LETTER
Ceftazidime-avibactam (CZA) is a newer β-lactam–β-lactamase inhibitor combination with in vitro activity against Gram-negative bacteria, including a range of carbapenemase-producing organisms (1). Quantitative MIC data are often necessary for the management of infections in critical care patients. MIC gradient tests are particularly valuable in these situations.
We compared the performance of the CZA Etest (bioMérieux SA, Marcy l'Etoile, France) with that of the reference broth microdilution (BMD) method against 200 Gram-negative bacteria, of which 140 were Enterobacterales strains (including 28 extended-spectrum-β-lactamase [ESBL]-producing organisms, 20 carbapenemase-producing organisms, and 3 organisms coproducing an ESBL and a carbapenemase) and 60 Pseudomonas aeruginosa strains (including 18 carbapenemase-producing organisms). BMD MICs of imipenem and meropenem for the carbapenemase-producing Enterobacterales strains ranged from 1 to >32 μg/ml and 0.5 to >32 μg/ml, respectively, while those for carbapenemase-producing P. aeruginosa strains were in the range of 32 to >32 μg/ml.
Powders of ceftazidime and avibactam as well as Etest strips were obtained from bioMérieux (Marcy l'Etoile, France). BMD MICs were determined according to international standard ISO 20776-1 on panels prepared in-house using cation-adjusted Mueller-Hinton broth (Becton Dickinson, Heidelberg, Germany) (2). Concentrations of ceftazidime ranged from 0.016 to 256 μg/ml. The concentration of avibactam was fixed at 4 μg/ml. The Etest was done according to the manufacturer's instructions using Mueller-Hinton E agar (MHE; bioMérieux SA, Marcy l'Etoile, France) as the growth medium. Etest endpoints were read by the naked eye. BMD and the Etest were performed using the same bacterial suspension, incubation temperature (35°C ± 1°C), and time (18 h). Escherichia coli ATCC 25922, Escherichia coli ATCC 35218, Klebsiella pneumoniae ATCC 700603 (ESBL positive), and P. aeruginosa ATCC 27853 were included for quality control.
Ceftazidime-avibactam MICs were interpreted using the breakpoints set by the U.S. Food and Drug Administration (FDA) (3), the Clinical and Laboratory Standards Institute (CLSI) (4), and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (5), as follows: for susceptible isolates, MICs were ≤8/4 μg/ml, respectively, and for resistant isolates, MICs were ≥16/4 μg/ml, respectively, according to the FDA and CLSI and >8/4 μg/ml, respectively, according to EUCAST. The production of ESBLs and carbapenemases was confirmed by phenotypic and molecular methods.
To allow a fair comparison of the two methods, Etest MICs lying in between the standard values were rounded up to the next concentration matching the 2-fold dilution scheme of the BMD method. All test organisms were included in the head-to-head analyses of Etest and BMD results for the category agreement (CA) evaluation (agreement of interpretative results) and the overall essential agreement (EA), while isolates exhibiting on-scale MICs from both the Etest and BMD (n = 178) were also included in the calculation of the EA based on evaluable results. EA was established when the Etest MIC agreed within ±1 log2 dilution with the BMD MIC.
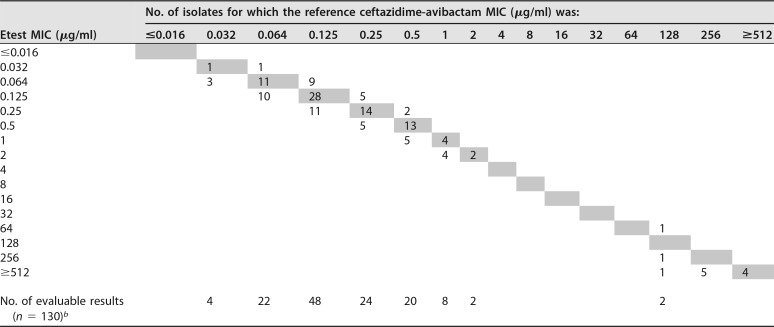
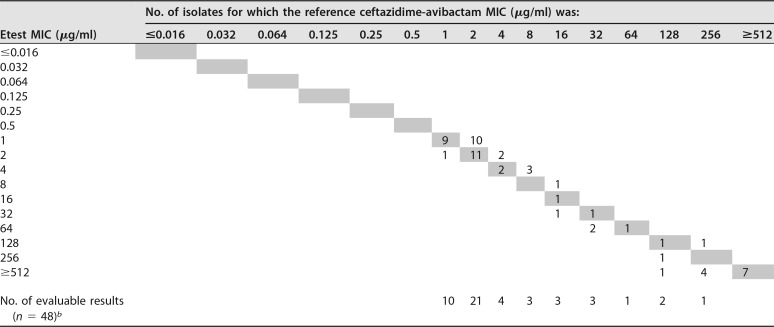
When the BMD method was used, 36.7% (n = 22) of the P. aeruginosa and 8.6% (n = 12) of the Enterobacterales strains were CZA resistant. The overall EA was 99% (98.3% for P. aeruginosa and 99.3% for Enterobacterales isolates), the EA of evaluable results was 100%, and the overall CA was 99.5% (98.3% for P. aeruginosa and 100% for Enterobacterales isolates) (Tables 1 to 3). There were no major errors (ME; false resistance), but there was one very major error (VME; false susceptibility) related to a carbapenem-resistant, non-carbapenemase-producing P. aeruginosa strain for which the BMD MIC was 16/4 μg CZA per ml (resistant) and for which the Etest MIC was 8/4 μg/ml (susceptible) (Table 1).
TABLE 1.
Scattergram of Etest MICs and BMD MICs for Enterobacterales isolatesa
One hundred forty isolates were tested. MICs are expressed as the ceftazidime concentration.
bTest organisms for which on-scale MICs were available from both the Etest and BMD.
TABLE 2.
Scattergram of Etest MICs and BMD MICs for P. aeruginosa isolatesa
Sixty isolates were tested. MICs are expressed as the ceftazidime concentration.
bTest organisms for which on-scale MICs wereavailable from both the Etest and BMD.
TABLE 3.
Evaluation of agreement and errors between results of the Etest and BMD
| Organism(s) | No. of strains tested | CAa |
MEb |
VMEc |
Overall EAd |
EA of evaluable resultse |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No.f | % | No. | % | No. | % | No. | % | No. | % | ||
| Enterobacterales | 140 | 140 | 100.0 | 0 | 0.0 | 0 | 0.0 | 139 | 99.3 | 130 | 100.0 |
| P. aeruginosa | 60 | 59 | 98.3 | 0 | 0.0 | 1 | 4.5 | 59 | 98.3 | 48 | 100.0 |
| Total | 200 | 199 | 99.5 | 0 | 0.0 | 1 | 0.5 | 198 | 99.0 | 178 | 100.0 |
CA, categorical agreement of interpretative results (susceptible, resistant) between the Etest and BMD. All test organisms were included in the calculation.
ME, major errors. Organisms were resistant by the Etest and susceptible by BMD.
VME, very major errors. Organisms were susceptible by the Etest and resistant by BMD.
Overall EA, essential agreement within ±1 log2 dilution between the Etest and BMD. All test organisms were included in the calculation.
EA of evaluable results, essential agreement within ±1 log2 dilution between results of the Etest and BMD. Test organisms for which on-scale MICs were available from both the Etest and BMD (Enterobacterales, n = 130; P. aeruginosa, n = 48) were included in the calculation.
No., number of isolates.
To the best of our knowledge, this is the first study that has compared the CZA Etest to the standard BMD method against a large collection of carbapenem-susceptible and non-carbapenem-susceptible Enterobacterales and P. aeruginosa isolates. The rates of CA and EA between BMD and Etest results for Enterobacterales in the present study were similar to those reported in an earlier study verifying the CZA Etest against a collection of 74 carbapenem-resistant Enterobacteriaceae. In that study, EA and CA between BMD and Etest results were 89% and 97%, respectively (6). The investigators noted VMEs for two CZA-resistant strains for which BMD MICs were 16/4 μg/ml and Etest MICs were 8/4 μg/ml, as was also observed in the present study.
In conclusion, our study provided excellent results in terms of EA and CA. Hence, the Etest seems to be a suitable test device to prove the susceptibility of Enterobacterales and P. aeruginosa isolates to CZA.
ACKNOWLEDGMENTS
This investigation was funded by bioMérieux, Marcy l'Etoile, France.
M.K. is a partner and CEO of Antiinfectives Intelligence GmbH, a research organization providing services to pharmaceutical companies. B.K.-I. was an employee of Antiinfectives Intelligence GmbH.
REFERENCES
- 1.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Organization for Standardization (ISO). 2006. Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. ISO 20776-1:2006. ISO, Geneva, Switzerland. [Google Scholar]
- 3.Food and Drug Administration (FDA). 12 December 2017. Ceftazidime avibactam injection. FDA-identified interpretive criteria. FDA, Silver Spring, MD: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm587129.htm. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute (CLSI). 2018. Performance standards for antimicrobial susceptibility testing, 28th ed CLSI supplement M100. CLSI, Wayne, PA. [Google Scholar]
- 5.The European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.0, 2018. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf.
- 6.Shields RK, Clancy CJ, Pasculle AW, Press EG, Haidar G, Hao B, Chen L, Kreiswirth BN, Nguyen MH. 2018. Verification of ceftazidime-avibactam and ceftolozane-tazobactam susceptibility testing methods against carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol 56:e01093-17. doi: 10.1128/JCM.01093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]