Chlamydia trachomatis serological assays with improved sensitivity over commercially available assays are needed to evaluate the burden of C. trachomatis infection and the effectiveness of prevention efforts. We evaluated the performance of a C. trachomatis outer membrane complex protein B (OmcB) enzyme-linked immunosorbent assay (ELISA) in the detection of anti-C. trachomatis antibody responses in C. trachomatis-infected women.
KEYWORDS: Chlamydia trachomatis, ELISA, OmcB
ABSTRACT
Chlamydia trachomatis serological assays with improved sensitivity over commercially available assays are needed to evaluate the burden of C. trachomatis infection and the effectiveness of prevention efforts. We evaluated the performance of a C. trachomatis outer membrane complex protein B (OmcB) enzyme-linked immunosorbent assay (ELISA) in the detection of anti-C. trachomatis antibody responses in C. trachomatis-infected women. OmcB ELISA was less sensitive than our C. trachomatis elementary body (EB) ELISA, but it was highly specific. The magnitude of the antibody response was higher in African-Americans and those with prior C. trachomatis infection. Unlike EB ELISA, the IgG1 response to C. trachomatis OmcB was short-lived and was not maintained by repeat C. trachomatis infection.
INTRODUCTION
Chlamydia trachomatis infection is the most prevalent sexually transmitted bacterial infection in the world (1) and can lead to significant reproductive complications, including infertility and increased risk for HIV transmission (2). Although CDC-recommended treatment for genital C. trachomatis infection is highly efficacious (3), the number of C. trachomatis cases in the United States reported to the Centers for Disease Control and Prevention (CDC) continues to rise (4). Thus, improved C. trachomatis infection prevention strategies, such as an effective C. trachomatis vaccine, are needed. C. trachomatis vaccine development will require a thorough understanding of the natural history of infection, as well as accurate C. trachomatis serological assays for determining the serostatus of the target population. Not only does C. trachomatis serology provide information on the burden of infection in a population, but it can also be useful in surveillance research, evaluating changes in C. trachomatis seroprevalence over time and the effectiveness of prevention efforts.
To date, several serological assays have been used for the serodiagnosis of C. trachomatis infection. Microimmunofluorescence, which detects antibodies to C. trachomatis elementary bodies (EBs), is neither highly sensitive nor specific (5, 6). Many commercially available enzyme-linked immunosorbent assays (ELISAs), including Medac (a commercial immunoassay; Medac, IL), SeroCT IgG ELISA (Savyon Diagnostics, Israel), and IgG EIA (Ani Labsystems, Finland) are based on peptides derived from the C. trachomatis major outer membrane protein, an abundant immunogenic C. trachomatis surface protein, and have been reported to have low sensitivity (usually <80%) (7, 8). We previously utilized a C. trachomatis EB ELISA to evaluate anti-C. trachomatis IgG1 and IgG3 responses in C. trachomatis-infected patients and found it to have a higher sensitivity for detection of C. trachomatis seropositivity than the Medac assay, which measures anti-C. trachomatis IgG (9). However, the C. trachomatis EB ELISA is a more laborious assay that is not widely available; therefore, there remains great interest in the development of highly sensitive and specific ELISA-based C. trachomatis serological assays that use immunodominant C. trachomatis antigens, particularly recombinant C. trachomatis proteins. Previously, the plasmid-encoded Pgp3 ELISA was developed for the seroepidemiological analysis of C. trachomatis infection and was found to be more sensitive than but with similar specificity to commercially available ELISAs (7). C. trachomatis outer membrane complex protein B (OmcB) is a highly abundant 60-kDa cysteine-rich protein (10, 11) that has been previously considered for use in serodiagnostic assays for C. trachomatis infection (12, 13) because of its strong immunogenicity with both B-cell and T-cell epitopes (14, 15). OmcB is highly conserved among Chlamydia species (70 to 80% overall identity) (13) and has been shown not only to function as an adhesin for bacterial entry into the host cell (16, 17) but also to contribute to cell wall structural stability (18).
To address the need for development of highly sensitive and specific ELISA-based C. trachomatis serological assays that use immunodominant C. trachomatis antigens instead of EBs that can be used for seroprevalence and surveillance studies, we conducted a study whose primary objective was to evaluate the performance of a C. trachomatis OmcB ELISA in the serodiagnosis of C. trachomatis infection in women. Secondary objectives were to (i) assess the influence of patient characteristics on the magnitude of the serum antibody response to C. trachomatis OmcB, (ii) evaluate changes in the magnitude of the antibody response to C. trachomatis OmcB over time, and (iii) investigate the influence of repeat C. trachomatis infection on the magnitude of the antibody response to C. trachomatis OmcB.
MATERIALS AND METHODS
Study participants and clinical procedures.
In a prospective study of immune responses to C. trachomatis infection, we enrolled women with a recent positive-screening C. trachomatis nucleic acid amplification test (NAAT) at the time they returned to the Jefferson County Department of Health (JCDH) sexually transmitted diseases clinic in Birmingham, AL, for C. trachomatis treatment. At the baseline visit, women were interviewed, and data were collected on age, race, sexual history, hormonal contraceptive use, antibiotic use, and symptoms and signs of infection. The subjects had had a cervical swab collected for repeat C. trachomatis NAAT (Aptima Combo 2 [AC2]; Hologic, Marlborough, MA) and blood from which serum was extracted, and were then treated with azithromycin (1 g). Study participants had a 6-month follow-up visit in which repeat NAAT on a cervical swab, an interview, and phlebotomy were performed. Written informed consent was obtained from each study participant before enrollment. The study was approved by University of Alabama at Birmingham Institutional Review Board (IRB) and JCDH. The CDC determined that CDC involvement did not constitute engagement in human subjects research, and therefore a CDC IRB review was not required.
C. trachomatis OmcB ELISA.
Serum IgG1 responses were measured with a C. trachomatis OmcB ELISA by a methodology adapted from a C. trachomatis EB ELISA described previously (9, 19, 20). Briefly, Immulon 2 HB U-bottom 96-well plates (Thermo Scientific) were coated with 100 μl of recombinant OmcB (MBS1432139 [vector tag and endotoxin free]; MyBioSource) per well (0.5 μg/ml) in Tris buffer (0.05 M Tris, 0.15 M NaCl), followed by incubation overnight at 4°C. The coating solution was discarded before the ELISA plates were blocked with 2% bovine serum albumin in wash buffer (0.12 M Tris, 1.4 M NaCl, 0.03 M KCl, 0.05% Tween 20) and then incubated for 1 h at 37°C. The plates were washed three times with wash buffer. Portions (100 μl) of each serum sample diluted 1:64 in Tris buffer were added per well, followed by incubation for 1 h at 37°C. IgG1 responses were detected using alkaline phosphatase-labeled mouse anti-human IgG1 (a pool of clones 4E3 [Southern Biotech] and HP6069 [Calbiochem]) at an optical density of 405 nm (OD405).
To establish the OD405 cutoff value for a positive anti-C. trachomatis IgG1 response with the C. trachomatis OmcB ELISA, we evaluated serum samples from 75 individuals (45 Caucasian and 30 African-American) that had previously been found to be C. trachomatis seronegative by our highly sensitive and specific C. trachomatis EB ELISA in a C. trachomatis seroprevalence study (9) that evaluated adult females (aged 18 to 30 years) in the Birmingham, Alabama, community who had serum samples collected at the time of screening for a phase III genital herpes vaccine trial (21). The cutoff value for a positive anti-C. trachomatis IgG1 response by the OmcB ELISA was determined to be 1.3, calculated by the following formula: mean OD405 value + 3.45 standard deviations (22). The reported serologic responses of all participants represent the mean of triplicate determinations. To evaluate for cross-reactivity with Chlamydia pneumoniae antibodies, C. trachomatis OmcB ELISA was performed on the serum samples of 85 individuals (45 Caucasians and 40 African-Americans) who were C. trachomatis EB ELISA seronegative from the same study as above (21) and in whom C. pneumoniae EB ELISA had been performed earlier (9).
Statistics.
Difference in the proportion of C. trachomatis-infected women (based on a positive cervical C. trachomatis NAAT) who were seropositive for anti-C. trachomatis IgG1 by the C. trachomatis OmcB ELISA versus the C. trachomatis EB ELISA was evaluated by the McNemar's test. Associations of participant characteristics and reinfection status at follow-up with the magnitude of the C. trachomatis OmcB ELISA response (OD405 value) were evaluated using the Wilcoxon rank sum test and Spearman's correlation test; participant characteristics associated with magnitude of the C. trachomatis OmcB ELISA response on univariate analyses (P < 0.1) were further evaluated with a multivariate regression model. The difference in the magnitude of the C. trachomatis OmcB ELISA response from baseline to follow-up was analyzed using a Wilcoxon signed-rank test. The difference in the magnitude of the C. trachomatis OmcB ELISA response (i.e., OD readings) in C. trachomatis EB ELISA-seronegative individuals who were C. pneumoniae seropositive versus C. pneumoniae seronegative (based on prior testing with a C. pneumoniae EB ELISA [9]) was analyzed using the Wilcoxon rank sum test. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Comparison of C. trachomatis OmcB ELISA versus C. trachomatis EB ELISA for the serodiagnosis of C. trachomatis infection.
Sera from 150 women with active urogenital C. trachomatis infections based on a positive C. trachomatis NAAT at the time of treatment were tested by a C. trachomatis OmcB ELISA and C. trachomatis EB ELISA. Baseline characteristics of these 150 women are summarized in Table 1. There were 119 (79.3%) women seropositive by the OmcB ELISA, which was significantly lower than the proportion seropositive by the EB ELISA (148 [98.6%]) (P < 0.0001). These findings suggest that C. trachomatis EB ELISA is a more sensitive assay than the C. trachomatis OmcB ELISA.
TABLE 1.
Study participant characteristics collected at the baseline visit (n = 150)
| Patient characteristics | No. (%) of study participantsa |
|---|---|
| Median age in yrs (range) | 22 (16–46) |
| Race | |
| African-American | 143 (95.3) |
| Non-African-American | 7 (4.7) |
| Symptoms | |
| Yes | 68 (45.3) |
| No | 82 (54.7) |
| Prior C. trachomatis infection | 82 (54.7) |
| Median no. of sex partners over 3 mo (range) | 1 (1–9) |
| Hormonal contraceptive use | 72 (48) |
| Cervicitis | 35 (23.3) |
| PID | 4 (2.7) |
Except where noted otherwise in column 1.
Evaluation of cross-reactivity of C. pneumoniae antibodies with the C. trachomatis OmcB ELISA.
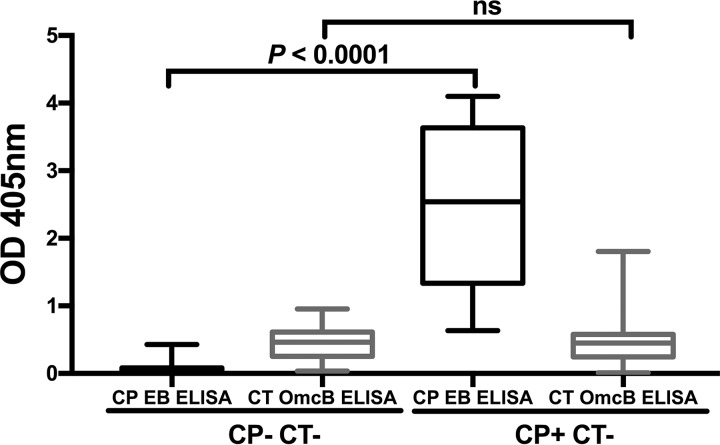
Since there is 71% identity between OmcB proteins of C. trachomatis and C. pneumoniae (13), we evaluated whether C. trachomatis seropositivity by C. trachomatis OmcB ELISA may have been confounded by cross-reacting antibodies to C. pneumoniae OmcB produced in response to C. pneumoniae infection. C. trachomatis OmcB ELISA was performed on serum samples from 85 C. trachomatis EB ELISA-seronegative individuals who were also previously tested by a C. pneumoniae EB ELISA (OD405 cutoff, 0.5) (9). There was no significant difference in the C. trachomatis OmcB ELISA median OD405 values in participants that were C. pneumoniae EB ELISA seropositive versus seronegative (0.463 versus 0.449; P = 0.920) (Fig. 1), suggesting that the C. trachomatis OmcB ELISA serological response is specific for C. trachomatis infection and not likely significantly confounded by an antibody response to C. pneumoniae infection.
FIG 1.
Cross-reactivity of C. pneumoniae antibodies with the C. trachomatis OmcB ELISA. C. trachomatis OmcB ELISA was performed and IgG1 response was measured on adults (n = 85) who were C. trachomatis seronegative based on C. trachomatis EB ELISA and either C. pneumoniae seropositive (CP+ CT−) or C. pneumoniae seronegative (CP− CT−) based on C. pneumoniae EB ELISA (9). Data for OD405 values are represented as a box-and-whiskers plot, with boxes denoting interquartile ranges and whiskers denoting the 5th and 95th percentiles. The median is shown as a horizontal line. Significance between the IgG1 responses was determined by the Wilcoxon rank sum test (ns, not significant).
Variations in the OmcB ELISA IgG1 response by patient characteristics.
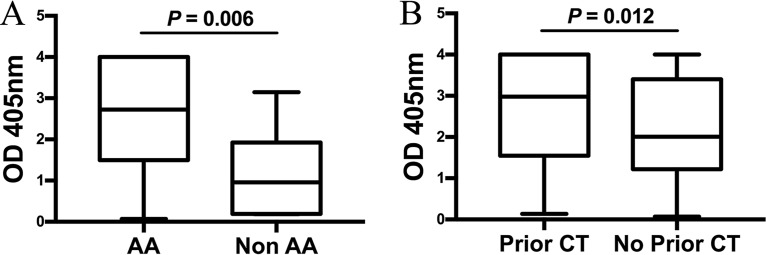
Among the 119 women who were seropositive by OmcB ELISA, the magnitude of the antibody response to C. trachomatis OmcB was significantly higher in African-American versus non-African-American women (median OD405, 2.724 versus 0.958; P = 0.006) (Fig. 2A) and in those with a history of prior C. trachomatis infection (either self-reported or by laboratory testing results documented in the medical record) versus no history of prior C. trachomatis infection (median OD405, 2.979 versus 2.008; P = 0.012) (Fig. 2B). There was no significant difference in the magnitude of the IgG1 response to C. trachomatis OmcB with respect to age, symptoms, hormonal contraception use, number of partners, or diagnosis of cervicitis or pelvic inflammatory disease (PID).
FIG 2.
Effect of race and prior C. trachomatis (CT) infection on the magnitude of the C. trachomatis OmcB ELISA IgG1 response. The magnitude of the C. trachomatis OmcB ELISA IgG1 response was compared between African-American (AA; n = 143) and non-African-American (Non AA; n = 7) women (2.724 versus 0.958; P = 0.006) (A) and women with versus without prior C. trachomatis infection based on self-report or documentation of laboratory test results (2.979 versus 2.008; P = 0.01) (B). Data for OD405 values are represented as a box-and-whiskers plot, with boxes denoting interquartile ranges and whiskers denoting the 5th and 95th percentiles. The median is shown as a horizontal line. Significance between the IgG1 responses was determined by a Wilcoxon rank sum test.
In a multivariate linear regression model that evaluated the independent effects of race and prior C. trachomatis infection on the magnitude of OmcB antibody response, race remained significantly associated with a higher magnitude of OmcB antibody response (P = 0.013), and there remained a trend toward an association of prior C. trachomatis infection with a higher C. trachomatis OmcB response (P = 0.070).
Change in the magnitude of the IgG1 response to C. trachomatis OmcB between the time of C. trachomatis infection treatment and a 6-month follow-up visit.
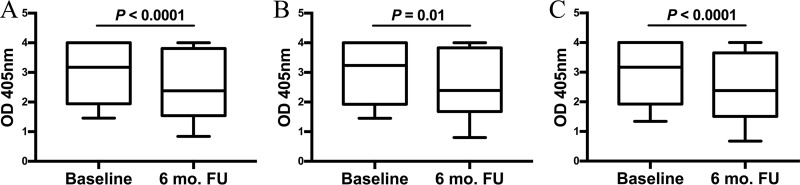
Among participants with active urogenital C. trachomatis infection who were C. trachomatis seropositive by the C. trachomatis OmcB ELISA at baseline (n = 119), we performed C. trachomatis OmcB ELISA on 95 of these participants who had sera available from a 6-month follow-up visit to evaluate for change in magnitude of the IgG1 response to C. trachomatis OmcB over a 6-month time period. There was a significant decline in the magnitude of the IgG1 response from baseline to follow-up (median OD, 3.175 versus 2.383; P < 0.0001), and only 83 (87.3%) participants remained C. trachomatis seropositive at follow-up (Fig. 3A). We also evaluated whether the presence of C. trachomatis reinfection at the follow-up visit (based on a positive cervical C. trachomatis NAAT) influenced the magnitude of the IgG1 response to OmcB. There were 21 (22.1%) participants who had C. trachomatis reinfection at follow-up. We found a similar decline in the magnitude of the IgG1 response between treatment and follow-up visits in those with reinfection (median OD405, 3.236 versus 2.393; P = 0.01) versus without reinfection (median OD405, 3.171 versus 2.380; P < 0.0001) (Fig. 3B and C); the fold reduction in the IgG1 response was the same for both the groups at 1.3, and the median OD405 of the IgG1 response at the follow-up visit did not differ in those with versus without reinfection (P = 0.77).
FIG 3.
Comparison of the magnitude of the C. trachomatis OmcB ELISA IgG1 response at a baseline versus 6-month follow-up visit. The magnitude of the C. trachomatis OmcB ELISA IgG1 response was compared between the baseline and follow-up (6 mo. FU) visits in all women (n = 95) (A), women with reinfection at follow-up (n = 21) (B), and women without reinfection at follow-up (n = 74) (C). Data for OD405 values are represented as a box-and-whiskers plot, with boxes denoting interquartile ranges and whiskers denoting the 5th and 95th percentiles. The median is shown as a horizontal line. Significance between the IgG1 responses was determined by a Wilcoxon signed-rank test.
DISCUSSION
To address the need for C. trachomatis serological assays with improved sensitivity over commercially available assays, we developed an ELISA using the highly immunogenic and abundant C. trachomatis outer membrane protein OmcB and evaluated its performance for the serodiagnosis of C. trachomatis infection in women. The C. trachomatis OmcB ELISA had a sensitivity of 83.3%, which was similar to earlier studies that had also evaluated OmcB ELISAs for the serodiagnosis of C. trachomatis infection (12, 23) and higher than the sensitivity of the commercially available Medac assay (∼70%) (8). However, the sensitivity of the C. trachomatis OmcB ELISA was significantly lower than our C. trachomatis EB ELISA.
Since OmcB is highly conserved among the chlamydial species, there is the potential for cross-reactivity of the C. trachomatis OmcB ELISA with the C. pneumoniae OmcB (24). Although some studies have shown a low specificity for the C. trachomatis OmcB ELISA due to cross-reacting antibodies (12, 23), other reports have suggested that high specificity of C. trachomatis OmcB ELISA can be achieved using a truncated C. trachomatis OmcB protein (13, 25). We found no significant difference in the C. trachomatis OmcB ELISA IgG1 responses in sera from individuals who were determined to be C. pneumoniae seropositive and C. trachomatis seronegative versus those who were determined to be C. pneumoniae seronegative and C. trachomatis seronegative by the C. pneumoniae and C. trachomatis EB ELISAs. The C. trachomatis-specific antibody responses our OmcB ELISA detected may have been facilitated in part by the fact that we used a tagless, endotoxin-free recombinant full-length mature C. trachomatis OmcB protein, in contrast to the previous studies reporting low specificity of their OmcB ELISA (12, 23). Recently, it was suggested that recombinant proteins with vector tags should be used with caution since they frequently show high reactivity with human sera (13). It is also possible that the specificity of our assay may have been further enhanced by using the predominant IgG subclass IgG1 rather than measuring total IgG, which may have more “background noise” in measuring anti-C. trachomatis antibody responses.
To our knowledge, this is the first study to investigate the influence of patient characteristics on the magnitude of IgG1 response to C. trachomatis OmcB. We found that the magnitude of the IgG1 response to C. trachomatis OmcB was higher in African-Americans and individuals with prior C. trachomatis infections. Interestingly, we previously demonstrated that African-American ethnicity and prior C. trachomatis infections were associated with a higher magnitude of the IgG1 response to C. trachomatis EBs (9), suggesting that these associations might be common with other C. trachomatis antigens as well. However, our study population was only women and predominantly African-American women, which may limit the generalizability of our findings to women of other races/ethnicities and to men.
Prior to our study, there was limited information about the duration of antibody response to C. trachomatis OmcB. Portig et al. showed in a single C. trachomatis patient that antibodies against OmcB had declined to background levels on repeat testing performed during a period of 6 months to 2 years after the previous test (a more specific time frame was not given) (23). Our finding that the IgG1 response to C. trachomatis OmcB declined significantly over a 6-month period after treatment of infection suggests that the IgG1 antibody response to this recombinant protein may not be long-lived, which is in contrast to the more stable IgG1 response to C. trachomatis EBs that we previously demonstrated (9). Interestingly, the decline in the magnitude of IgG1 antibody response to C. trachomatis OmcB was similar in those with versus without reinfection at follow-up, suggesting that IgG1 levels were not maintained even by early reinfection. Studies have previously demonstrated lower C. trachomatis organism loads with subsequent infections (26), and perhaps this may in part explain why there was not a greater increase in the magnitude of the response with reinfection at 6 months.
While the short-lived IgG1 response to C. trachomatis OmcB could be useful to study timing of C. trachomatis infections in combination with other assays that detect a more long-lived IgG1 response, such as the EB ELISA, the C. trachomatis OmcB ELISA may not be useful in evaluating the cumulative burden of C. trachomatis infection or effectiveness of C. trachomatis prevention efforts. Thus, there remains the need to develop sensitive and specific assays for the serodiagnosis of C. trachomatis infection that use recombinant C. trachomatis proteins, which will be less labor-intensive and more feasible than C. trachomatis EB ELISA for large-scale C. trachomatis seroepidemiology studies and surveillance efforts. Interestingly, recent antibody studies reported that C. trachomatis OmcB was the C. trachomatis protein most often recognized in women with tubal factor infertility (27, 28), raising the possibility that this C. trachomatis antigen could contribute to immunopathology during C. trachomatis infection. Our future studies will include investigating C. trachomatis OmcB responses in women with C. trachomatis-related infertility.
ACKNOWLEDGMENTS
We thank Hanne Harbison and Cynthia Poore for their assistance in the collection of specimens and clinical data. We also thank Shelly Lensing for her assistance with the manuscript review.
This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI09369 to W.M.G.) and a CDC grant (1U48DP005037 to W.M.G.). The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Institutes of Health or Centers for Disease Control and Prevention.
REFERENCES
- 1.World Health Organization. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Fleming DT, Wasserheit JN. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisler WM, Uniyal A, Lee JY, Lensing SY, Johnson S, Perry RC, Kadrnka CM, Kerndt PR. 2015. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med 373:2512–2521. doi: 10.1056/NEJMoa1502599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2016. Sexually transmitted diseases surveillance 2015. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 5.Bas S, Muzzin P, Ninet B, Bornand JE, Scieux C, Vischer TL. 2001. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J Clin Microbiol 39:1368–1377. doi: 10.1128/JCM.39.4.1368-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong YK, Sueur JM, Fall CH, Orfila J, Ward ME. 1999. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring IgG antibodies against Chlamydia pneumoniae. J Clin Pathol 52:99–102. doi: 10.1136/jcp.52.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills GS, Horner PJ, Reynolds R, Johnson AM, Muir DA, Brown DW, Winston A, Broadbent AJ, Parker D, McClure MO. 2009. Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol 16:835–843. doi: 10.1128/CVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morre SA, Munk C, Persson K, Kruger-Kjaer S, van Dijk R, Meijer CJ, van Den Brule AJ. 2002. Comparison of three commercially available peptide-based immunoglobulin G (IgG) and IgA assays to microimmunofluorescence assay for detection of Chlamydia trachomatis antibodies. J Clin Microbiol 40:584–587. doi: 10.1128/JCM.40.2.584-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisler WM, Morrison SG, Doemland ML, Iqbal SM, Su J, Mancevski A, Hook EW III, Morrison RP. 2012. Immunoglobulin-specific responses to Chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J Infect Dis 206:1836–1843. doi: 10.1093/infdis/jis621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen JE, Stephens RS. 1989. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol 171:285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newhall WJ., V 1987. Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun 55:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bas S, Muzzin P, Vischer TL. 2001. Chlamydia trachomatis serology: diagnostic value of outer membrane protein 2 compared with that of other antigens. J Clin Microbiol 39:4082–4085. doi: 10.1128/JCM.39.11.4082-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frikha-Gargouri O, Gdoura R, Znazen A, Gargouri B, Gargouri J, Rebai A, Hammami A. 2008. Evaluation of an in silico predicted specific and immunogenic antigen from the OmcB protein for the serodiagnosis of Chlamydia trachomatis infections. BMC Microbiol 8:217. doi: 10.1186/1471-2180-8-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman KS, Chowdhury EU, Poudel A, Ruettger A, Sachse K, Kaltenboeck B. 2015. Defining species-specific immunodominant B cell epitopes for molecular serology of Chlamydia species. Clin Vaccine Immunol 22:539–552. doi: 10.1128/CVI.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodall JC, Beacock-Sharp H, Deane KH, Gaston JS. 2001. Recognition of the 60-kilodalton cysteine-rich outer membrane protein OMP2 by CD4+ T cells from humans infected with Chlamydia trachomatis. Clin Exp Immunol 126:488–493. doi: 10.1046/j.1365-2249.2001.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadel S, Eley A. 2007. Chlamydia trachomatis OmcB protein is a surface-exposed glycosaminoglycan-dependent adhesin. J Med Microbiol 56:15–22. doi: 10.1099/jmm.0.46801-0. [DOI] [PubMed] [Google Scholar]
- 17.Fechtner T, Stallmann S, Moelleken K, Meyer KL, Hegemann JH. 2013. Characterization of the interaction between the chlamydial adhesin OmcB and the human host cell. J Bacteriol 195:5323–5333. doi: 10.1128/JB.00780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett KD, Hatch TP. 1995. Architecture of the cell envelope of Chlamydia psittaci 6BC. J Bacteriol 177:877–882. doi: 10.1128/jb.177.4.877-882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner AZ, Diamond MP, Legro RS, Schlaff WD, Barnhart KT, Casson PR, Christman GM, Alvero R, Hansen KR, Geisler WM, Thomas T, Santoro N, Zhang H, Eisenberg E, Reproductive Medicine Network. 2015. Chlamydia trachomatis immunoglobulin G3 seropositivity is a predictor of reproductive outcomes in infertile women with patent fallopian tubes. Fertil Steril 104:1522–1526. doi: 10.1016/j.fertnstert.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey A, Di Canzio J, Zurakowski D. 1998. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods 221:35–41. doi: 10.1016/S0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 23.Portig I, Goodall JC, Bailey RL, Gaston JS. 2003. Characterization of the humoral immune response to Chlamydia outer membrane protein 2 in chlamydial infection. Clin Diagn Lab Immunol 10:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein M, Kotz A, Bernardo K, Kronke M. 2003. Detection of Chlamydia pneumoniae-specific antibodies binding to the VD2 and VD3 regions of the major outer membrane protein. J Clin Microbiol 41:1957–1962. doi: 10.1128/JCM.41.5.1957-1962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mygind P, Christiansen G, Persson K, Birkelund S. 1998. Analysis of the humoral immune response to Chlamydia outer membrane protein 2. Clin Diagn Lab Immunol 5:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vodstrcil LA, McIver R, Huston WM, Tabrizi SN, Timms P, Hocking JS. 2015. The epidemiology of Chlamydia trachomatis organism load during genital infection: a systematic review. J Infect Dis 211:1628–1645. doi: 10.1093/infdis/jiu670. [DOI] [PubMed] [Google Scholar]
- 27.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]