Manuel Martinez, Isidro Gonzales, and Herbert Saavedra (Instituto Nacional de Ciencias Neurológicas, Lima, Perú); Manuela Verastegui, Javier A. Bustos, Mirko Zimic, Holger Mayta, Yesenia Castillo, and Yagahira Castro (Universidad Peruana Cayetano Heredia, Lima, Perú); Maria T. Lopez and Cesar M. Gavidia (School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima, Perú); Luz M. Moyano, Ricardo Gamboa, Claudio Muro, and Percy Vilchez (Cysticercosis Elimination Program, Tumbes, Perú); Theodore E. Nash and Siddhartha Mahanty (NIAID, NIH, Bethesda, MD); and Jon Friedland (Imperial College, London, UK).
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors have no competing interests to declare.
Contributor Information
Colleen Suzanne Kraft, Emory University.
for The Cysticercosis Working Group in Peru:
Robert H. Gilman, Armando E. Gonzalez, Victor C. W. Tsang, Silvia Rodriguez, Manuel Martinez, Isidro Gonzales, Herbert Saavedra, Manuela Verastegui, Javier A. Bustos, Mirko Zimic, Holger Mayta, Yesenia Castillo, Yagahira Castro, Maria T. Lopez, Cesar M. Gavidia, Luz M. Moyano, Ricardo Gamboa, Claudio Muro, Percy Vilchez, Theodore E. Nash, Siddhartha Mahanty, and Jon Friedland
REFERENCES
- 1.Garcia HH, Nash TE, Del Brutto OH. 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13:1202–1215. doi: 10.1016/S1474-4422(14)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton CR, Garcia HH. 2012. Epilepsy in poor regions of the world. Lancet 380:1193–1201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- 3.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, Dickey M, Reynolds S, Stoner JA. 2010. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis 4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neal SE, Flecker RH. 2015. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg Infect Dis 21:969–976. doi: 10.3201/eid2106.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshino K. 1933. Studies on the post-embryonal development of Taenia solium: III. On the development of Cysticercus cellulosae within the definitive intermediate host. J Med Assoc Formosa 32:166–169. [Google Scholar]
- 6.Dixon HB, Lipscomb FM. 1961. Cysticercosis: an analysis and follow-up of 450 cases, vol 299 Medical Research Council, London, United Kingdom. [Google Scholar]
- 7.White AC Jr, Robinson P, Kuhn R. 1997. Taenia solium cysticercosis: host-parasite interactions and the immune response. Chem Immunol 66:209–230. doi: 10.1159/000058663. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakhar S, Singh G. Taenia solium: a historical note, p 157–168. In Singh G, Prabhakhar S (ed), Taenia solium cysticercosis: from basic to clinical science. CABI Publishing, Oxon, United Kingdom. [Google Scholar]
- 9.Tsang VC, Brand JA, Boyer AE. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Arambulo PV III, Walls KW, Bullock S, Kagan IG. 1978. Serodiagnosis of human cysticercosis by microplate enzyme-linked immunospecific assay (ELISA). Acta Trop 35:63–67. [PubMed] [Google Scholar]
- 11.Coker-Vann M, Brown P, Gajdusek DC. 1984. Serodiagnosis of human cysticercosis using a chromatofocused antigenic preparation of Taenia solium cysticerci in an enzyme-linked immunosorbent assay (ELISA). Trans R Soc Trop Med Hyg 78:492–496. doi: 10.1016/0035-9203(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 12.Diwan AR, Coker-Vann M, Brown P, Subianto DB, Yolken R, Desowitz R, Escobar A, Gibbs CJ Jr, Gajdusek DC. 1982. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibody to cysticerci of Taenia solium. Am J Trop Med Hyg 31:364–369. doi: 10.4269/ajtmh.1982.31.364. [DOI] [PubMed] [Google Scholar]
- 13.Costa JM, Ferreira AW, Makino MM, Camargo ME. 1982. Spinal fluid immunoenzymatic assay (ELISA) for neurocysticercosis. Rev Inst Med Trop Sao Paulo 24:337–341. [PubMed] [Google Scholar]
- 14.Tellez Giron E, Ramos MC, Dufour L, Montante M. 1984. Use of the ELISA method in the diagnosis of cysticercosis. Bol Oficina Sanit Panam 97:8–13. (In Spanish.) [PubMed] [Google Scholar]
- 15.Correa D, Sandoval MA, Harrison LJ, Parkhouse RM, Plancarte A, Meza-Lucas A, Flisser A. 1989. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg 83:814–816. doi: 10.1016/0035-9203(89)90340-4. [DOI] [PubMed] [Google Scholar]
- 16.Harrison LJS, Joshua GWP, Wright SH, Parkhouse RME. 1989. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol 11:351–370. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 17.Brandt JRA, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, Falla N. 1992. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol 22:471–477. doi: 10.1016/0020-7519(92)90148-E. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez S, Dorny P, Tsang VCW, Pretell EJ, Brandt J, Lescano AG, Gonzalez AE, Gilman RH, Garcia HH. 2009. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis 199:1345–1352. doi: 10.1086/597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamora H, Castillo Y, Garcia HH, Pretell J, Rodriguez S, Dorny P, Gonzalez AE, Gilman RH, Tsang VCW, Brandt J. 2005. Drop in antigen levels following successful treatment of subarachnoid neurocysticercosis. Am J Trop Med Hyg 73(6):S41. [Google Scholar]
- 20.Bobes RJ, Hernández M, Márquez C, Fragoso G, García E, Parkhouse RME, Harrison LJS, Sciutto E, Fleury A. 2006. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med IntHealth 11:943–950. doi: 10.1111/j.1365-3156.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel S, Blocher J, Dorny P, Abatih EN, Schmutzhard E, Ombay M, Mathias B, Winkler AS. 2012. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis 6:e1851. doi: 10.1371/journal.pntd.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paredes A, Sáenz P, Marzal MW, Orrego MA, Castillo Y, Rivera A, Mahanty S, Guerra-Giraldez C, García HH, Nash TE. 2016. Anti-Taenia solium monoclonal antibodies for the detection of parasite antigens in body fluids from patients with neurocysticercosis. Exp Parasitol 166:37–43. doi: 10.1016/j.exppara.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo Y, Rodriguez S, García HH, Brandt J, Van Hul A, Silva M, Rodriguez-Hidalgo R, Portocarrero M, Melendez DP, Gonzalez AE, Gilman RH, Dorny P, Cysticercosis Working Group in Peru. 2009. Urine antigen detection for the diagnosis of human neurocysticercosis. Am J Trop Med Hyg 80:379–383. [PubMed] [Google Scholar]
- 24.Rodriguez S, Wilkins P, Dorny P. 2012. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health 106:286–298. doi: 10.1179/2047773212Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia HH, Castillo Y, Gonzales I, Bustos JA, Saavedra H, Jacob L, Del Brutto OH, Wilkins PP, Gonzalez AE, Gilman RH, Cysticercosis Working Group in Peru. 2018. Low sensitivity and frequent cross-reactions in commercially available antibody detection ELISA assays for Taenia solium cysticercosis. Trop Med Int Health 23:101–105. doi: 10.1111/tmi.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YM, Handali S, Hancock K, Pattabhi S, Kovalenko VA, Levin A, Rodriguez S, Lin S, Scheel CM, Gonzalez AE, Gilman RH, Garcia HH, Tsang VC. 2011. Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in QuickELISA. Am J Trop Med Hyg 84:587–593. doi: 10.4269/ajtmh.2011.10-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-González A, Noh J, Perteguer MJ, Garate T, Handali S. 2017. Comparison of T24H-his, GST-T24H and GST-Ts8B2 recombinant antigens in Western blot, ELISA and multiplex bead-based assay for diagnosis of neurocysticercosis. Parasit Vectors 10:237. doi: 10.1186/s13071-017-2160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hancock K, Khan A, Williams FB, Yushak ML, Pattabhi S, Noh J, Tsang VCW. 2003. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J Clin Microbiol 41:2577–2586. doi: 10.1128/JCM.41.6.2577-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noh J, Rodriguez S, Lee YM, Handali S, Gonzalez AE, Gilman RH, Tsang VCW, Garcia HH, Wilkins PP. 2014. Recombinant protein- and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol 52:1429–1434. doi: 10.1128/JCM.03260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock K, Pattabhi S, Greene RM, Yushak ML, Williams F, Khan A, Priest JW, Levine MZ, Tsang VCW. 2004. Characterization and cloning of GP50, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol 133:115–124. doi: 10.1016/j.molbiopara.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, Gonzalez AE, Gilman RH, Tsang VCW. 2006. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol 147:109–117. doi: 10.1016/j.molbiopara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Bueno EC, Vaz AJ, Machado LD, Livramento JA. 2000. Neurocysticercosis: detection of IgG, IgA and IgE antibodies in cerebrospinal fluid, serum and saliva samples by ELISA with Taenia solium and Taenia crassiceps antigens. Arq Neuropsiquiatr 58:18–24. doi: 10.1590/S0004-282X2000000100003. [DOI] [PubMed] [Google Scholar]
- 33.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, Gonzalez AE, Tsang VC, Gilman RH, Garcia HH, Cysticercosis Working Group in Peru. 2005. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology 65:229–233. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 34.Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, Verastegui M, Wilkins P, Tsang VCW, Cysticercosis Working Group in Peru. 2001. Short report: transient antibody response in Taenia solium infection in field conditions-a major contributor to high seroprevalence. Am J Trop Med Hyg 65:31–32. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 35.Arroyo G, Rodriguez S, Lescano AG, Alroy K, Bustos JA, Santivanez S, Gonzales I, Saavedra H, Pretell EJ, Gonzalez AE, Gilman RH, Tsang VCW, Garcia HH, Cysticercosis Working Group in Peru. 2018. Antibody banding patterns of the enzyme-linked immunoelectrotransfer blot (EITB) and brain imaging findings in patients with neurocysticercosis. Clin Infect Dis 66:282–288. doi: 10.1093/cid/cix774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VCW, Martinez M, Altamirano J, Trelles L, Cuba JM, Alvarado M, Alban G, Estrada H, Rios-Saavedra N, Soto M, Torres MP, Boero J, Gavidia C, Barron E. 1997. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. J Infect Dis 175:486–489. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]