No gold standard exists for histopathological diagnosis of a prosthetic joint infection (PJI). The historical criterion considers the presence of neutrophil infiltration upon examination of periprosthetic tissue.
KEYWORDS: prosthetic joint Infection, periprosthetic interface membrane, neutrophil threshold
ABSTRACT
No gold standard exists for histopathological diagnosis of a prosthetic joint infection (PJI). The historical criterion considers the presence of neutrophil infiltration upon examination of periprosthetic tissue. Morawietz et al. proposed a classification of periprosthetic membranes (Morawietz et al., Clin Pathol 59:591–597, 2006, https://doi.org/10.1136/jcp.2005.027458) and a more recently described classification with a new cutoff value of 23 neutrophils in 10 high-power fields (Morawietz et al., Histopathology 54:847–853, 2009. https://doi.org/10.1111/j.1365-2559.2009.03313.x). We performed a multicenter prospective study, which compared both methods for the diagnosis of PJI. All suspicions of PJI (n = 264) between December 2010 and March 2012 in seven centers were prospectively included. Five perioperative specimens were collected per patient for cultures, and one was collected for histology. Diagnosis of PJI was made according to the Infectious Diseases Society of America (IDSA) guidelines. Histopathological analysis classified the patients according to the threshold of 23 neutrophils and according to the classification of Morawietz. Performances of both methods were compared by using clinical and/or bacteriological criteria as the gold standard. Among 264 patients with suspected PJI, a diagnosis of infection was confirmed in 215 and unconfirmed in 49 patients. Histopathological analysis was available for 150 confirmed PJI and 40 unconfirmed PJI cases. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 78.7%, 90.0%, 96.7%, 52.9%, and 81.1%, respectively, for the Morawietz classification, and 82.0%, 90.0%, 96.9%, 57.1%, and 83.7%, respectively, for the 23-neutrophil threshold. The new algorithm using a threshold of 23 neutrophils can be proposed as a new gold standard for the histopathological diagnosis of PJI.
INTRODUCTION
Prosthetic joint infection (PJI) is one of the most serious complications of orthopedic surgery, increasing the risk of morbidity and mortality (1, 2). Clinical practice guidelines were produced between 2009 and 2013 by several societies, including the Infectious Diseases Society of America (IDSA), the Musculoskeletal Infection Society (MSIS), the Société de Pathologie Infectieuse de Langue Française (joining 10 other French specialty societies), and the Italian Society of Infectious and Tropical Diseases (3–6). Recently, an International Consensus Meeting on Periprosthetic Joint Infection was supported by the European Bone and Joint Infection Society (EBJIS), the MSIS, and a large number of other groups from around the world, who proposed an international consensus definition of PJI (7). Evidence of PJI is considered definitive when a sinus tract exists in communication with the prosthesis, when the same pathogen is growing from two different periprosthetic tissue or fluid samples, or when at least 3 of 6 minor criteria are present. Purulence around prosthesis, which was a major criterion in the IDSA definition and a minor criterion in the MSIS definition, was unfortunately removed from the International Consensus Meeting definition of PJI. However, according to IDSA guidelines, purulence caused by adverse reaction to metal debris must be excluded from PJI criteria (3). The observation of acute inflammation with neutrophilic infiltration upon histopathological examination of periprosthetic tissue is considered highly suggestive of PJI. The most widely used analysis is based on the count of neutrophils. The number of neutrophils per high-power field (HPF) strongly correlating with infection has historically differed among authors from more than 1 to more than 5 neutrophils per HPF (8–12). However, the most consensual criterion is the presence of at least 5 neutrophils per HPF in at least 5 separate microscopic fields (9, 11). This criterion is that of the MSIS consensus, whereas the IDSA consensus includes acute inflammation without defining a precise neutrophil score (3, 4).
As underlined in consensual guidelines, histopathological analysis may be rendered difficult by a heterogeneous appearance of periprosthetic membrane. A prospective study analyzing specimens from pseudocapsule and from interface membrane showed that periprosthetic interface membrane is the best specimen for the histopathological diagnosis of prosthetic joint infection (13). Morawietz classification was proposed to enable a standardized typing of periprosthetic membrane into four different types, among which two are infectious types (14). This new classification is interesting, insofar as it provides histopathological findings other than those related to the inflammatory response sensu stricto. It was preliminarily applied to a study collecting 370 samples from revision surgery, underlining the importance of noninfectious, non-particle-induced loosening of prostheses in orthopedic surgery (type IV membrane), which was diagnosed in 15% of patients (14). In a second prospective study, which included 50 patients suspected of PJI, Morawietz classification of periprosthetic membrane showed the existence of PJI in 37 patients, despite a high proportion of poor-virulence cutaneous bacteria isolated from tissue samples (15). The two main limitations of this study were, first, the lack of comparison between neutrophil count and Morawietz classification, and second, the low number of PJI cases studied.
More recently, the same team of pathologists from Berlin proposed a new algorithm for histopathological diagnosis of PJI. By enumerating the neutrophils in 10 HPF without counting more than 10 neutrophils in each HPF, they finally proposed a threshold of 23 neutrophils, above which the case can be considered an infectious type and below which it would not be an infectious type (16). In this study, the authors used immunohistochemistry with an anti-CD15 antibody for specific identification of neutrophils.
Another important limitation of histopathological analysis is the large reading variation among different pathologists, which is underlined in consensus guidelines. Finally, given that most of the studies analyzing histology in the diagnosis of PJI are single-center studies that include a relatively low number of patients, a large multicenter study was missing.
Our West French network organization (Centre de Référence des Infections Ostéo-articulaires du Grand Ouest [CRIOGO]) for the multidisciplinary diagnosis and treatment of bone and joint infections in seven referral centers allowed us to carry out the first prospective multicenter study related to the molecular and histopathological diagnosis of PJI. The contribution of broad-range PCR to the diagnosis was recently published (17, 18).
The main objective of this study was to compare the Morawietz histopathological classification of the periprosthetic interface membrane with the threshold of 23 neutrophils in 10 HPF to allow better differentiation between septic and aseptic processes in the diagnosis of PJI.
MATERIALS AND METHODS
Study design and ethics approval.
The study was designed as a multicenter, prospective, observational, cross-sectional study of adult patients suspected of having PJI. The study protocol (Programme Hospitalier de Recherche Clinique Interrégional API/N/041) was approved by the institutional review board or ethics committee at every site. Informed consent was obtained from each patient before inclusion.
Study population.
Consecutive patients with clinical signs suggesting acute or chronic PJI in seven French university hospitals between December 2010 and March 2012 were included. Six tissue samples were collected during surgery, consisting of five samples for culture and molecular diagnosis and one periprosthetic membrane sample for histopathological analysis.
Definition of PJI.
Early postoperative PJI was suspected for patients with pain, disunion, necrosis, or wound dehiscence within the first month following prosthesis implantation. Late chronic PJI was suspected in the presence of chronic pain without systemic symptoms that occurs more than 1 month after the index surgery, as well as that of a loosened prosthesis (19, 20). According to the IDSA guidelines, PJI was diagnosed when at least one of the following criteria was positive: (i) clinical criterion with the presence of a sinus tract communicating with the prosthesis and/or purulence around the prosthesis, and/or (ii) bacteriological criterion for infection (as specified below).
Microbiological methods.
For each patient, 5 perioperative specimens (periprosthetic tissue or synovial fluid) collected in sterile vials were submitted to the microbiology laboratory. Each of the 5 bead-milled suspensions was inoculated into a pediatric blood culture bottle and into Schaedler broth, then onto a blood agar plate, a chocolate agar plate, and a blood agar plate supplemented with hemin and vitamin K1. Isolated bacteria were identified according to the standard laboratory procedures. Discordant results were resolved using partial 16S rRNA gene sequencing. Antibiotic susceptibility testing was determined according to the EUCAST recommendations (21). The bacteriological results were considered positive if at least one culture yielded a strict pathogen (such as Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacteriaceae spp., or anaerobes) or two cultures yielded a pathogen that was a skin commensal (such as coagulase-negative staphylococci [CoNS] or Cutibacterium acnes) (3).
Molecular methods.
PCR assays were performed in a highly standardized manner with the 5 perioperative patient specimens (periprosthetic tissue or synovial fluid) at all sites. All PCRs were performed in parallel with cultures from the same bead-milled suspension. Real-time PCR was performed to target the 5′ part of the 16S rRNA gene. The corresponding amplicons were sequenced in both strands and assembled, and the consensus sequences were compared with those in the Bioinformatics Bacteria Identification (BIBI) and BLAST databases. The rates of concordance between 16S rRNA gene PCR and bacteriological results were based on results at the genus (≥96% similarity) and species (≥98% similarity) levels. The criterion for molecular diagnosis was modeled on the bacteriological criterion (≥1 positive sample for strict pathogens and ≥2 positive samples for commensal skin flora). Molecular data were analyzed according to the diagnosis of PJI (for more information, see references 17 and 18).
Histopathological analysis.
The periprosthetic membrane samples were fixed in buffered formalin, and paraffin block sections (3- to 5-μm slice thickness; slide area 30 × 25 mm) were stained with hematoxylin and eosin. The slides were postoperatively studied under normal and polarized light microscopy. The periprosthetic membrane specimens were analyzed in two manners, as follows.
The 23-neutrophil method.
The 23-neutrophil method uses the neutrophil score after examination of 10 high-power fields (HPF). As recommended by Morawietz, a maximum of 10 neutrophils were counted in each HPF (16). Thus, a wider, less focal distribution of neutrophils may be analyzed, avoiding focal neutrophilic aggregates produced by extravasation from blood vessels overcalling acute inflammation and thus PJI (16). The total sum of neutrophils, determined for 10 HPF, was between 0 and 100. Finally, each case with a neutrophil count of ≥23 was considered an infective type, and each case with a neutrophil count of <23 was considered a noninfective type (16).
Periprosthetic membrane classification.
The periprosthetic membrane classification system defined by Morawietz has four different types of periprosthetic membranes, as follows: type I, periprosthetic membrane of the wear-particle-induced type, characterized by predominant infiltration of macrophages and multinuclear giant cells containing polyethylene particles; type II, periprosthetic membrane of the infectious type, containing activated fibroblasts, proliferation of small blood vessels, edema, and inflammatory infiltrate of neutrophilic granulocytes; type III, periprosthetic membrane of the combined type, which is a combination of the histopathological aspects described for types (I and II) and is also considered an infectious type; and type IV, periprosthetic membrane of the indeterminate type (noninfected, not induced by wear particles), described as a connective tissue low in cells and rich in collagen fibers (14). In each of the seven hospitals, the slides were assessed by a pathologist who was blinded to the clinical data and to the results of the cultures. An electronic case report form was created to collect each of the items necessary to the periprosthetic membrane classification system and to the neutrophil score.
Statistical analysis.
To evaluate the contribution of histopathological criterion in the diagnosis of PJI, the clinical and bacteriological criteria were analyzed, namely, the presence of clinical and/or bacteriological criteria in favor of an infection versus neither of these two criteria.
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the Morawietz classification with respect to the presence of clinical and/or bacteriological criteria were calculated.
The neutrophil counts were described using quartiles (median, 25th, and 75th percentiles) for patients having the clinical and/or bacteriological criteria and for patients with neither of the two criteria. Neutrophil counts were also binarized according to the threshold of 23 neutrophils proposed by Morawietz et al. (16). Then sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the 23-neutrophil threshold with respect to the presence of clinical and/or bacteriological criteria were calculated. Bacterial documentation was described for the discordant cases.
Finally, a univariate logistic model was used to estimate the discriminating quality of the neutrophil count in the diagnosis of PJI (area under the receiver operating characteristic [ROC] curve) and to plot the associated ROC curve.
RESULTS
Diagnosis of infection.
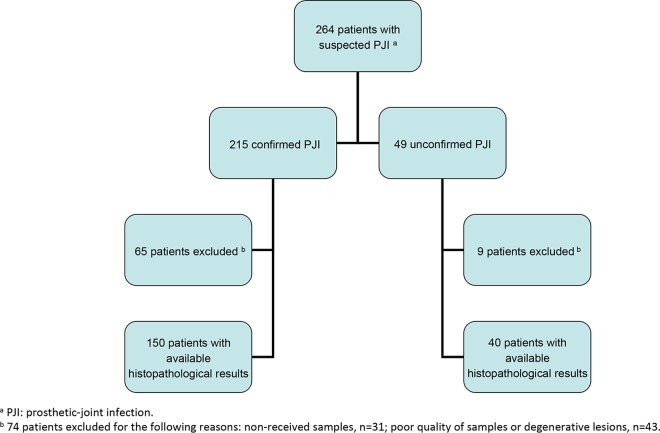
Of the 264 suspected cases of PJI, 127 (48%) occurred in male patients, and the median age at the time of diagnosis was 73 years. The suspected cases of PJI included 165 hip arthroplasty infections (63%), 88 knee arthroplasty infections (33%), and 11 shoulder and elbow arthroplasty infections (4%). The patients presented with symptoms of early postoperative infection in 19% of cases and late chronic infection in 81% of cases. Seventy-six patients (29%) received antibiotics for 2 weeks before surgery (17). After analysis of clinical and bacteriological criteria, a definitive diagnosis of infection was confirmed in 215 out of 264 suspected cases of PJI (Fig. 1). The PJI were late chronic for 168/215 (78%) and early postoperative infection for 47/215 (22%) of cases.
FIG 1.
Flowchart.
Of the 215 patients with confirmed PJI, 192 (89%) had a positive bacteriological criterion, with monomicrobial infection in 163 (85%) cases and polymicrobial infection in 29 (15%) cases (17).
Of the 163 monomicrobial infections, staphylococci were isolated in 108 cases, streptococci and enterococci in 22 cases, Gram-negative bacilli in 16 cases, anaerobes in 13 cases, and other bacteria in 4 cases (for detailed results, see Table S1 in the supplemental material). Of the 29 polymicrobial infections, 21 involved 2 or more different aerobic bacteria, 7 involved both aerobic and anaerobic bacteria, and 1 exclusively involved anaerobes (17).
Analysis of histopathological criterion.
Among the 264 suspicions of PJI, 74 patients were excluded for the following reasons: histology results were not received for 31 patients or could not be interpreted due to degenerative lesions or poor quality of samples (small size, osseous muscular tissue, and/or fibrinous or fibrinohemorrhagic deposits) in 43 cases, leaving 190 patients available for histopathological analysis (Fig. 1).
Histopathological analysis using the periprosthetic membrane classification system.
Of 190 available histopathological results, 89 (46.8%) were of the infectious type II, 33 (17.4%) of the combined type III, 42 (22.1%) of the wear-particle-induced type I, and 26 (13.7%) of the indeterminate type IV. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the Morawietz classification with respect to the presence of clinical and/or bacteriological criteria were 78.7% (95% confidence interval [CI], 71.2 to 84.9), 90.0% (76.3 to 97.2), 96.7% (91.8 to 99.1), 52.9% (40.4 to 65.2), and 81.1% (74.7 to 86.4), respectively (Table 1).
TABLE 1.
Assessment of the Morawietz classification with respect to the presence of clinical and/or bacteriological criteria
| Clinical and/or bacteriological criteria of infection | Morawietz classification (no. of patients) |
Total | |
|---|---|---|---|
| Type II and III membranesa | Type I and IV membranesb | ||
| Presence | 118 | 32 | 150 |
| Absence | 4 | 36 | 40 |
| Total | 122 | 68 | 190 |
Types II and III were considered to be associated with infection.
Types I and IV were not associated with infection.
Histopathological analysis using a threshold of 23 neutrophils.
The median neutrophil number was 86.5 (95% CI, 43 to 100) for the 150 patients having the clinical and/or bacteriological criteria and 0 (95% CI, 0) for the 40 patients with neither of the two criteria. The threshold of 23 neutrophils for the histopathological diagnosis of PJI with respect to the presence of clinical and/or bacteriological criteria led to a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 82.0% (95% CI, 74.9 to 87.8), 90.0% (76.3 to 97.2), 96.9% (92.1 to 99.1), 57.1% (44.0 to 69.5), and 83.7% (77.6 to 88.6), respectively (Table 2). The receiver operating characteristic curve of the neutrophil count showed the performances of the 23-neutrophil threshold with a satisfactory area under the curve of 0.907 (Fig. 2).
TABLE 2.
Assessment of the threshold of 23 neutrophils with respect to the presence of clinical and/or bacteriological criteria
| Clinical and/or bacteriological criteria of infection | No. of patients with neutrophil count of: |
Total | |
|---|---|---|---|
| ≥23 | <23 | ||
| Presence | 123 | 27 | 150 |
| Absence | 4 | 36 | 40 |
| Total | 127 | 63 | 190 |
FIG 2.
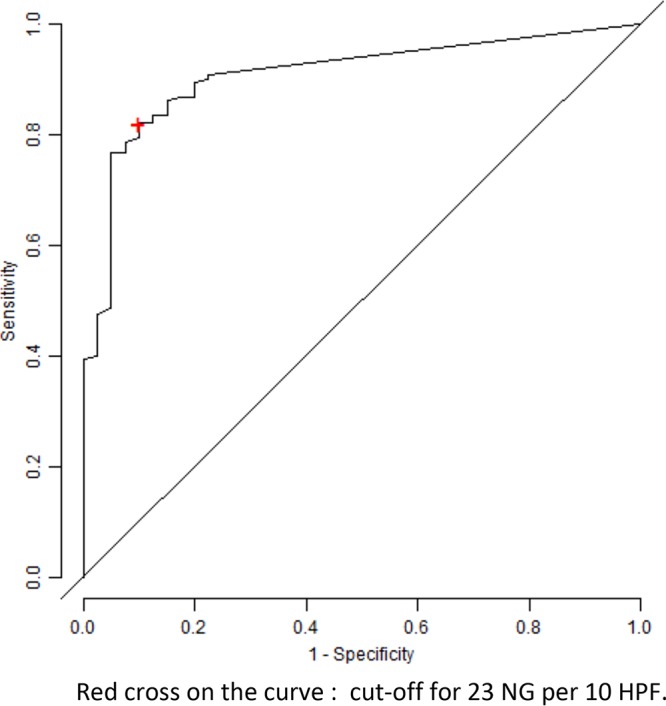
Receiver-operating characteristic curve of the neutrophil (NG) count according to the clinical and/or bacteriological criteria.
Bacteriological documentation of discordant results between the 23-neutrophil threshold and clinicobacteriological criterion.
Twenty-seven patients with the presence of clinical and/or bacteriological criteria of infection presented with a neutrophil count of less than 23. Half of them (56%) were due to bacteria belonging to cutaneous flora (CoNS, n = 13; Cutibacterium acnes, n = 1), or to a non-beta-hemolytic Streptococcus sp. (n = 1). In culture, 6 of the 27 (22%) patients remained negative, among which 3 treated patients were positive by PCR. At least the 6 remaining patients (22%) had a PJI involving virulent pathogens, namely, S. aureus in 4 cases and P. aeruginosa in 2 cases.
DISCUSSION
The classification proposed by Morawietz defines histopathological criteria to establish a concise typing of the periprosthetic interface membrane that would be applicable to routine investigation (14). Detailed histopathological characterization provides information about the cause of prosthesis loosening, allowing differentiation between infected and noninfected loosening. It proposes in particular to search for wear particles, predominantly polyethylene particles, which are classified in the noninfectious type I classification when they are associated with macrophages and multinuclear giant cells, or in the infectious type III classification when they are associated with infiltrates of neutrophilic granulocytes and activated fibroblasts. Focus on wear particles is particularly interesting because they can be responsible for inflammation, which is sometimes difficult to distinguish from infection. Hence, this classification helps to differentiate purulence caused by inflammatory reaction to metal debris from true PJI, whereas purulence and wear particles are associated with periprosthetic tissue samples positive in culture for bacteria. et al. showed that histopathology type III was associated with prosthetic-joint infections, such as type II, taking the two infectious types into consideration (14). Another interesting type in the Morawietz classification is type IV, which is noninfected and not induced by wear particles, and which is described as a connective tissue rich in collagen fibers. The histopathological classification of Morawietz is a precise and detailed analysis that is currently routinely used in German-speaking countries (14–16, 22).
The recent algorithm developed by the same team using a threshold of 23 neutrophils in 10 HPF allows a rapid and precise histopathological diagnosis of PJI. Indeed, counting only a maximum of 10 neutrophils before moving to the next field makes it possible to scan more different fields in cases of difficult diagnosis. In our multicenter study, specificity and positive predictive value of the two methods showed very similar performances. On the other hand, the threshold of 23 neutrophils exhibited a slightly higher sensitivity (82%) with slightly greater precision (83.7%) than those of the interface membrane classification (78.7% and 81.1%, respectively). It can be emphasized that these good performances were obtained without CD15 immunohistochemistry. CD15 is a sensitive antibody that facilitates the identification of neutrophils by staining them red. Unfortunately, we could not use this technique for our study because it required at least 30 min for its realization and was relatively expensive. Since then, this immunohistochemical staining can be performed in a fully automated staining system, which remains still expensive and cannot be routinely used in all histology services (23). Under our analysis conditions, it was reassuring to obtain this good performance on a large number of patients without using immunohistochemistry. Using this 23-neutrophil threshold without CD15 staining, we found a sensitivity of 82% compared with bacteriological and/or clinical criteria, against 73% compared with microbiological diagnosis or 77% compared with clinical diagnosis in the study using CD15 immunochemistry (16). Both methods showed a lack of sensitivity, as 21% (32/150) versus 18% (27/150) of PJI were not well-characterized by the Morawietz classification and the threshold of 23 neutrophils, respectively. Most of these discordant cases were explained by the nonpyogenic nature of the cutaneous bacteria involved, as previously described (13).
In conclusion, our multicenter prospective study found the threshold of 23 neutrophils to be at least as discriminating as the Morawietz classification for the diagnosis of PJI. Therefore, the 23-neutrophil threshold could be proposed as a new histopathological gold standard for the diagnosis of PJI.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Karine Fèvre and Line Happi for their help with the study and technical assistance. We are indebted to Jane Cottin for her enthusiasm until the end. We are also very grateful to all the members of the CRIOGO for their continuing support. Finally, we thank Jeffrey Arsham, an American medical translator, for reviewing our original English-language manuscript.
The CRIOGO study group included the following (all cities indicated are in France): J. Cottin, P. Bizot, P. Abgueguen, V. Balan (Angers); E. Stindel, S. Ansart, A. Greves (Brest); S. Touchais, F. Gouin, D. Boutoille, N. Asseray, L. Happi (Nantes); J. Guinard, F. Razanabola, C. Mille (Orléans); L.E. Gayet, G. Le Moal, C. Thomas (Poitiers); J.L. Polard, C. Arvieux, A. Meheut (Rennes); L. Bernard, P Rosset, G. Gras, J. Druon, K. Fèvre (Tours).
This study was funded by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique Interrégionale API/N/041) and by a grant from the CRIOGO (Centre de Référence des Infections Ostéo-articulaires du Grand Ouest).
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00536-18.
Contributor Information
Robin Patel, Mayo Clinic.
for the CRIOGO (Centre de Référence des Infections Ostéo-articulaires du Grand Ouest) Study Team:
J. Cottin, P. Bizot, P. Abgueguen, V. Balan, E. Stindel, S. Ansart, A. Greves, S. Touchais, F. Gouin, D. Boutoille, N. Asseray, L. Happi, J. Guinard, F. Razanabola, C. Mille, L.E. Gayet, G. Le Moal, C. Thomas, J.L. Polard, C. Arvieux, A. Meheut, L. Bernard, P Rosset, G. Gras, J. Druon, and K. Fèvre
REFERENCES
- 1.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 2.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. 2011. New definition for periprosthetic joint infection: from the workgroup of the musculoskeletal infection society. Clin Orthop 469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupon M, Dutronc H, Perpoint T, Berthelot P, Bouscarra J, Chidiac C, Drape JL, Eyrolle L, Grimprel E, Morelec I, Cyteval C, Dubost JJ, Gaudias J, Jenny JY, Lebtahi R, Rogues AM, Senneville E, Arvieux C, Baron R, Bobieux A, Claverie JP, Codine P, Daquet V, Devillers A, Epifanie JL, Fajon O, Fernandez P, Fessy MH, Gromb S, Guggenbuhl P, Hajjar J, Huglo D, Lesens O, Lucht F, Lusting S, Macouillard G, Morand P, Petiot S, Railhac J, Rambaud C, Riegel P, Salmon D, Sarlangue J, Tavernier T, Bernard L, Besnier JM, Boeri C, Bonnet E, Bouscarra J, Claudot F. 2010. Recommendations for bone and joint prosthetic device infections in clinical practice (prosthesis, implants, osteosynthesis). Société de Pathologie Infectieuse de Langue Française (SPILF). Med Mal Infect 40:185–211. doi: 10.1016/j.medmal.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Esposito S, Leone S, Bassetti M, Borrè S, Leoncini F, Meani E, Venditti M, Mazzotta F, Bone Joint Infections Committee for the Italian Society of Infectious Tropical Diseases (SIMIT). 2009. Italian guidelines for the diagnosis and infectious disease management of osteomyelitis and prosthetic-joint infections in adults. Infection 37:478–496. doi: 10.1007/s15010-009-8269-2. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Gehrke T, Chen AF. 2013. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 95(Suppl B):1450–1452. doi: 10.2106/JBJS.L.00251. [DOI] [PubMed] [Google Scholar]
- 8.Athanasou NA, Pandey R, de Steiger R, Crook D, Smith McLardy P. 1995. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Br 77:28–33. [PubMed] [Google Scholar]
- 9.Feldman DS, Lonner JH, Desai P, Zuckerman JD. 1995. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am 77:1807–1813. [DOI] [PubMed] [Google Scholar]
- 10.Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. 1999. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am 81:672–683. [DOI] [PubMed] [Google Scholar]
- 11.Mirra JM, Marder RA, Amstutz HC. 1982. The pathology of failed total joint arthroplasty. Clin Orthop Relat Res 170:175–183. [PubMed] [Google Scholar]
- 12.Musso AD, Mohanty K, Spencer-Jones R. 2003. Role of frozen section of histology in diagnosis of infection during revision arthroplasty. Postgrad Med J 79:590–593. doi: 10.1136/pmj.79.936.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bori G, Munoz-Mahamud E, Garcia S, Mallofre C, Gallart X, Bosch J, Garcia E, Riba J, Mensa J, Soriano A. 2011. Interface membrane is the best sample for histopathological study to diagnose prosthetic joint infection. Modern Pathology 24:579–584. doi: 10.1038/modpathol.2010.219. [DOI] [PubMed] [Google Scholar]
- 14.Morawietz L, Classen RA, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V. 2006. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol 59:591–597. doi: 10.1136/jcp.2005.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. 2008. Diagnosis of periprosthetic infection following total hip arthroplasty-evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg Res 3:31. doi: 10.1186/1749-799X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder JH, Perka C, Krenn V. 2009. Twenty-three neutrophils granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology 54:847–853. doi: 10.1111/j.1365-2559.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 17.Bémer P, Plouzeau C, Tande D, Léger J, Giraudeau B, Valentin AS, Jolivet-Gougeon A, Vincent P, Corvec S, Gibaud S, Juvin ME, Héry-Arnaud G, Lemarié C, Kempf M, Bret L, Quentin R, Coffre C, de Pinieux G, Bernard L, Burucoa C. 2014. Evaluation of 16S rRNA gene PCR sensitivity and specificity for diagnosis of prosthetic joint infection: a prospective multicenter cross-sectional study. J Clin Microbiol 52:3583. doi: 10.1128/JCM.01459-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plouzeau C, Bémer P, Valentin AS, Héry-Arnaud G, Tandé D, Jolivet-Gougeon A, Vincent P, Kempf M, Lemarié C, Guinard J, Bret L, Cognée AS, Gibaud S, Burucoa C, Corvec S. 2015. First experience of a multicenter external quality assessment of molecular 16S rRNA gene detection in bone and joint infections. J Clin Microbiol 53:419–424. doi: 10.1128/JCM.02413-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukayama DT, Estrada R, Gustilo RB. 1996. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 78:512–523. [DOI] [PubMed] [Google Scholar]
- 20.Tsukayama DT, Goldberg VM, Kyle R. 2003. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am 85(Suppl 1):S75–S80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 21.European Committee on Antimicrobial Susceptibility Testing. 2012. Breakpoint tables for interpretation of MICs and zone diameters, version 2.0, valid from 2012-01-01. European Committee on Antimicrobial Susceptibility Testing, Vaxjo, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf. [Google Scholar]
- 22.Krenn V, Morawietz L, Perino G, Kienapfel H, Ascherl R, Hassenpflug GJ, Thomsen M, Thomas P, Huber M, Kendoff D, Baumhoer D, Krukemeyer MG, Natu S, Boettner F, Zustin J, Kölbel B, Rüther W, Kretzer JP, Tiemann A, Trampuz A, Frommelt L, Tichilow R, Söder S, Müller S, Parvizi J, Illgner U, Gehrke T. 2014. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract 210:779–786. doi: 10.1016/j.prp.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Krenn VT, Liebisch M, Kölbel B, Renz N, Gehrke T, Huber M, Krukemeyer MG, Trampuz A, Reschg H, Krenn V. 2017. CD15 focus score: infection diagnosis and stratification into low-virulence and high-virulence microbial pathogens in periprosthetic joint infection. Pathol Res Pract 213:541–547. doi: 10.1016/j.prp.2017.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.