The incidence of candidemia caused by Candida albicans and Candida glabrata is constantly increasing and is accompanied by the rising use of the few available antifungals. The widespread use of echinocandins and azoles for the treatment of invasive candidemia has enhanced the development of antifungal resistance, resulting in an increasing health care problem.
KEYWORDS: Candida spp., MALDI-TOF MS, antifungal susceptibility testing
ABSTRACT
The incidence of candidemia caused by Candida albicans and Candida glabrata is constantly increasing and is accompanied by the rising use of the few available antifungals. The widespread use of echinocandins and azoles for the treatment of invasive candidemia has enhanced the development of antifungal resistance, resulting in an increasing health care problem. Hence, the rapid detection of resistant strains is required. This study aimed to evaluate the detection of C. albicans and C. glabrata strains resistant to caspofungin by the matrix-assisted laser desorption ionization Biotyper antibiotic susceptibility test rapid assay (MBT ASTRA). This novel semiquantitative technique facilitates the detection of caspofungin-resistant strains within 6 h. MBT ASTRA results were compared to the data obtained by the use of Clinical and Laboratory Standards Institute (CLSI) guidelines. Clinical isolates of C. albicans (n = 58) and C. glabrata (n = 57) were analyzed by MBT ASTRA and the CLSI microdilution method. Antifungal susceptibility testing against caspofungin by the CLSI microdilution method classified the C. albicans isolates into 36 susceptible and 22 resistant strains and the C. glabrata isolates into 5 susceptible, 33 resistant, and 19 intermediate strains. For C. albicans, the comparison of MBT ASTRA and the CLSI method revealed an excellent categorical agreement of 100%. A sensitivity and a specificity between MBT ASTRA and the CLSI microdilution method of 94% and 80%, respectively, were detected for C. glabrata strains, based on categorical agreement. In conclusion, the results obtained by MBT ASTRA indicate that this is a very promising approach for the rapid detection of Candida isolates resistant to caspofungin.
INTRODUCTION
Invasive fungal diseases are a prevalent life-threatening complication in patients who are immune suppressed, are receiving chemotherapy, suffer from hematological disorders, have received transplants, and are admitted to intensive care units (1, 2). Several Candida species cause opportunistic fungal infections and are responsible for 70 to 90% of all invasive fungal infections. Among the Candida spp., Candida albicans is the major species isolated from patients (1, 3), followed by Candida glabrata (2, 4). C. albicans is estimated to cause mortality at rates as high as 45%, which can be due to a lack of rapid diagnostic methods and/or inappropriate antifungal treatment (5). The incidence of candidemia caused by C. albicans and C. glabrata is increasing globally (5), which is corresponding to the application of only a few classes of antifungals (6, 7). Therefore, there is an apprehension that the efficacy of the major antifungals will be reduced, resulting in limited therapeutic options in future (6, 8). Caspofungin, which belongs to the class of echinocandin drugs, is one of the modern lipopeptide antifungals and is often used as initial therapy against invasive candidiasis. This drug inhibits β-1,3 glucan synthase, which is essential to the production of β-1,3 glucans, one of the important components of the fungal cell wall. In C. albicans, resistance to caspofungin is mostly a consequence of mutations in two hot spot regions in the FKS1 gene. Additionally, Suwunnakorn et al. (13) have shown that CAT1 overproduction could increase the MIC of caspofungin 2-fold. Another study demonstrated that the negative regulation of one copy of a chromosome 5 (Ch5) gene may be associated with echinocandin tolerance in C. albicans (9–13), while resistance to caspofungin in C. glabrata is related to mutations in the FKS1 and the FKS2 genes (9, 14, 15).
The fast and reliable detection of C. albicans and C. glabrata isolates resistant to caspofungin is a major prerequisite for appropriate patient management. In spite of the advantages of conventional susceptibility tests, such as the microdilution method performed according to Clinical and Laboratory Standards Institute (CLSI) or European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines, these standard methods require long incubation times and have received considerable critical attention in clinical laboratories. Recent trends in the replacement of standard microdilution methods by commercial approaches promoted antifungal susceptibility tests (AFST) that provide reliable results within somewhat shortened incubation times (16–19).
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) is an easy, rapid, cost-effective, and high-throughput technology with outstanding accuracy in species identification (20, 21). Subsequent to the identification of microorganisms, this technique has recently been applied for antimicrobial susceptibility testing (20, 22). Previously, the MALDI Biotyper antibiotic susceptibility test rapid assay (MBT ASTRA) has been described to be a novel semiquantitative technique for susceptibility testing in bacteria (23). This approach is a phenotypic assay comparing cell growth in the presence of an antibiotic to cell growth in a control setup without antibiotic. Due to the high sensitivity of MALDI-TOF MS, differences between susceptible and resistant strains are detectable within a few hours (3 to 5 h for bacteria) by semiquantitative analysis of the acquired mass spectra. In this study, MBT ASTRA was optimized and applied to the susceptibility testing of yeasts, such as C. albicans and C. glabrata.
MATERIALS AND METHODS
Strains.
Clinical isolates of C. albicans (n = 58) and C. glabrata (n = 57) obtained from three different clinical origins (Division of Hygiene and Microbiology, Medical University of Innsbruck, Innsbruck, Austria; Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; and the Max von Pettenkofer-Institut, Munich, Germany) were analyzed. Species were confirmed by the MALDI Biotyper (Bruker Daltonik GmbH, Bremen, Germany). Antifungal susceptibility testing against caspofungin by the CLSI microdilution method classified the C. albicans isolates into 36 susceptible and 22 resistant strains and the C. glabrata isolates into 5 susceptible, 33 resistant, and 19 intermediate strains. Isolates stored at −80°C were directly cultivated on Sabouraud dextrose agar (SDA) overnight at 35°C before use. The reference organisms C. parapsilosis ATCC 22019 and C. krusei (Pichia kudriavzevii) ATCC 6258, recommended by CLSI, were used as quality control strains for susceptibility testing by microdilution (CLSI). C. albicans ATCC 64548 and C. albicans ATCC 64550, recommended by EUCAST as quality control strains for caspofungin susceptibility testing, were used in testing by both microdilution and MBT ASTRA (24, 25).
Antifungal susceptibility testing.
In vitro antifungal susceptibility testing was performed by the classical microdilution method, employing 2-fold serial dilutions of caspofungin (Sigma-Aldrich, Germany) according to current CLSI guideline M60 (November 2017) (24). Breakpoints were applied according to current CLSI guideline M60 (November 2017) for C. albicans (clinical breakpoints, ≤0.25 μg/ml for susceptible, ≥1 μg/ml for resistant, and 0.5 μg/ml for intermediate) and C. glabrata (clinical breakpoints, ≤0.125 μg/ml for susceptible, ≥0.5 μg/ml for resistant, and 0.25 μg/ml for intermediate) (24). Slow-growing Candida strains that could not be visually evaluated were analyzed by use of a microplate reader (CLARIOstar/BMG Labtech, Germany) in flat-bottom microplates, in which growth was measured at 450 nm after 48 h of incubation at 37°C without shaking.
MBT ASTRA.
A cell suspension at a 0.5 McFarland standard (Grant-bio-DEN-1; Grant Instruments, England) prepared from fresh overnight cultures of individual isolates was diluted 1:20 into RPMI 1640 medium (Sigma-Aldrich, Germany) supplemented with a final concentration of 0.165 mol/liter morpholinepropanesulfonic acid (MOPS; Sigma-Aldrich, Germany) and 2% glucose (Sigma-Aldrich, Germany) and adjusted to pH 7.0 (25). Cells were incubated at 37°C for 0 h, 3 h, 6 h, and 24 h. Ten microliters of the cell suspension was used at each time point for cell counting in a Neubauer hemocytometer (Marienfeld, Germany). The average for four replicates was calculated.
The same suspension used for cell counting was also applied to the MALDI-TOF MS-based resistance assay. Twofold serial dilutions of caspofungin ranging from 0.125 up to 4 μg/ml plus a control without antifungal containing 0.5 × 105 to 2.5 × 105 cells/liter in a volume of 600 μl were prepared (25). Incubation was performed at 37°C for 6 h in a ThermoMixer (Eppendorf, Germany) for 4 h without agitation, followed by 2 h under agitation at 300 rpm. Multiwell filter plates (1-ml well; 0.45-μm-pore-size filter with a GH Polypro (GHP) membrane; Pall, USA) were employed to collect the cells after incubation by centrifugation at 4,000 × g for 5 min (5804 R centrifuge; Eppendorf, Germany). The cells were rinsed twice with 200 μl sterile deionized water and once with 100 μl 75% ethanol. Cell lysis was performed according to the MALDI Biotyper standard protocol (26) using 10.5 μl 70% formic acid (Merck, Germany) and 10.5 μl 100% acetonitrile (Roth, Germany) directly on the filter. Cell lysis using formic acid and acetonitrile was repeated twice. For MALDI-TOF MS measurements, 1 μl lysate from each setup was spotted in duplicate onto a polished steel target plate (Bruker Daltonik, Germany) and overlaid with 1 μl MALDI matrix (10 mg/ml of α-cyano-4-hydroxy-cinnamic acid [α-HCCA] in 50% acetonitrile–2.5% trifluoroacetic acid; Bruker Daltonik, Germany) containing MBT ASTRA standard II (Bruker Daltonik, Germany). MALDI-TOF MS spectra were acquired on a Microflex LT/SH mass spectrometer (Bruker Daltonik GmbH, Germany) calibrated with a bacterial test standard (BTS; Bruker Daltonik GmbH, Germany) in a mass range of from 2,000 to 20,000 Da (23).
Data analysis.
The acquired spectra were analyzed by MS ASTRA prototype software written in R according to the procedure described elsewhere (23, 27, 28) resulting in the area under the curve (AUC) for each incubation setup. The relative growth (RG) was individually calculated for each concentration of caspofungin by determining the ratio of the AUC of the antifungal setup (AUC with RPMI 1640 medium plus caspofungin [AUCRPMI + caspofungin]) to the AUC of the control (AUC with RPMI 1640 medium [AUCRPMI]): RG = (AUCRPMI + caspofungin)/(AUCRPMI).
The RG cutoff was set to 0.6 RG unit. Accordingly, strains showing RG above this threshold were considered resistant, and those revealing RG below this cutoff were considered susceptible. Further development will be necessary to improve this software and provide it to customers. The CLSI microdilution method was used as a gold standard, and the sensitivity and specificity for MBT ASTRA were calculated based on this method.
RESULTS
Optimization of MBT ASTRA.
MBT ASTRA was originally developed to detect the resistance of bacterial strains to different antibiotics within 1 to 3 h. Adaptation of this protocol for the analysis of yeasts required the definition of the minimal and sufficient incubation time in which both species showed significant growth. A collection of 20 strains (10 C. albicans and 10 C. glabrata strains), including 5 susceptible and 5 resistant strains of each species, was selected for counting of the yeast cells as described above. Monitoring of cell growth revealed that C. albicans strains resistant to caspofungin showed faster growth than susceptible strains (data not shown). The growth rate of susceptible and resistant C. glabrata strains was approximately identical. The results demonstrated that detectable cell growth started after 3 h for both species, but significant growth was observed between 5 and 6 h for all strains. An incubation time of 6 h was defined for MBT ASTRA, fulfilling the growth requirements for C. albicans and C. glabrata strains.
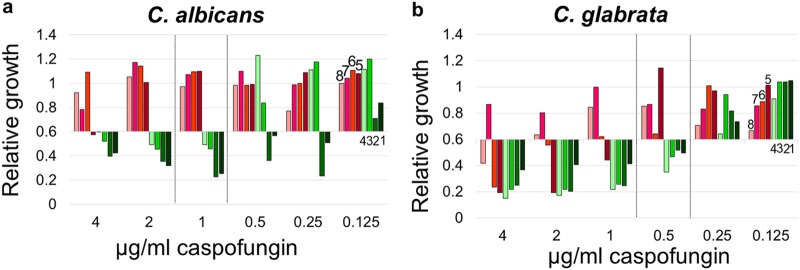
A total of 16 strains, including 4 susceptible isolates and 4 isolates resistant to caspofungin from each species, were used to optimize MBT ASTRA. Before testing, the FKS1 and FKS2 genes of these isolates were sequenced as described elsewhere (Table 1) (29), and identification was performed by the MALDI Biotyper to confirm the species identity. MBT ASTRA was performed for these strains as described above. The acquired spectra were processed by MBT ASTRA prototype software, which first calculated the area under the curve (AUC), which directly corresponds to the growth of each strain within the respective setup. This analysis revealed that the growth (AUC) varied for each strain and each analytical setup, depending on the individual growth capacity and on the resistance status of the strain. Detailed analysis of the data demonstrated that it was necessary to introduce a threshold for the minimum growth of the control setups without antibiotic to achieve a reliable categorization of the strains. For each strain passing this threshold, relative growth was calculated as a measure of the growth of the respective strain in the presence of the antifungal. A cutoff value (RG) of 0.6 was defined for both species. The titration experiments revealed that each strain changed its behavior from growth to no growth at an individual caspofungin concentration. The RG values of all C. albicans strains previously characterized as susceptible by gene sequencing were below the threshold of 0.6 (RG < 0.6) at a caspofungin concentration of 1 μg/ml or below. In contrast, the RG values of C. albicans strains that had been characterized as resistant by gene sequencing showed this switch only at caspofungin concentrations above 1 μg/ml (Fig. 1a). For C. glabrata, the same behavior was observed, with the difference being that the caspofungin breakpoint concentration for susceptible strains was 0.5 μg/ml or below and that for resistant strains was above 0.5 μg/ml (Fig. 1b). These results imply that MBT ASTRA-specific breakpoint concentrations were required for categorization of the strains. Compared to the CLSI breakpoints, these novel MBT ASTRA breakpoint concentrations were 1 dilution step above the CLSI breakpoints.
TABLE 1.
FKS1 and FKS2 gene sequencing results for C. albicans and C. glabrata strains
| Species (no. of isolates) | Strain | Genotype |
|
|---|---|---|---|
| FKS1 | FKS2 | ||
| C. albicans (8) | ATCC 64548 | WTa | |
| ATCC 64550 | WT | ||
| ATCC 24433 | WT | ||
| CBS_8758 | WT | ||
| CLF_11 | S645P | ||
| CLF_41 | F641S | ||
| CLF_52 | S645Y | ||
| CLF_82 | P649H | ||
| C. glabrata (8) | ATCC 2001 | WT | |
| CBS_1518 | WT | ||
| CBS_2175 | WT | ||
| 11876 | WT | ||
| CLF_4 | Fks2p-F659S | ||
| CLF_24 | S629P | ||
| CLF_34 | D632G | ||
| CLF_83 | Fks2p-S663F | ||
WT, wild type.
FIG 1.
Optimization of MBT ASTRA for susceptibility testing of yeasts. Relative growth values of 2-fold serial dilutions of caspofungin ranging from 0.125 to 4 μg/ml for C. albicans (a) and C. glabrata (b) derived by MBT ASTRA are shown. An MBT ASTRA caspofungin breakpoint was defined for C. albicans (a) and C. glabrata (b). (a) C. albicans strains with MICs equal to or below 1 μg/ml caspofungin were categorized as susceptible (strains 1 to 4), and strains with MICs above 1 μg/ml were categorized as resistant (strains 5 to 8). (b) For C. glabrata, strains with MICs equal to or below 0.5 μg/ml caspofungin were categorized as susceptible (strains 1 to 4), and strains with MICs above 0.5 μg/ml caspofungin were categorized as resistant (strains 5 to 8).
Reproducibility of MBT ASTRA.
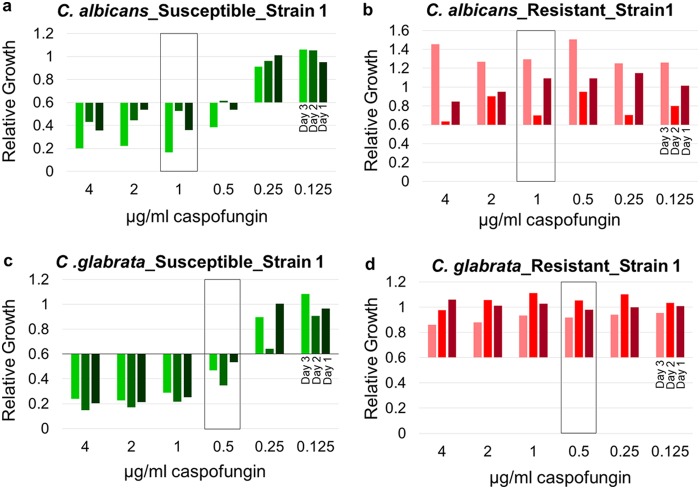
The reproducibility of this novel approach was tested on three different days by analysis of 20 isolates, including 5 susceptible and 5 resistant strains of each species randomly selected (Fig. 2). For C. albicans, agreement between the results of these five individual experiments was observed for all susceptible strains, showing a significant relative growth reduction below the RG threshold of 0.6 at a caspofungin concentration equal to or below 1 μg/ml. Although the absolute values varied, the strains were correctly categorized as susceptible (Fig. 2a; see also Fig. S1a to d in the supplemental material). For resistant C. albicans strains, four strains were categorized as resistant in all three analyses (Fig. 2b and S1e, g, and h). Only one strain had a relative growth reduction at a caspofungin concentration of 1 μg/ml and showed an RG value slightly below the RG cutoff value; it was thereby categorized as susceptible in one experiment (Fig. S1f). Surprisingly, the growth rate of this strain again increased at a concentration 2 μg/ml. For C. glabrata, four susceptible strains were constantly categorized as susceptible in all three different experiments (Fig. 2c and S1j to l). One susceptible strain showed a variable behavior within the three different experiments (Fig. S1i). In contrast, all five resistant strains were accurately detected (Fig. 2d and S1m to p). Intermediate C. glabrata strains revealed variable results between the susceptible and resistant categorizations (Fig. S1q to s).
FIG 2.
Example of the reproducibility of MBT ASTRA. Relative growth values were obtained by 2-fold serial dilutions of caspofungin for susceptible (a) and resistant (b) C. albicans strains and susceptible (c) and resistant (d) C. glabrata strains on three different days. C. albicans and C. glabrata revealed consistent results at the breakpoint concentration (boxes) for the different experiments.
Analysis of clinical isolates by MBT ASTRA.
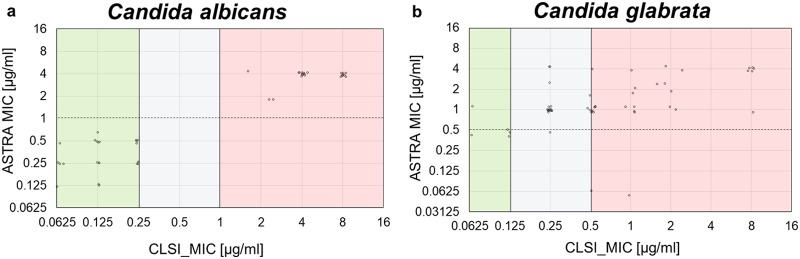
After assay optimization, 115 clinical isolates were analyzed by MBT ASTRA and MALDI Biotyper according to the protocol described above. The caspofungin concentration resulting in an RG value equal to or below 0.6 was taken as the individual MIC of each strain. Figure 3 presents a comparison of the CLSI microdilution method-derived MICs and the MBT ASTRA-derived MICs. For C. albicans, complete agreement between the two approaches was observed. All isolates that were categorized as susceptible by microdilution were also detected as susceptible by MBT ASTRA, and all strains categorized as resistant by microdilution were found to be resistant by MBT ASTRA. No intermediate strains were detected by microdilution or MBT ASTRA. In total, 29 susceptible and 22 resistant C. albicans strains were correctly categorized by MBT ASTRA within 6 h (Fig. 3a; Table 2). Seven strains showed insufficient growth in the control setup and were excluded from further evaluation, resulting in a validity of the MBT ASTRA approach of 88%. When the seven slow-growing strains of C. albicans were excluded, the sensitivity and specificity of MBT ASTRA compared to the results of the CLSI microdilution method were 100% (Table 3). For C. glabrata, 31 of 33 strains categorized as resistant by microdilution were also found to be resistant by MBT ASTRA. Four of five susceptible strains were successfully detected by MBT ASTRA. Nineteen strains were categorized as intermediate by microdilution. Overall, strains showing intermediate behavior in the microdilution assay were considered resistant by MBT ASTRA. Eighteen of these strains were categorized as resistant by MBT ASTRA, as no intermediate range could be defined by MBT ASTRA. Only one intermediate strain was classified as susceptible (Table 4; Fig. S1q). The sensitivity, specificity, and validity of MBT ASTRA compared to the results of the microdilution method were calculated to be 94%, 80%, and 95%, respectively (Table 3).
FIG 3.
Comparison of MBT ASTRA and microdilution results for 51 clinical C. albicans isolates and 57 clinical C. glabrata isolates. For each strain, the MIC obtained by microdilution (x axis) was plotted against the MIC value derived by MBT ASTRA (y axis). The colored boxes indicate the MIC ranges according to CLSI breakpoint; green, susceptible; gray, intermediate; and red, resistant. The horizontal dashed line indicates the breakpoint concentration defined for the MBT ASTRA MIC. (a) For C. albicans, total agreement was observed between the two approaches. (b) For C. glabrata, concordant results were observed for 53 strains by the two methods. Only one susceptible strain was detected as resistant and two resistant isolates were misclassified as susceptible by MBT ASTRA.
TABLE 2.
In vitro caspofungin susceptibility testing using CLSI microdilution method and MBT ASTRA for 58 isolates of C. albicans
| C. albicans strainsa | MIC (μg/ml) |
||
|---|---|---|---|
| CLSI microdilution methodb |
MBT ASTRA, 6 h | ||
| 24 h | 48 h | ||
| 1 | 8 | 8 | 4 |
| 2 | 8 | >8 | 4 |
| 3 | 2 | 4 | 4 |
| 4 | 4 | 8 | 4 |
| 5 | ? | 4 | 4 |
| 6 | ? | 2 | 2 |
| 7 | ? | 2 | 2 |
| 8 | ? | 2 | 4 |
| 9 | >8 | >8 | 4 |
| 10 | >8 | >8 | 4 |
| 11 | >8 | >8 | 4 |
| 12 | 0.5 | 4 | 4 |
| 13 | 1 | 4 | 4 |
| 14 | >8 | >8 | 4 |
| 15 | >8 | >8 | 4 |
| 16 | 4 | 4 | 4 |
| 17 | 2 | 4 | 4 |
| 18 | 2 | 4 | 4 |
| 19 | 4 | 4 | 4 |
| 20 | 4 | 4 | 4 |
| 21 | 8 | 8 | 4 |
| 22 | 4 | 4 | 4 |
| 23 | 0.06 | 0.06 | ≤0.25 |
| 24 | 0.06 | 0.06 | ≤0.25 |
| 25 | 0.06 | 0.125 | ≤0.5 |
| 26 | 0.125 | 0.25 | ≤0.125 |
| 27 | 0.06 | 0.06 | ≤1 |
| 28 | 0.06 | 0.125 | ≤0.5 |
| 29 | 0.06 | 0.06 | ≤0.125 |
| 30 | 0.125 | 0.5 | ≤0.25 |
| 31 | 0.25 | 0.25 | ≤0.5 |
| 32 | 0.25 | 0.25 | ≤0.5 |
| 33 | 0.25 | 0.25 | ≤0.5 |
| 34 | 0.25 | 0.25 | ≤0.25 |
| 35 | 0.25 | 0.5 | ≤0.25 |
| 36 | 0.125 | 0.5 | ≤0.25 |
| 37 | 0.25 | 0.5 | ≤0.5 |
| 38 | 0.25 | 0.5 | ≤0.25 |
| 39 | 0.125 | 0.25 | ≤1 |
| 40 | 0.125 | 0.25 | ≤0.5 |
| 41 | 0.125 | 0.25 | ≤0.5 |
| 42 | 0.125 | 0.25 | ≤0.5 |
| 43 | 0.06 | 0.06 | ≤0.5 |
| 44 | 0.125 | 0.06 | ≤0.25 |
| 45 | NAc | 0.5d | ≤0.5 |
| 46 | 0.06 | 0.06 | ≤0.25 |
| 47 | 0.25 | 0.125 | ≤0.25 |
| 48 | 0.125 | 0.25 | ≤0.125 |
| 49 | 0.06 | 0.06 | ≤0.5 |
| 50 | 0.125 | 0.06 | ≤0.5 |
| 51 | 0.06 | 0.06 | ≤0.25 |
| 52 | 0.125 | 0.125 | NA |
| 53 | 0.125 | 0.25 | NA |
| 54 | 0.25 | 0.25 | NA |
| 55 | 0.06 | 0.125 | NA |
| 56 | 0.25 | 0.25 | NA |
| 57 | 0.125 | 0.25 | NA |
| 58 | 0.06 | 0.06 | NA |
| ATCC 64548 | 0.125 | 0.125 | ≤1 |
| ATCC 64550 | 0.25 | 0.25 | ≤1 |
| ATCC 22019e | 1 | 2 | |
| ATCC 6258e | 1 | 1 | |
Strains 1 to 22 are resistant, and strains 23 to 58 are susceptible.
The evaluation was performed visually.
NA, not available.
The evaluation was performed by use of a microplate reader after 48 h.
These quality control strains were tested only by the CLSI microdilution method.
TABLE 3.
Sensitivity, specificity, and validity of MBT ASTRA in comparison to CLSI microdilution method results for caspofungin
| Species | Sensitivity (%) | Specificity (%) | Validity (%) |
|---|---|---|---|
| C. albicans | 100 | 100 | 88 |
| C. glabrata | 94.2 | 80 | 95 |
TABLE 4.
In vitro caspofungin susceptibility testing using CLSI microdilution method and MBT ASTRA for 57 isolates of C. glabrata
| C. glabrata straina | MIC (μg/ml) |
||
|---|---|---|---|
| CLSI microdilution methodb |
MBT ASTRA, 6 h | ||
| 48 h | 72 h | ||
| 1 | 8 | 8 | 4 |
| 2 | 2 | 2 | 4 |
| 3 | 1 | 1 | 4 |
| 4 | 1 | 1 | 2 |
| 5 | 4 | 8 | 4 |
| 6 | 2 | 2 | 2 |
| 7 | NAc | 2 | 4 |
| 8 | NA | 2 | 1 |
| 9 | 2 | 2 | 2 |
| 10 | NA | 2 | 2 |
| 11 | 0.5 | 0.5 | 1 |
| 12 | 0.5 | 0.5 | 1 |
| 13 | 0.5 | 1 | 1 |
| 14 | 0.5 | 0.5 | 1 |
| 15 | 0.5 | 1 | 1 |
| 16 | 0.5 | 0.5 | 4 |
| 17 | >8 | >8 | 4 |
| 18 | >8 | >8 | 4 |
| 19 | 0.5 | 0.5 | 1 |
| 20 | 0.5 | 1 | 2 |
| 21 | NA | 0.5 | 1 |
| 22 | 0.5 | 0.5 | 1 |
| 23 | 0.5 | 0.5 | ≤0.5 |
| 24 | 0.5 | 1 | 1 |
| 25 | 2 | 2 | 1 |
| 26 | 0.5 | 1 | ≤0.5 |
| 27 | 0.5 | 0.5 | 1 |
| 28 | 0.5 | 0.5 | 1 |
| 29 | 0.5 | 0.5 | 1 |
| 30 | 0.5 | 1 | 1 |
| 31 | ? | 0.5 | 2 |
| 32 | >8 | >8 | 1 |
| 33 | >8 | >8 | 4 |
| 34 | 0.25 | 0.5 | 1 |
| 35 | 0.25 | 0.5 | 1 |
| 36 | 0.25 | 0.5 | 2 |
| 37 | 0.25 | 0.25 | 1 |
| 38 | 0.25 | 0.25 | ≤0.5 |
| 39 | 0.25 | 0.5 | 1 |
| 40 | 0.25 | 0.5 | 1 |
| 41 | 0.25 | 0.5 | 1 |
| 42 | 0.25 | 0.25 | 1 |
| 43 | 0.25 | 1 | 1 |
| 44 | 0.25 | 0.5 | 1 |
| 45 | 0.25 | 0.5 | 1 |
| 46 | 0.25 | 0.25 | 1 |
| 47 | 0.25 | 0.25 | 1 |
| 48 | 0.25 | 0.5 | 1 |
| 49 | 0.25 | 0.25 | 1 |
| 50 | 0.25 | 0.5 | 1 |
| 51 | 0.25 | 0.25 | 4 |
| 52 | 0.25 | 0.25 | 4 |
| 53 | 0.06 | 0.5 | 1 |
| 54 | 0.06 | 0.5 | ≤0.5 |
| 55 | 0.125 | 0.5 | ≤0.5 |
| 56 | 0.125 | 0.25 | ≤0.5 |
| 57 | 0.125 | 0.25 | ≤0.5 |
Strains 1 to 33 are resistant, and strains 34 to 52 are susceptible.
The evaluation was performed visually.
NA, not available.
Strains and routine antifungal susceptibility methods.
Tables 2 and 4 summarize the caspofungin MIC results obtained by the CLSI method compared to those obtained by MBT ASTRA for C. albicans and C. glabrata, respectively. In general, the microdilution result was taken as the gold standard and was used to evaluate the MICs obtained by MBT ASTRA. Initially, the MICs of C. albicans and C. glabrata based on the CLSI microdilution method were determined after 24 h and 48 h, respectively. However, due to some slow-growing strains, the MIC reading was prolonged up to 72 h for both species. Application of the CLSI breakpoint for caspofungin for C. albicans isolates classified this species into 36 susceptible and 22 resistant strains. C. glabrata was categorized into 5 susceptible, 33 resistant, and 19 intermediate strains.
DISCUSSION
MALDI-TOF MS has become a rapid technology broadly applied in clinical laboratories to identify yeasts and yeast-like isolates. The rapidness and accuracy of this method predestine it to be used in AFST. Standard AFST by microdilution methods takes at least 24 h, but the application of MALDI-TOF MS may accelerate susceptibility testing. So far, rapid susceptibility testing by MALDI-TOF MS has mainly been described for bacteria (23, 27, 30–33). One research group has reported on a rapid AFST approach by analyzing the similarities of the MALDI-TOF MS profile spectra derived from different setups containing increasing antibiotic concentrations (34–36). MBT ASTRA is a semiquantitative method that was first applied for the detection of meropenem susceptibility in bacteria derived from culture plates or from positive blood cultures within 1 to 4 h (32). Subsequently, additional studies demonstrating the successful applicability of MBT ASTRA for detection of strains resistant to gentamicin, cefotaxime (37), and rifampin (31, 38) were performed. In all these studies, the time to the result was significantly reduced from at least 24 h to 3 to 6 h by use of the MALDI-TOF MS approach. In this study, MBT ASTRA was optimized to facilitate the detection of resistance against caspofungin in two different yeast species, C. albicans and C. glabrata.
Since yeasts grow significantly more slowly than bacteria, a major improvement of MBT ASTRA was the reduction of the incubation time. The usually required incubation time of 24 h up to even 72 h for yeast resistance detection by microdilution was reduced to a short incubation time of about 6 h. To achieve MBT ASTRA results, the concentration of the internal standard was adapted to the profile spectra derived from yeasts, which revealed intensities and numbers of peaks different from those for the spectra derived from bacteria.
MBT ASTRA is a phenotypic assay that directly monitors growth in the presence of antifungals and compares this to the growth of a control setup without antifungal. In contrast, the approach of Vella and colleagues (34) analyzed changes in the MS profile spectra induced by antifungals. A recent study of the same group (36) has shown that this approach does not accurately work for C. glabrata strains that are resistant to anidulafungin and that have known FKS2 mutations (36). The complete assay setup of MBT ASTRA is like that of the commonly accepted microdilution method, which is generally accepted as the gold standard. A threshold for relative growth of 0.6 was defined as the cutoff value for categorization. Isolates with RG values equal to or below 0.6 were considered susceptible, and those with RG values above 0.6 were categorized as resistant. The breakpoint concentrations categorizing susceptible and resistant strains defined for MBT ASTRA were close to the breakpoint concentrations defined by CLSI and varied in this study by 2 doubling dilutions. For MBT ASTRA, breakpoints for caspofungin were defined as follows: for C. albicans, ≤1 μg/ml for susceptible and >1 μg/ml for resistance, and for C. glabrata, ≤0.5 μg/ml for susceptible and >0.5 μg/ml for resistance. Employing MBT ASTRA, no intermediate range could be defined.
Performing the MBT ASTRA on serial dilutions of a certain antifungal, an individual, assay-specific breakpoint for each strain was determined, and it was possible to categorize strains as susceptible or resistant by reading the respective MBT ASTRA MIC which was 1 doubling dilution higher. On the other hand, MBT ASTRA can be performed at the breakpoint concentration of an antifungal, which facilitates categorization of a strain into susceptible. The overall time to the result for MBT ASTRA was about 7 h. Thereby, the time to the result for MBT ASTRA is shorter than that of established routine methods for AFST, and resistant strains can be detected in less than one clinical laboratory's day (24, 25, 39). Even automated commercially available systems like Vitek 2 (bioMérieux, France) or Sensititre Yeast One (Thermo Fischer Scientific, USA) are significantly slower than MBT ASTRA (17–19, 40). Molecular approaches that are generally faster have to deal with the major obstacle that they do not provide any information about the phenotype of the strains. Additionally, altered gene sequences or new resistance mechanisms may not be detected by PCR analysis.
In this study, an absolute concordance of the MBT ASTRA results with those of microdilution was found for C. albicans. For seven slow-growing strains, insufficient growth of the control setups was observed, preventing a reliable classification. This demonstrated the importance of first checking the growth in the control setups. To realize this, a threshold for the minimum required growth calculated by the prototype software was introduced. Only strains with sufficient growth for the control were considered for evaluation of the sensitivity and specificity of MBT ASTRA. Seven slow-growing strains that could not be evaluated were detected, resulting in an assay validity of 88%. These strains were also tested by the Vitek 2 system to find out whether they belong to other species, like C. africana or C. stellatoidea, which cannot be detected by MALDI. The results confirmed that all seven strains were C. albicans and susceptible to echinocandins. Therefore, the poor growth rate cannot be explained by the fact that they belong to other species. Additionally, caspofungin is known to show a high variability in its MIC distribution in different labs, causing the wrong reporting of wild-type strains as nonsusceptible for most common Candida species (41). This could explain the few cases of disagreement found in this study between MBT ASTRA and CLSI. Beyond the growth rate, technical errors like insufficient extraction, the use of inadequate culture medium, as well as the use of an inaccurate amount of internal standard could lead to poor-quality spectra, leading to a risk of misclassification. Besides, the reproducibility of this study revealed acceptable results for C. albicans, with the result for only 1 out of 30 analyses being incorrect. Detailed analysis of the spectrum for that strain revealed a general problem with the MALDI-TOF MS measurements on that day for that strain, because the spectra were acquired from only one spot instead of two spots. Visual inspection of these spectra revealed poor-quality spectra. This could have been the reason for the misclassification. Further improvements to the software algorithm will be necessary to evaluate the spectrum quality and to exclude those spectra with an insufficient quality.
For C. glabrata, sensitivity, specificity, and validity were calculated to be 94%, 80%, and 95%, respectively. Four strains were found to be susceptible to caspofungin by MBT ASTRA, whereas 5 strains were categorized as susceptible by microdilution. The relatively low specificity found in this study could be explained by the low number of only 5 susceptible C. glabrata strains included. One of these 5 susceptible strains was categorized as resistant by MBT ASTRA. In contrast, 2 of 33 resistant strains were incorrectly detected as susceptible. The reproducibility study for C. glabrata revealed 1 deviation out of 30 analyses. One susceptible and two intermediate strains were miscategorized. Furthermore, in this study, MBT ASTRA could not identify intermediate strains. For C. albicans, no intermediate strains were tested, and for C. glabrata, except for one strain, all intermediates were categorized as resistant. This might be further improved by modifications to the assay setup in the future.
Moreover, one susceptible strain and one resistant strain of C. albicans indicated an increase of growth in the presence of caspofungin at a concentration of 4 μg/ml. The same paradoxical effect that has been described for microdilution probably also occurred for MBT ASTRA (42) (see Fig. S1a and f in the supplemental material). In principle, this approach has the potential to be applicable for susceptibility testing of further yeast species and different antifungals. Further investigations, possibly additional optimization, and validation will be necessary to develop the approach for routine application workflows.
In summary, this study provided promising results for the use of MBT ASTRA for AFST of C. albicans and C. glabrata. The hands-on time for the setup of MBT ASTRA is, in principle, identical to that of conventional microdilution methods. However, sample preparation requires some extra steps. After incubation, additional work, including centrifugation, extraction, and spotting of the lysate on the target, is necessary and takes about 15 min per strain. Taking into account that the resistance result is available on the same day, these efforts seem to be justified. In summary, the excellent outcome with respect to sensitivity and specificity, the simple setup, and the short incubation time predestine this MALDI-TOF MS-based approach to be an interesting alternative to conventional AFST. Since MALDI-TOF MS instruments are already part of many clinical laboratories, no additional investment (excluding a centrifuge to deal with microtiter plates, which is available in most clinical labs) will be required to perform this cost-efficient and fast method in the future.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Aimilia Stavrou at the Westerdijk Fungal Biodiversity Institute and the collaborators of the Division of Hygiene and Microbiology, Medical University of Innsbruck, Innsbruck, Austria, for providing some of the strains used in this study.
This work was funded by the European Union (OPATHY, project 642095; program H2020-EU.1.3.1.).
M.K., K.S., and M.V. are employees of the mass spectrometry company Bruker Daltonik GmbH.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00420-18.
REFERENCES
- 1.Cui J, Ren B, Tong Y, Dai H, Zhang L. 2015. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 6:362–371. doi: 10.1080/21505594.2015.1039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delaloye J, Calandra T. 2014. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent J-L. 2016. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care 20:125. doi: 10.1186/s13054-016-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendrup MC. 2010. Epidemiology of invasive candidiasis. Curr Opin Crit Care 16:445–452. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- 5.Mikulska M, Del Bono V, Ratto S, Viscoli C. 2012. Occurrence, presentation and treatment of candidemia. Expert Rev Clin Immunol 8:755–765. doi: 10.1586/eci.12.52. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Dagi HT, Findik D, Senkeles C, Arslan U. 2016. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann Clin Microbiol Antimicrob 15:36. doi: 10.1186/s12941-016-0153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce CG, Lopez-Ribot JL. 2013. Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs. Expert Opin Drug Discov 8:1117–1126. doi: 10.1517/17460441.2013.807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanglard D, Coste A, Ferrari S. 2009. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res 9:1029–1050. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- 10.Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat 10:121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Román E, Prieto D, Martin R, Correia I, Mesa Arango AC, Alonso-Monge R, Zaragoza O, Pla J. 3 October 2016. Role of catalase overproduction in drug resistance and virulence in Candida albicans. Future Microbiol doi: 10.2217/fmb-2016-0067. [DOI] [PubMed] [Google Scholar]
- 13.Suwunnakorn S, Wakabayashi H, Rustchenko E. 2016. Chromosome 5 of human pathogen Candida albicans carries multiple genes for negative control of caspofungin and anidulafungin susceptibility. Antimicrob Agents Chemother 60:7457–7467. doi: 10.1128/AAC.01888-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katiyar SK, Alastruey-Izquierdo A, Healey KR, Johnson ME, Perlin DS, Edlind TD. 2012. Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrob Agents Chemother 56:6304–6309. doi: 10.1128/AAC.00813-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. 2014. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 59:819–825. doi: 10.1093/cid/ciu407. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EM. 2008. Issues in antifungal susceptibility testing. J Antimicrob Chemother 61(Suppl 1):i13–i18. doi: 10.1093/jac/dkm427. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Automated quantitative or qualitative antifungal susceptibility test of Candida species to caspofungin. Report number K101566. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 18.Peterson JF, Pfaller MA, Diekema DJ, Rinaldi MG, Riebe KM, Ledeboer NA. 2011. Multicenter comparison of the Vitek 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing caspofungin, micafungin, and posaconazole against Candida spp. J Clin Microbiol 49:1765–1771. doi: 10.1128/JCM.02517-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuenca-Estrella M, Gomez-Lopez A, Alastruey-Izquierdo A, Bernal-Martinez L, Cuesta I, Buitrago MJ, Rodriguez-Tudela JL. 2010. Comparison of the Vitek 2 antifungal susceptibility system with the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution reference methods and with the Sensititre YeastOne and Etest techniques for in vitro detection of antifungal resistance in yeast isolates. J Clin Microbiol 48:1782–1786. doi: 10.1128/JCM.02316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostrzewa M, Schubert S. 2016. MALDI-TOF mass spectrometry in microbiology. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 21.Croxatto A, Prod'hom G, Greub G. 2012. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev 36:380–407. doi: 10.1111/j.1574-6976.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- 22.Posteraro B, Sanguinetti M. 2014. The future of fungal susceptibility testing. Future Microbiol 9:947–967. doi: 10.2217/fmb.14.55. [DOI] [PubMed] [Google Scholar]
- 23.Lange C, Schubert S, Jung J, Kostrzewa M, Sparbier K. 2014. Quantitative matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid resistance detection. J Clin Microbiol 52:4155–4162. doi: 10.1128/JCM.01872-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2017. Performance standards for antifungal susceptibility testing of yeasts; approved standard, 1st ed CLSI document M60. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2017. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. EUCAST E.DEF 7.3.1; European Committee for Antimicrobial Susceptibility Testing. [Google Scholar]
- 26.Sauer S, Freiwald A, Maier T, Kube M, Reinhardt R, Kostrzewa M, Geider K. 2008. Classification and identification of bacteria by mass spectrometry and computational analysis. PLoS One 3:e2843. doi: 10.1371/journal.pone.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Foundation for Statistical Computing. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 28.Gibb S, Strimmer K. 2012. MALDIquant: a versatile R package for the analysis of mass spectrometry data. Bioinformatics 28:2270–2271. doi: 10.1093/bioinformatics/bts447. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother 53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparbier K, Lange C, Jung J, Wieser A, Schubert S, Kostrzewa M. 2013. MALDI Biotyper-based rapid resistance detection by stable-isotope labeling. J Clin Microbiol 51:3741–3748. doi: 10.1128/JCM.01536-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxson T, Taylor-Howell CL, Minogue TD. 2017. Semi-quantitative MALDI-TOF for antimicrobial susceptibility testing in Staphylococcus aureus. PLoS One 12:e0183899. doi: 10.1371/journal.pone.0183899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparbier K, Schubert S, Kostrzewa M. 2016. MBT-ASTRA: a suitable tool for fast antibiotic susceptibility testing? Methods 104:48–54. doi: 10.1016/j.ymeth.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Oviaño M, Bou G. 2017. Imipenem-avibactam: a novel combination for the rapid detection of carbapenemase activity in Enterobacteriaceae and Acinetobacter baumannii by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Diagn Microbiol Infect Dis 87:129–132. doi: 10.1016/j.diagmicrobio.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Vella A, De Carolis E, Vaccaro L, Posteraro P, Perlin DS, Kostrzewa M, Posteraro B, Sanguinetti M. 2013. Rapid antifungal susceptibility testing by matrix-assisted laser desorption ionization–time of flight mass spectrometry analysis. J Clin Microbiol 51:2964–2969. doi: 10.1128/JCM.00903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Carolis E, Vella A, Florio AR, Posteraro P, Perlin DS, Sanguinetti M, Posteraro B. 2012. Use of matrix-assisted laser desorption ionization–time of flight mass spectrometry for caspofungin susceptibility testing of Candida and Aspergillus species. J Clin Microbiol 50:2479–2483. doi: 10.1128/JCM.00224-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vella A, De Carolis E, Mello E, Perlin DS, Sanglard D, Sanguinetti M, Posteraro B. 2017. Potential use of MALDI-ToF mass spectrometry for rapid detection of antifungal resistance in the human pathogen Candida glabrata. Sci Rep 7:9099. doi: 10.1038/s41598-017-09329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung JS, Hamacher C, Gross B, Sparbier K, Lange C, Kostrzewa M, Schubert S. 2016. Evaluation of a semiquantitative matrix-assisted laser desorption ionization–time of flight mass spectrometry method for rapid antimicrobial susceptibility testing of positive blood cultures. J Clin Microbiol 54:2820–2824. doi: 10.1128/JCM.01131-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceyssens P-J, Soetaert K, Timke M, den Bossche AV, Sparbier K, Cremer KD, Kostrzewa M, Hendrickx M, Mathys V. 2017. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for combined species identification and drug sensitivity testing in mycobacteria. J Clin Microbiol 55:624–634. doi: 10.1128/JCM.02089-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song YB, Suh MK, Ha GY, Kim H. 2015. Antifungal susceptibility testing with Etest for Candida species isolated from patients with oral candidiasis. Ann Dermatol 27:715–720. doi: 10.5021/ad.2015.27.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cretella D, Barber KE, King ST, Stover KR. 2016. Comparison of susceptibility patterns using commercially available susceptibility testing methods performed on prevalent Candida spp. J Med Microbiol 65:1445–1451. doi: 10.1099/jmm.0.000383. [DOI] [PubMed] [Google Scholar]
- 41.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Flörl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SRBS, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rueda C, Cuenca-Estrella M, Zaragoza O. 2014. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother 58:1071–1083. doi: 10.1128/AAC.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.