Abstract
Nodal cytotoxic molecule (CM)‐positive peripheral T‐cell lymphoma (CTL) has recently been recognized as a clinicopathologically distinct disease. To further characterize this disease, here we compared 58 patients with Epstein‐Barr virus (EBV)‐negative CTL to 48 patients with EBV‐positive CTL. The two groups did not differ in histopathology, T‐cell receptor (TCR) expression or rearrangement incidences, or survival curves. However, patients with EBV‐negative CTL less frequently showed hepatic involvement (P = .007), B symptoms (P = .020), hemophagocytosis (P = .024), and detectable CD4 (P = .002) and CD5 (P = .009). Univariate and multivariate analyses identified three factors that independently predicted favorable survival, onset age <60 years (P = .002), CD5 expression (P = .002), and mixed morphology (P = .013), TCRαβ was not an independent predictor (P = .30), but was strongly linked with long survivorship among patients younger than 60 years old. A prognostic model incorporating these factors worked well for prognostic delineation, independently of the International Prognostic Index (P = .007 vs P = .082) and Prognostic Index for PTCL (P = .020 vs P = .15). Moreover, this constellation of findings indicated two nodal indolent diseases: CD5+ TCRαβ (n = 13), and CD5+ NK‐cell type lacking TCR expression or clonal TCRγ rearrangement (n = 4). The survival curves for these two groups were significantly superior to others (n = 29, P < .001). These diseases appear to be unique in their indolent clinical behavior, and should be managed differently from other diseases.
Keywords: cytotoxic molecule, Epstein‐Barr virus, onset age, peripheral T‐cell lymphoma‐not otherwise specified, TCR phenotype
1. INTRODUCTION
World Health Organization (WHO) classifications report that peripheral T‐cell lymphoma not otherwise specified (PTCL‐NOS) is the most common subtype of mature T‐cell and natural killer (NK) cell neoplasms1, 2 and is a heterogeneous disease with a generally poor prognosis, but not a distinctive immunophenotype.3
Cytotoxic molecules (CMs) such as granzyme B, and perforin are cellular lytic granules that are secreted from CD8‐positive T cells and NK cells.4, 5, 6 The CMs, which are expressed in extranodal T‐cell lymphoma subtypes such as extranodal NK/T‐cell lymphoma, nasal type (ENKL), and hepatosplenic T‐cell lymphoma, are generally associated with poor prognosis.7, 8 Research over the last decade has led to the identification of previously unrecognized indolent cytotoxic T‐cell and NK‐cell lymphomas/lymphoproliferative disorders (LPDs), including indolent T‐cell LPD of the gastrointestinal tract, NK‐cell enteropathy/lymphomatoid gastroenteropathy, and primary cutaneous acral CD8+ T‐cell lymphoma.1, 2 CM expression has also been noted in some nodal PTCLs. We have reported that nodal CM‐positive PTCL (CTL) shows a more aggressive clinical course than CM‐negative PTCL, such that CMs constitute a useful biomarker for PTCL.7 To our knowledge, nodal indolent cytotoxic lymphomas have not been addressed in the past.
There is an accumulation of genetic mutations in TCR signaling molecules in PTCLs, demonstrating that the TCR and its downstream signaling pathway are critically important for their development.9, 10, 11, 12, 13, 14, 15 TCR comprises an αβ or γδ heterodimer, and αβ is a major component in PTCL as well as T‐lymphocytes.16, 17 PTCL subtypes derived from γδ T cells—such as primary cutaneous γδ T‐cell lymphoma, hepatosplenic T‐cell lymphoma, and monomorphic intestinal T‐cell lymphoma—show aggressive clinical behavior.1, 2, 18, 19, 20, 21, 22 We recently documented that nodal EBV‐positive CTL has poor prognosis often with a γδ phenotype.7, 8, 23 On the other hand, recent studies shed a light on an indolent cytotoxic T‐cell lymphoma/LPD, characterized by a TCRαβ phenotype and younger onset age, referred to as subcutaneous panniculitis‐like T‐cell lymphoma with an αβ T‐cell phenotype.24, 25, 26, 27
The available data suggest that CMs, EBV infection, and TCRαβ or γδ phenotype status likely affect the pathophysiology of PTCL‐NOS. While we previously described the clinicopathological features of nodal EBV‐positive CTL, EBV‐negative CTL remains uncharacterized.8, 23 In our present study, we retrospectively investigated the pathological and clinical features of nodal EBV‐negative CTL, which led to the identification of nodal indolent CD5‐positive diseases predominantly affecting patients younger than 60 years old.
2. MATERIALS AND METHODS
2.1. Patients
This study enrolled patients with PTCL‐NOS consecutively diagnosed by lymph node biopsy according to the WHO classification2 between January 1982 and April 2015. They were also clinically evaluated for nodal disease. Inclusion criteria were the absence of B‐cell markers, and positivity for at least one T‐cell antigen (CD3, CD4, CD5, CD8, or CD45RO) according to either immunohistochemistry or flow cytometry. All were positive for expression of at least one CM. We evaluated the presence of EBV using EBV‐encoded small nuclear early region in situ hybridization with a cut‐off of >50% positivity of neoplastic cells. The analysis excluded patients with lymphoepithelioid (Lennert) lymphoma, angioimmunoblastic T‐cell lymphoma (AITL), anaplastic lymphoma kinase (ALK)‐positive or ALK‐negative anaplastic large cell lymphoma (ALCL), ATLL, primary cutaneous T‐cell lymphoma, or ENKL. Tumors showing morphologically within the spectrum of ALK‐positive ALCL, with strong and uniform expression of CD30 were ruled out from our analysis as ALCL.
We identified 58 evaluable cases of nodal EBV‐negative CTL with paraffin blocks available for analyses, including 39 in our previous study.8 As a control group, we analyzed data from 48 nodal EBV‐positive CTL cases, including 39 previously reported.8, 23
Our study protocol was approved by the institutional review board of Nagoya University (No.1066‐3).
2.2. Histopathology
Tissue samples were fixed in 10% formalin, embedded in paraffin (FFPE). The cases were reviewed by three pathologists (D.Y., S.K., and S.N.), and were divided into four morphologic groups based on cell nuclei shape: centroblastoid, pleomorphic, mixed, and unspecified (Figure 1A‐C). In the centroblastoid group, >50% of the neoplastic cells were large and had oval‐to‐round vesicular nuclei with fine chromatin, morphologically resembling diffuse large B‐cell lymphoma (DLBCL) cells. In the pleomorphic group, over two‐thirds of the tumor cells had pleomorphic features with irregular nuclei folding. In the mixed group, the tumors comprised a mixture of medium and large cells. Despite the variation in cell size, cases with mixed morphology showed lower cellular atypia than cases with pleomorphic morphology. Finally, the unspecified group included cases for which the biopsy specimens were too small to reach a good consensus regarding morphology. We also evaluated cells for the presence of elongated nuclei.
Figure 1.
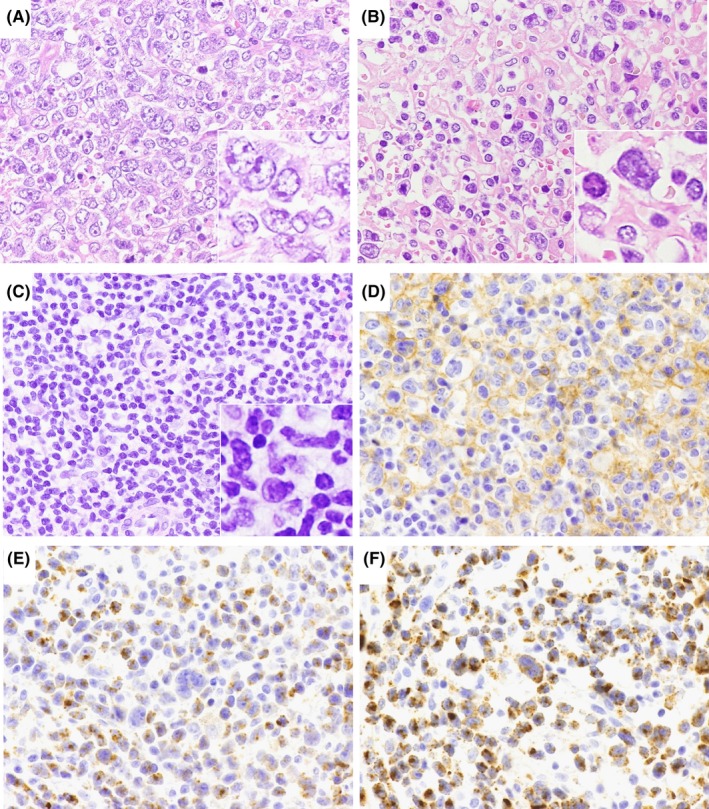
Light microscopy images of nodal Epstein‐Barr virus ‐negative cytotoxic molecule(CM)‐positive peripheral T‐cell lymphoma samples. Hematoxylin and eosin staining was performed to examine nuclear morphology, revealing centroblastoid morphology (A), pleomorphic morphology (B) and mixed morphology (C). Other samples were immunostained for CD4 (D), TIA‐1 (E), and granzyme B (F). Original magnification: 400×. Inset: enlarged view of tumor cells
2.3. Immunophenotypic and ISH analysis
FFPE sections were subjected to immunoperoxidase analysis with monoclonal antibodies as follows: CD2, CD4, CD5, and CD56 (Novocastra Laboratories, Newcastle, UK); CD3, CD8, UCHL‐1/CD45RO, L26/CD20, Ber‐H2/CD30, and ALK1 (Dako, Santa Clara, CA); βF1 (T‐cell receptor [TCR] β chain; T Cell Science, Cambridge, MA); TCR 1153 (TCR‐γ; clone γ 3.20) and TCRδ constant region (clone 5A6.E9; Thermo Fisher Scientific),23 TIA‐1 (Coulter Immunology, Hialeah, FL), granzyme B (Monosan, Uden, the Netherlands), PD‐L1 (clone SP142; Spring Bioscience, Pleasanton, CA), and ALK 5A4.28 The reactions were considered positive with a cut‐off of 30% (Figure 1D‐F). Tumor cell and microenviroment PD‐L1 expression was considered positive when ≥10% of the tumor cells and nonmalignant stromal cells showed membranous and/or cytoplasmic PD‐L1 staining, resepectively.29, 30
To evaluate the presence of EBV small ribonucleic acids, we subjected formalin‐fixed, paraffin‐embedded sections to in situ hybridization using EBV‐encoded small nuclear early region (EBER) oligonucleotides, as previously reported.8
2.4. TCRγ PCR analysis
Paraffin‐embedded tissue was examined by using a QiaAmp kit for DNA extraction from tissue (QIAGEN GmbH, Hilden, Germany) for PCR analysis of the TCRγ gene according to the BIOMED2 protocol with the QIAGEN Multiplex PCR Kit and GeneScan Analysis Software (Perkin Elmer Biosystems, Weiterstadt, Germany) as described previously.31
2.5. Statistical analysis
We evaluated correlations between the two groups using Fisher's exact test and Student's t test. Patient survival data were analyzed using the Kaplan‐Meier method and the log‐rank test. Survivors with a follow‐up period <6 months were excluded from analysis. We performed univariate and multivariate analyses using a Cox proportional hazard regression model. All statistical analyses were performed using the graphical user interface for R, EZR32 (The R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Clinicopathological characteristics of nodal CTLs
Compared to nodal EBV‐positive CTL (n = 48), nodal EBV‐negative CTL (n = 58) was more commonly associated with favorable clinical parameters at presentation (Table 1). The latter showed lower frequencies of hepatic involvement (10% vs 32%, P = .007); B symptoms (47% vs 72%, P = .022), and hemophagocytosis (13% vs 35%, P = .024) compared to the former.
Table 1.
Differences in the clinicopathological characteristics between nodal EBV‐negative and ‐positive CTL
| Nodal EBV‐negative CTL(n = 58) (n [%]) | Nodal EBV‐positive CTL(n = 48) (n [%]) | P | |
|---|---|---|---|
| Age at diagnosis (median[range]) (y) | 65 (29‐88) | 62 (0‐80) | .22 |
| Age at diagnosis > 60 y | 36/58 (62) | 27/48 (56) | .56 |
| Sex (male/female) | 30/28 | 33/15 | .11 |
| PS > 1 | 21/49 (42) | 23/43 (53) | .40 |
| Clinical stage III/IV | 44/57 (77) | 40/46 (86) | .31 |
| B symptoms present | 25/53 (47) | 31/43 (72) | .022 |
| Extranodal site > 1 site | 13/58 (22) | 7/47 (15) | .45 |
| Extranodal sites | |||
| Bone marrow | 11/55 (20) | 11/45 (24) | .63 |
| Liver | 6/58 (10) | 15/46 (32) | .007 |
| Skin and/or soft tissue | 7/58 (12) | 1/46 (2) | .074 |
| GI tract | 3/58 (5) | 1/46 (2) | .63 |
| Hemophagocytosis | 7/51 (13) | 14/40 (35) | .024 |
| IPI_high‐intermediate/high | 34/54 (62) | 29/45 (64) | 1.0 |
| PIT group 3/4 | 38/54 (70) | 30/45 (66) | .83 |
| Hb < 13 g/dL (male) or Hb < 11 g/dL (female) | 25/51 (49) | 23/40 (57) | .53 |
| Platelets < 130 × 109/L | 17/51 (33) | 22/40 (55) | .055 |
| Serum LDH > normal | 42/56 (75) | 35/45 (77) | .81 |
| CRP > normal | 41/48 (85) | 22/25 (88) | 1.0 |
| Prior immunosuppressive drug therapy | 4/47 (8) | 4/25 (16) | .44 |
| History of autoimmune disease | 3/47 (6) | 4/29 (13) | .23 |
| Treatment | |||
| No therapy | 2/58 (3) | 8/45 (17) | .02 |
| CT with anthracycline | 46/55 (83) | 32/45 (71) | .35 |
| CT without anthracycline | 7/55 (12) | 5/45 (11) | 1.0 |
| ASCT | 8/58 (13) | 6/45 (13) | 1.0 |
| Response | |||
| CR | 20/49 (41) | 11/35 (31) | .49 |
| PR | 10/49 (20) | 8/35 (22) | .79 |
| NR | 19/49 (39) | 16/35 (45) | .65 |
| Morphology | |||
| Centroblastoid | 20/50 (40) | 24/46 (52) | .31 |
| Pleomorphic | 14/50 (29) | 12/46 (26) | .91 |
| Mixed | 12/50 (24) | 5/46 (10) | .092 |
| Unspecified | 4/50 (8) | 5/46 (10) | .73 |
| Immunophenotype | |||
| nPD‐L1 | 3/19 (16) | 2/22 (9) | .65 |
| miPD‐L1 | 10/19 (53) | 11/22 (50) | 1.0 |
| TIA‐1 | 50/58 (86) | 46/48 (95) | .11 |
| Granzyme B | 38/55 (69) | 45/47 (95) | <.001 |
| cyCD3 | 49/57 (85) | 46/48 (95) | .11 |
| CD4 | 27/54 (50) | 9/47 (19) | .002 |
| CD5 | 31/55 (56) | 14/47 (29) | .009 |
| CD8 | 14/55 (25) | 30/47 (63) | <.001 |
| CD30 | 24/45 (53) | 12/32 (37) | .25 |
| CD56 | 9/58 (15) | 6/48 (12) | .78 |
ASCT, autologous stem cell transplantation; CR, complete remission; CRP, C‐reactive protein; CT, chemotherapy; CTL, cytotoxic molecule(CM)‐positive peripheral T‐cell lymphoma; cyCD3, cytoplasmic CD3; EBV, Epstein‐Barr virus; GI tract, gastrointestinal tract; Hb, hemoglobin; IPI, International Prognostic Index; LDH, lactate dehydrogenase; miPD‐L1, microenvironmental PD‐L1; nPD‐L1, neoplastic PD‐L1; NR, no response; PIT, prognostic index for PTCL; PR, partial remission; PS, performance status.
Treatment for nodal EBV‐negative CTL comprised chemotherapeutic regimens with or without anthracycline (46 and 7 patients, respectively). Eight patients underwent high‐dose chemotherapy with autologous hematopoietic stem cell transplantation (ASCT). None of the patients underwent allogeneic transplants. Two exhibited a rapidly lethal clinical course within 3 weeks before any treatment.
Morphological findings did not differ between the EBV‐positive and ‐negative groups. Compared to the latter, the former less frequently showed expression of CD4 (50% vs 19%, P = .002) and CD5 (56% vs 29%, P = .009), but more frequently exhibited CD8 (25% vs 63%, P < .001) and granzyme B (69% vs 95%, P < .001).
Anaplastic lymphoma kinase expression was not detected in our series using the conventional ALK1 antibody. This absence was further verified in 26 EBV‐negative cases by using a highly sensitive immunohistochemistry assay. We also did not detect EBV‐harboring tumor cells in the present series, although two were accompanied by a small number (5%‐15%) of EBV+ B lymphocytes.
Immunohistochemistry showed that nodal EBV‐negative and EBV‐positive CTL cases had similar ratios for neoplastic positive PD‐L1 expression (16% vs 9%, P = .65) and microenvironmental positive PD‐L1 expression (53% vs 50%, P = 1.0). Our series of EBV‐negative CTL cases was also consisted of TIA‐1+ granzyme B+ (n = 33) and TIA‐1+ granzyme B− types (n = 15), the latter of which showed higher frequencies of gastrointestinal tract involvement (20% vs 0%, P = .026) and lower CR ratio (14% vs 53%, P = .020) than the former, despite their overall overlapping survival curves with median survival times of 8 and 11 months, respectively (Table S1 and Figure S1).
3.2. TCR phenotype of nodal CTLs
Among the 47 nodal EBV‐negative cases, 23 showed TCRβ positivity (ie, TCRαβ type) (Table 2). Four were determined to be TCRγδ type based on TCRγ and/or TCRδ positivity and TCRβ negativity. Twelve showed clonal TCRγ gene rearrangement without expression of TCR β, γ, or δ, and were thus designated TCR‐silent type. Finally, eight were NK‐cell type, as they showed no TCR protein expression or clonal TCRγ gene rearrangement. Overall, 39 cases (82%) of nodal EBV‐negative CTL were categorized as T‐cell types based on their TCR protein expression and/or clonal TCRγ gene rearrangement. Among EBV‐positive CTLs, 80% were categorized as T‐cell types. EBV‐negative and EBV‐positive CTLs did not significantly differ in the incidences of the TCR subtypes.
Table 2.
Differences in the TCR phenotype between nodal EBV‐negative and EBV‐positive CTL
| Nodal EBV‐negative CTL (n = 47) (n [%]) | Nodal EBV‐positive CTL (n = 41) (n [%]) | P | |
|---|---|---|---|
| T‐cell type | 39/47 (82) | 33/41 (80) | .79 |
| αβ T (TCR β positive) | 23/47 (48) | 18/41 (43) | .67 |
| γδ T (TCR γ positive and/or δ positive) | 4/47 (8) | 5/41 (12) | .73 |
| TCR‐silent | 12/47 (25) | 10/41 (24) | 1.0 |
| NK‐cell type | 8/47 (17) | 8/41 (19) | .79 |
CTL, cytotoxic molecule (CM)‐positive peripheral T‐cell lymphoma; EBV, Epstein‐Barr virus; NK, natural killer; TCR, T‐cell receptor.
Patients with T‐cell type showed positivity for TCR protein expression and/or TCRγ gene rearrangement. TCR‐silent cases were negative for TCRβ, γ, and δ expression but positive for clonal TCRγ gene rearrangement. Patients with NK‐cell type did not have any of the TCR protein expression or clonal TCRγ gene rearrangement. One case of nodal EBV‐negative CTL had TCRβ and γ double positive type.
3.3. Overall survival for patients with nodal CTLs
The unadjusted OS curves for patients with nodal EBV‐positive and EBV‐negative CTLs showed an aggressive clinical course, with median survival times of 8 and 11 months, respectively (Figure 2A). The survival curves for 24 months after diagnosis overlapped (P = .14), as was previously documented by our group.8
Figure 2.
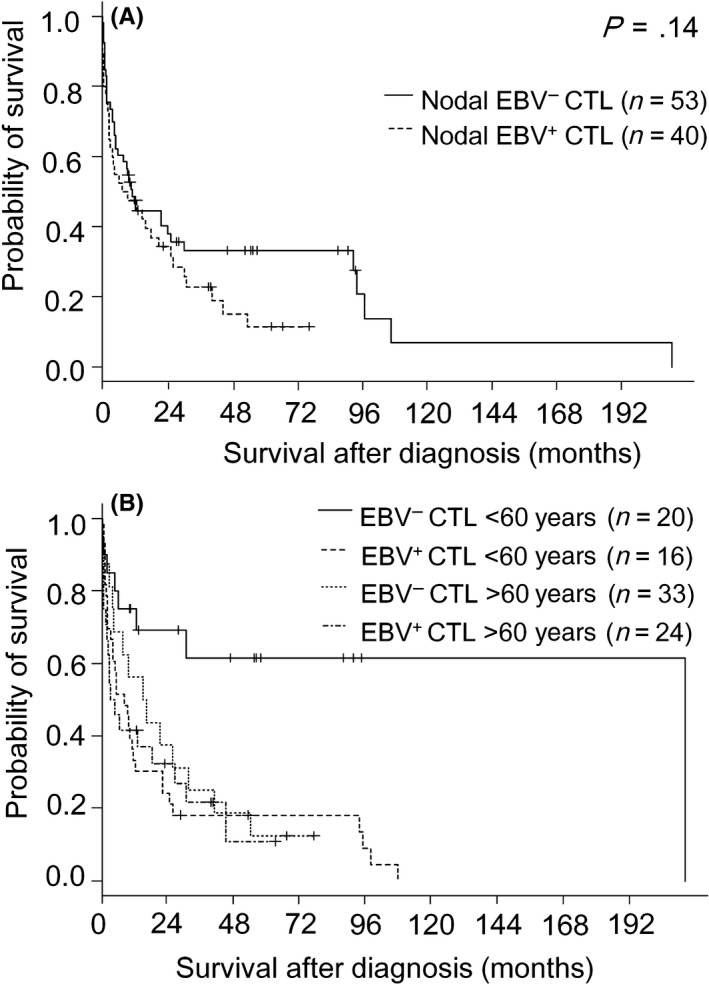
Survival curves for nodal Epstein‐Barr virus (EBV)‐negative and EBV‐positive cytotoxic molecule(CM)‐positive peripheral T‐cell lymphoma (CTL) patients (A) and nodal EBV‐negative and EBV‐positive CTL patients with a cut‐off age of 60 y (B)
3.4. Univariate and multivariate analysis of overall survival for nodal CTL cases
In the univariate analysis of our whole group consisting of EBV‐positive (n = 48) and ‐negative cases (n = 58), variables that predicted poor OS included Prognostic Index for PTCL (PIT) group 3 or 4, with a hazard ratio (HR) of 2.88 (P < .001); International Prognostic Index (IPI) high‐intermediate/high risk group (HR = 2.79, P < .001); extranodal involvement at >1 site (HR = 3.39, P < .001); bone marrow involvement (HR = 2.62, P < .001); thrombocytopenia (HR = 2.36, P < .001); presence of B symptoms (HR = 2.60, P < .001); hemophagocytosis (HR = 2.42, P = .002); liver involvement (HR = 2.32, P = .002); above‐normal serum lactic dehydrogenase (LDH) (HR = 2.54, P = .004); performance status (PS) > 1 (HR = 2.01, P = .006); CRP high (HR = 11.8, P = .015); and gastrointestinal involvement (HR = 2.84, P = .046); but not EBV‐harboring (HR = 1.44, P = .14).Variables that predicted a favorable outcome included CD5 positivity (HR = 0.40, P < .001); onset age younger than 60 years (HR = 0.48, P = .005); and mixed morphology (HR = 0.40, P = .011), but not ASCT (HR = 0.51, P = .093) (Table S2). In this cohort, multivariate analysis showed that CD5 was independent from both of IPI and PIT, and that CD5 and mixed appearance, but not TCRαβ or onset age younger than 60 years, were significant among these 4 variables (Table 3A).
Table 3.
Multivariate Cox analysis for prognostic factors affecting overall survival of (A) nodal CTL, (B) nodal EBV‐negative CTL
| Factor | Multivariate analysis HR (95% CI) | P |
|---|---|---|
| (A) | ||
| Comparison with factors | ||
| Mixed morphology | 0.34 (0.16‐0.73) | .006 |
| Onset age <60 y | 0.70 (0.39‐1.27) | .24 |
| CD5 positivity | 0.21 (0.09‐0.49) | <.001 |
| Comparison with factors | ||
| Onset age <60 y | 0.67 (0.37‐1.21) | .19 |
| CD5 positivity | 0.36 (0.19‐0.68) | .002 |
| Mixed morphology | 0.38 (0.18‐0.82) | .013 |
| TCRαβ | 0.75 (0.43‐1.32) | .32 |
| Comparison with factors | ||
| CD5 positivity | 0.45 (0.27‐0.77) | .003 |
| IPI | 2.95 (1.66‐5.23) | <.001 |
| Comparison with factors | ||
| CD5 positivity | 0.50 (0.30‐0.85) | .011 |
| PIT | 2.70 (1.48‐4.94) | .001 |
| (B) | ||
| Comparison with factors | ||
| Mixed morphology | 0.27 (0.10‐0.72) | .009 |
| Onset age <60 y | 0.31 (0.12‐0.76) | .011 |
| CD5 positivity | 0.36 (0.16‐0.82) | .014 |
| Comparison with factors | ||
| Onset age <60 y | 0.20 (0.07‐0.61) | .005 |
| CD5 positivity | 0.30 (0.12‐0.76) | .011 |
| Mixed morphology | 0.27 (0.09‐0.79) | .018 |
| TCRαβ | 0.50 (0.21‐1.17) | .11 |
| Comparison with factors | ||
| Our prognostic model | 0.45 (0.25‐0.80) | .007 |
| IPI | 1.36 (0.96‐1.94) | .082 |
| Comparison with factors | ||
| Our prognostic model | 0.48 (0.26‐0.90) | .020 |
| PIT | 1.37 (0.89‐2.11) | .15 |
CI, confidence interval; CTL, cytotoxic molecule (CM)‐positive peripheral T‐cell lymphoma; EBV indicates Epstein‐Barr virus; IPI, International Prognostic Index; PIT, prognostic index for PTCL; TCR; TCR, T‐cell receptor.
In the analysis limited for nodal EBV ‐negative CTL cases, variables that predicted poor OS included PIT group 3 or 4 (HR = 5.34, P < .001); IPI high‐intermediate/high risk group (HR = 4.26, P < .001); extranodal involvement at >1 site (HR = 3.71, P < .001); presence of B symptoms (HR = 2.79, P = .004); hemophagocytosis (HR = 3.32, P = .006); above‐normal serum LDH (HR = 3.28, P = .009); PS > 1 (HR = 2.29, P = .025); and bone marrow involvement (HR = 2.16, P = .038). Variables that predicted a favorable outcome included onset age younger than 60 years (HR = 0.27, P = .002), CD5 positivity (HR = 0.32, P = .002), and mixed morphology (HR = 0.29, P = .013) (Table S3). Interestingly, multivariate analysis revealed that these favorable prognostic factors were independently significant (Table 3B). Receiving ASCT was not a significant factor predicting favorable outcome (HR = 0.37, P = .099).
3.5. Comparison of the clinicopathological characteristics of nodal EBV‐negative CTL patients using a cut‐off age of 60 years
After unexpectedly finding that younger onset age was a favorable prognostic indicator, we analyzed the clinicopathological characteristics of nodal EBV‐negative patients using a cut‐off of 60 years (Table 4). The available clinical parameters did not significantly differ between groups, except that the younger subgroup was less likely to be in the IPI high‐intermediate/high risk group (31% vs 84%, P < .001) and PIT group 3/4 (31% vs 96%, P < .001).
Table 4.
Clinicopathological features of nodal EBV‐negative CTL with younger and older onset age
| <60 y (n = 22) (n [%]) | >60 y (n = 36) (n [%]) | P | |
|---|---|---|---|
| Age at diagnosis (years) median (range) | 52 (29‐60) | 72 (61‐88) | <.001 |
| Sex (male/female) | 12/10 | 18/18 | .79 |
| PS >1 | 9/20 (45) | 12/29 (41) | 1.0 |
| Clinical stage III/IV | 14/22 (63) | 30/35 (85) | .10 |
| B symptoms | 6/20 (30) | 19/33 (57) | .088 |
| Extranodal site > 1 site | 5/22 (23) | 8/36 (22) | 1.0 |
| Extranodal sites | |||
| Bone marrow | 2/21 (9) | 9/34 (26) | .17 |
| Liver | 2/22 (9) | 4/36 (11) | 1.0 |
| Skin and/or soft tissue | 1/22 (4) | 6/36 (16) | .24 |
| GI tract | 2/22 (9) | 1/36 (2) | .55 |
| Hemophagocytosis | 1/18 (5) | 6/33 (18) | .40 |
| IPI_high‐intermediate/high | 7/22 (31) | 27/32 (84) | <.001 |
| PIT group 3/4 | 7/22 (31) | 31/32 (96) | <.001 |
| Hb < 13 g/dL (male) or Hb < 11 g/dL (female) | 12/18 (66) | 13/33 (39) | .083 |
| Platelets < 130 × 109/L | 3/18 (16) | 14/33 (42) | .073 |
| Serum LDH > normal | 13/21 (61) | 29/35 (82) | .11 |
| CRP > normal | 13/18 (72) | 28/30 (93) | .086 |
| Prior immunosuppressive drug therapy | 2/18 (11) | 2/29 (6) | .63 |
| History of autoimmune disease | 1/18 (5) | 2/29 (6) | 1.0 |
| Treatment | |||
| No therapy | 1/22 (5) | 1/36 (2) | 1.0 |
| CT with anthracycline | 13/19 (68) | 32/36 (88) | .17 |
| CT without anthracycline | 5/19 (26) | 3/36 (8) | .22 |
| ASCT | 7/22 (31) | 1/36 (2) | .003 |
| Response | |||
| CR | 13/20 (65) | 7/29 (24) | .007 |
| PR | 2/20 (10) | 8/29 (27) | .17 |
| NR | 5/20 (25) | 14/29 (48) | .25 |
| Morphology | |||
| Centroblastoid | 8/19 (44) | 12/31 (38) | .77 |
| Pleomorphic | 6/19 (33) | 8/31 (26) | .84 |
| Mixed | 5/19 (26) | 7/31 (22) | 1.0 |
| Unspecified | 0/19 (0) | 4/31 (12) | .28 |
| Immunophenotype | |||
| nPD‐L1 | 2/7 (28) | 1/12 (8) | .52 |
| miPD‐L1 | 3/7 (42) | 7/12 (58) | .65 |
| TIA‐1 | 19/22 (86) | 31/36 (86) | 1.0 |
| Granzyme B | 16/21 (76) | 22/34 (64) | .55 |
| cyCD3 | 19/21 (90) | 30/36 (83) | .70 |
| CD4 | 10/21 (47) | 17/33 (51) | 1.0 |
| CD5 | 15/22 (68) | 16/33 (48) | .18 |
| CD8 | 3/21 (14) | 11/34 (32) | .21 |
| CD30 | 10/17 (58) | 14/28 (50) | .76 |
| CD56 | 5/22 (22) | 4/36 (11) | .28 |
| TCR phenotype | |||
| αβ | 7/17 (41) | 16/30 (53) | .55 |
| γδ | 2/17 (11) | 2/30 (6) | .61 |
| TCR‐silent | 4/17 (23) | 8/30 (26) | 1.0 |
| NK‐cell type | 4/17 (23) | 4/30 (13) | .44 |
ASCT, autologous stem cell transplantation; CR, complete remission; CRP, C‐reactive protein; CT, chemotherapy; CTL, cytotoxic molecule(CM)‐positive peripheral T‐cell lymphoma; cyCD3, cytoplasmic CD3; EBV, Epstein‐Barr virus; GI tract, gastrointestinal tract; Hb, hemoglobin; IPI, International Prognostic Index; LDH, lactate dehydrogenase; miPD‐L1, microenvironmental PD‐L1; NK, natural killer; nPD‐L1, neoplastic PD‐L1; NR, no response; PIT, prognostic index for PTCL; PR, partial remission; PS, performance status; TCR, T‐cell receptor.
Among EBV‐negative CTL cases, the younger subgroup had a significantly more favorable prognosis than the older (median survival of 209 vs 8 months, P < .001) (Figure 2B). But, among EBV‐positive CTL cases, prognosis did not significantly differ between these two age‐based subgroups (median survival of 15 vs 3 months, P = .44).
Detailed analysis of the younger patients revealed that five TCRαβ type patients were alive at a follow‐up time of 10‐86 months. Only one had died of the disease, with a long clinical course of 209 months. This was in contrast to the lethal clinical course within 24 months after diagnosis in 10 (71%) of the 14 patients who were older at diagnosis (Figure 3).
Figure 3.
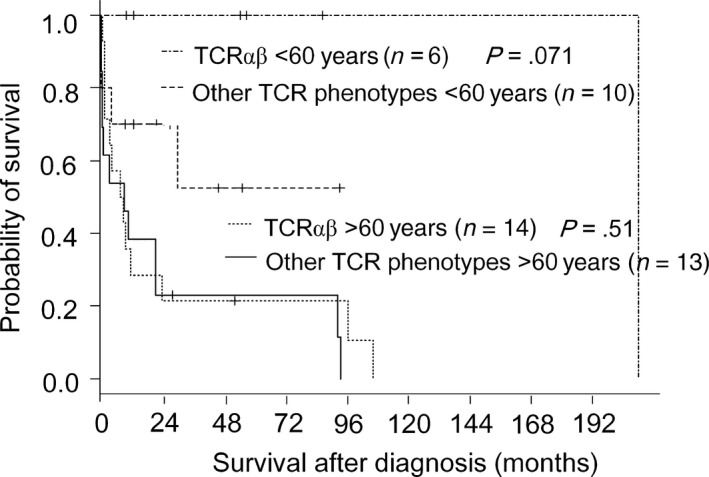
Survival curves for nodal Epstein‐Barr virus‐negative cytotoxic molecule(CM)‐positive peripheral T‐cell lymphoma of the T‐cell receptor (TCR)αβ type and of other TCR phenotypes, with a cut‐off age of 60 y
3.6. Prognostic model for nodal EBV‐negative CTL
Patients in the IPI low/low intermediate risk group (n = 18) had much longer survival than those in the IPI high intermediate/high risk group (n = 31), with a median survival of 209 vs 4 months (P < .001) (Figure 4A). Patients in PIT group 1 or 2 (n = 17) had much longer survival than those in PIT group 3 or 4 (n = 33), with a median survival of 106 vs 5 months (P < .001) (Figure 4A). Multivariate analysis revealed three independently significant favorable prognostic factors: mixed morphology, younger onset age, and CD5 positivity, (Table 3). TCRαβ was not identified as an independent prognostic factor, but has been noted to be highly associated with indolent clinical course among younger patients. We used these four variables to construct a prognostic model as follows: high‐risk group, patients with no favorable factors (n = 5); intermediate‐risk group, patients with one or two factors (n = 35); and low‐risk group, patients with three or four factors (n = 9; Table 5, case # 1‐7, 13, and 14). This prognostic model efficiently identified three groups of patients with different outcomes (Figure 4B, P < .001). Patients in the high‐, intermediate‐, and low‐risk groups had median OS times of 1, 10, and 158 months, respectively. Of note, all of the 8 patients in low risk group of our prognostic model were still alive at 106 months after diagnosis. On the other hand, 7 out of 18 patients in low/low intermediate risk group of IPI died within 30 months. This prognostic model was identified as a variable that predicted a favorable outcome (Table S3, HR = 0.31, P < .001). Multivariate analysis performed for our prognostic model and IPI or PIT revealed that our model was independent from IPI (P = .007 vs P = .082) and PIT (P = .020 vs P = .15, Table 3B).
Figure 4.
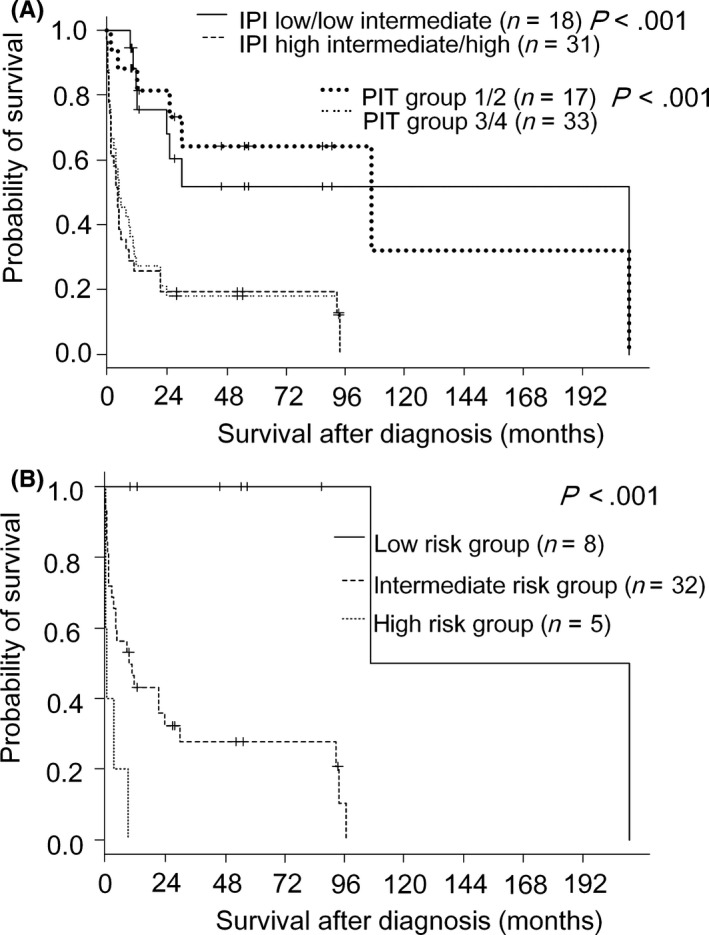
Overall survival curves for nodal Epstein‐Barr virus‐negative patients according to International Prognostic Index or PIT (A), and according to a prognostic model based on four variables: onset age < 60 y, mixed morphology, CD5 expression, and TCRαβ (B)
Table 5.
(A) Clinical findings and follow‐up of 17 patients with CD5 positive TCR αβ or NK‐cell phenotypes of nodal EBV‐negative CTL. (B) Pathological findings of 17 patients with CD5 positive TCR αβ or NK‐cell phenotypes of nodal EBV‐negative CTL
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Age/sex | Score | Stage | IPI | PIT | Chemotherapty | ASCT | Response rate | Follow‐up (mo) |
| 1 | 54/F | 3 | II | 0 | 0 | CHOP, VP‐16 | without | CR | Alive (86) |
| 2 | 59/F | 3 | II | 2 | 2 | NA | without | CR | NA |
| 3 | 56/F | 3 | III | 2 | 1 | THP‐COP | without | CR | Alive (10) |
| 4 | 51/M | 3 | III | 2 | 1 | NA | with | CR | Alive (12) |
| 5 | 57/M | 4 | II | NA | 0 | THP‐COP | without | CR | Alive (57) |
| 6 | 49/M | 4 | III | 2 | 1 | VENP | without | CR | Dead (209) |
| 7 | 29/M | 4 | III | 3 | 2 | CHOP | with | PR | Alive (54) |
| 8 | 61/M | 2 | III | NA | NA | VEPA | without | PR | Dead (96) |
| 9 | 62/M | 2 | III | 3 | 4 | mPSL | NA | NA | NA |
| 10 | 72/M | 2 | IV | 3 | 5 | CHOP | without | NR | Dead (3.6) |
| 11 | 77/F | 2 | IV | 2 | 4 | NA | without | NR | Dead (1.4) |
| 12 | 77/F | 2 | III | 3 | 4 | NA | without | NR | Dead (1.6) |
| 13 | 79/F | 3 | III | NA | NA | THP‐COP | with | CR | Dead (106) |
| 14 | 29/M | 3 | III | 1 | 2 | CHOP, VP16 | without | CR | Alive (46) |
| 15 | 40/M | 2 | III | 2 | 3 | CHOP | with | CR | Alive (93) |
| 16 | 63/F | 1 | III | 4 | 5 | CHOP | with | CR | Dead (93) |
| 17 | 74/F | 1 | III | NA | NA | CHOP | NA | NA | NA |
| (B) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | CD3 | CD4 | CD5 | CD8 | CD30 | CD56 | TIA‐1 | Granzyme | Perforin | TCR phenotype | Morphology |
| 1 | + | + | + | − | + | +a | + | + | NA | αβ | Centroblastoid |
| 2 | + | − | + | − | − | − | + | + | NA | αβ | Pleomorphic |
| 3 | + | − | + | − | − | − | + | + | + | αβ | Centroblastoid |
| 4 | + | + | + | − | + | − | − | + | NA | αβ | Pleomorphic |
| 5 | + | − | + | − | + | − | + | + | NA | αβ | Mixed |
| 6 | + | + | + | − | + | − | + | + | + | αβ | Mixed |
| 7 | − | + | + | − | + | − | − | − | + | αβ | Mixed |
| 8 | + | − | + | + | − | − | + | − | NA | αβ | Unspecified |
| 9 | + | − | + | + | − | − | + | + | + | αβ | Pleomorphic |
| 10 | + | − | + | − | NA | − | − | + | + | αβ | Centroblastoid |
| 11 | + | + | + | − | + | − | + | + | NA | αβ | Pleomorphic |
| 12 | + | + | + | − | NA | − | + | + | NA | αβ | Pleomorphic |
| 13 | + | NA | + | − | NA | − | + | + | NA | αβ | Mixed |
| 14 | + | − | + | − | − | − | + | + | − | NK‐cell | Mixed/pleomorphic |
| 15 | + | + | + | + | NA | − | + | + | + | NK‐cell | Pleomorphic |
| 16 | + | + | + | − | + | − | + | − | NA | NK‐cell | Centroblastoid |
| 17 | + | + | + | + | − | − | − | + | + | NK‐cell | Centroblastoid |
−, negative; +, positive; ASCT, autologous stem cell transplantation; CHOP, cyclophosphamide doxorubicin vincristine and predonisone; CR, complete response; CTL, cytotoxic molecule(CM)‐positive peripheral T‐cell lymphoma; EBV, Epstein‐Barr virus; F, female; IPI, International Prognostic Index; M, male; mPSL, methylprednisolone; NA, not available; NK, natural killer; NR, no response; PIT, prognostic index for PTCL; PR, partial response; TCR, T‐cell receptor; THP‐COP, pirarubicin cyclophosphamide vincristine predonisolone; VENP, vincristine cyclophosphamide procarbazine predonisolone; VP‐16, etoposide.
Addtionally positive for CD16.
3.7. Identification of nodal indolent diseases
Based on the above‐described clinicopathologic findings, we retrospectively identified two subgroups defined by their detailed immunophenotype: CD5+ TCRαβ type (n = 13), and CD5+ NK‐cell type without TCR expression or clonal TCRγ rearrangement (n = 4). These patient groups are summarized in Table 5A,B. They showed survival curves that were significantly superior to other groups (P < .001, Figure 5A,B). The CD5+ TCRαβ type group included 7 men and 6 women, with a median age of 59 years (range, 29‐79 years) and showed pleomorphic (n = 5), mixed (n = 4), centroblastoid (n = 3), and unspecified (n = 1) appearance. In addition to their constant expression of CD5, TCRαβ, and CMs, 6 cases were CD4+/CD8−, 2 were CD4−/CD8+, and 4 were CD4−/CD8−. Among the patients younger than 70 years with available follow‐up data (case # 1‐9), seven were alive without disease, and two had died of the disease with long clinical courses of 96 months (case #8) and 209 months (case #6). Among the four elder patients, three (case # 10‐12) showed a rapidly lethal clinical course within 4 months after their diagnosis (Figure S2, P = .029).
Figure 5.
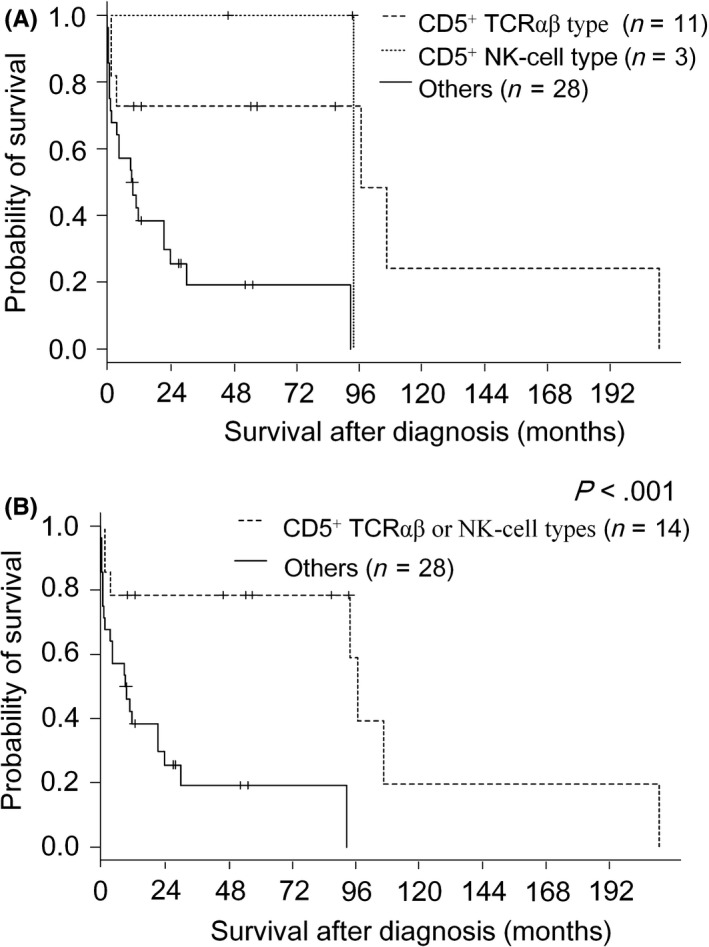
Overall survival curves for nodal Epstein‐Barr virus‐negative patients according to CD5‐positive T‐cell receptor (TCR)αβ type, CD5‐positive NK‐cell type, and others (A); and according to CD5‐positive TCRαβ or NK‐cell types, and others (B)
The CD5+ NK‐cell type cases (# 14‐17) comprised two men and two women with an age range of 29‐74 years. Aside from their constant CD5 positivity, two were CD4+/CD8+, one was CD4+/CD8−, and one was CD4−CD8−. Two cases (# 14 and 15) were alive at follow‐up. One (# 16) had died of the disease with a clinical course of 93 months.
4. DISCUSSION
Here we reported the clinicopathological characteristics of 58 patients with nodal EBV‐negative CTL, the biological behavior and prognostic diversity of which have not previously been described in detail due to the relatively small number of reported cases.7, 8, 23, 33 To our knowledge, this is the largest published series to date.
Patients with nodal EBV‐negative CTL could be divided into two subgroups based on onset age. Patients with an onset age of <60 years (38%) had an unexpectedly favorable clinical course (P < .001). On the other hand, prognosis did not significantly differ between these two age‐based subgroups among the EBV‐positive cases (P = .44). We initially thought that this more favorable prognosis might be biased due to the therapeutic option of ASCT, which was not identified as a prognostic indicator in our univariate analysis (HR = 0.37, P = .099). However, among patients with an onset age of <60 years, prognosis did not significantly differ between patients with vs without ASCT (P = .60; Figure S3). Rather, the unexpected finding that onset age was a prognostic factor was due to the fact that patients with a younger onset age showed a better response to conventional chemotherapy.
Analysis of TCR protein expression and TCRγ gene rearrangement revealed that 82% of the nodal EBV‐negative CTL cases was suggested to be of T‐cell origin. This was almost identical to that (80%) of EBV‐positive tumors, and was significantly higher than that of ENKTL cases (26%, P < .001).23 Moreover, the percentage of T‐cell type in our series of nodal CTLs was similar to those (71%‐84%) previously reported in major T‐cell lymphoma subtypes.34 These data suggest that EBV‐positive and EBV‐negative nodal CTLs constitute a unique subcategory under the umbrella diagnostic term PTCL‐NOS, and should be considered separately from ENKTL. Notably, the diagnostic criteria and definitions of some extranodal CTLs listed in the 2016 WHO classification are based on the distinction between TCRαβ and γδ types.1, 2 This issue is further highlighted by the distinctions between indolent T‐cell LPD of the gastrointestinal tract vs monomorphic epitheliotropic intestinal T‐cell lymphoma, and between subcutaneous panniculitis‐like T‐cell lymphoma of TCRαβ type vs primary cutaneous γδ T‐cell lymphoma.1, 2, 19, 20, 25, 27, 35, 36, 37 Interestingly, patients with these TCRαβ diseases are generally characterized by a young onset age (<60 years), and often show a relatively indolent clinical course. Among the younger patients of our series, TCRαβ type also appeared to be strongly linked to longer survivorship, although this difference was not statistically significant due to the paucity of enrolled cases. Overall, our findings suggested that nodal EBV‐negative CTL is heterogeneous, and that patients with an earlier age of onset and the TCR αβ phenotype may constitute a unique subgroup.
We initially reported that nodal EBV‐positive CTL was cytopathologically characterized by large lymphoid cells, often showing centroblastoid morphology resembling that of DLBCL.38 We subsequently reported that over half (56%) of these cases included cells with centroblastoid morphology.23 In contrast, only 15% of ENKTL cases have cells with centroblastoid morphology (P = .001). In the present study, nodal EBV‐negative and EBV‐positive CTLs showed similar incidences of centroblastoid (P = .31), pleomorphic (P = .91), and mixed morphology (P = .092). In nodal EBV‐negative CTL, mixed morphology was a good prognostic indicator (HR = 0.29, P = .013), but was not associated with differences in any other clinicopathological parameters.
Analysis of this constellation of clinicopathologic findings—based on CD5, TCRαβ, mixed appearance, and an onset age younger than 60 years as prognostic indicators—led to the identification of two nodal indolent diseases: CD5+ TCRαβ type (n = 13), and CD5+ NK‐cell type without TCR expression or clonal TCRγ rearrangement (n = 4). They showed an indolent clinical course that was significantly distinct from the other groups (P < .001). Notably, the CD5+ TCRαβ type group appeared to be divided into two clinical subgroups based on age‐related outcomes even with a cut‐off age of 70 years, with young patients showing an indolent course and elderly patients showing an aggressive course (P = .029, Figure S2). We previously emphasized that loss of CD5 expression is the most prognostically significant adverse factor among nodal EBV‐positive CTL patients.8 Pongpruttipan et al39 further indicated that TCRαβ is related with an indolent clinical behavior in EBV‐positive nasal type tumor. An indolent prognosis of the tumor with CD5+ TCRαβ type in the present series appears to be coincidental with those findings.
The CD5+ NK‐cell type may look like an ambiguous nosological term, but represents the cases showing CD5 positivity and lacking TCR expression and clonal TCR rearrangement, which may be regarded as discordance between immunophenotype and genotype. Although no definite conclusions can be drawn due to the paucity of enrolled cases, the follow‐up data for 3 of our 4 cases revealed a long clinical course of 46‐93 months. Further studies are needed to clarify the clinicopathologic significance of these nodal CD5+ CTLs.
Kwong et al40 recently reported that pembrolizumab is highly effective in patients with relapsed or refractory NK/T‐cell lymphoma, and that 80% of these cases showed uniformly strong PD‐L1 expression in neoplastic and/or microenvironmental cells, meaning that the therapeutic approach for this EBV‐positive lymphoma is revolutionarily changed. In our series, PD‐L1 expression on neoplastic and/or microenvironmental cells was detected in 63% of EBV‐negative CTL and 59% of EBV‐positive CTL cases. However, strong PD‐L1 positivity of >50% of neoplastic and/or microenvironmental cells was detected in only two EBV‐positive CTL cases (9%), and in no EBV‐negative cases. This was less common than the 80% rate reported by Kwong et al.40 The results could be biased due to difference in the utilized antibodies (clone SP142 vs clone SP263) or the selection of refractory patients in the prior study. Future studies should further investigate this matter.
In conclusion, nodal EBV‐negative CTL is heterogeneous, and there may be prognostically indolent subgroups defined by immunophenotype and genotype—ie, CD5+ TCRαβ and CD5+ NK‐cell types—which have not previously been highlighted. The clinicopathological and biological diversity of this disease presents diagnostic and therapeutic challenges to pathologists and hematologists, respectively. Much remains unknown about nodal EBV‐negative CTL, and further investigation is needed to better understand this disease.
CONFLICT OF INTEREST
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Supporting information
ACKNOWLEDGEMENTS
The authors thank Y. Katayama, Y. Inagaki, K. Matsubara, and K. Kito for technical assistance and the following collaborators for providing patient clinical data and specimens: Aichi Cancer Center Hospital, Aichi Medical University Hospital, Hamamatsu University Hospital, Hyogo Cancer Center, Ichinomiya Municipal Hospital, Kanazawa Medical University Hospital, Konan Kosei Hospital, Nagoya City West Medical Center, Okayama University Hospital, Ogaki Minicipal Hospital, Rinku General Medical Center, SeireiHamamatsu General Hospital, Shinshu University Hospital, and University Hospital Kyoto Prefectural University School of Medicine. This work was supported partly by Grants‐in‐Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Grant Number JP15K19052).
Yamashita D, Shimada K, Takata K, et al. Reappraisal of nodal Epstein‐Barr Virus‐negative cytotoxic T‐cell lymphoma: Identification of indolent CD5+ diseases. Cancer Sci. 2018;109:2599–2610. 10.1111/cas.13652
REFERENCES
- 1. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edn Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 3. Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T‐cell lymphoma. Blood. 2014;123:2915‐2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamashita Y, Yatabe Y, Tsuzuki T, et al. Perforin and granzyme expression in cytotoxic T‐cell lymphomas. Mod Pathol 1998;11:313‐323. [PubMed] [Google Scholar]
- 5. Kanavaros P, Boulland ML, Petit B, et al. Expression of cytotoxic proteins in peripheral T‐cell and natural killer‐cell (NK) lymphomas: association with extranodal site, NK or Tgammadelta phenotype, anaplastic morphology and CD30 expression. Leuk Lymphoma. 2000;38:317‐326. [DOI] [PubMed] [Google Scholar]
- 6. Afonina IS, Cullen SP, Martin SJ. Cytotoxic and non‐cytotoxic roles of the CTL/NK protease granzyme B. Immunol Rev. 2010;235:105‐116. [DOI] [PubMed] [Google Scholar]
- 7. Asano N, Suzuki R, Kagami Y, et al. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T‐cell lymphoma, unspecified. Am J Surg Pathol. 2005;29:1284‐1293. [DOI] [PubMed] [Google Scholar]
- 8. Kato S, Takahashi E, Asano N, et al. Nodal cytotoxic molecule (CM)‐positive Epstein‐Barr virus (EBV)‐associated peripheral T cell lymphoma (PTCL): a clinicopathological study of 26 cases. Histopathology. 2012;61:186‐199. [DOI] [PubMed] [Google Scholar]
- 9. Aifantis I, Mandal M, Sawai K, et al. Regulation of T‐cell progenitor survival and cell‐cycle entry by the pre‐T‐cell receptor. Immunol Rev. 2006;209:159‐169. [DOI] [PubMed] [Google Scholar]
- 10. Pechloff K, Holch J, Ferch U, et al. The fusion kinase ITK‐SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med. 2010;207:1031‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serwold T, Hochedlinger K, Swindle J, et al. T‐cell receptor‐driven lymphomagenesis in mice derived from a reprogrammed T cell. Proc Natl Acad Sci USA. 2010;107:18939‐18943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Werneck MB, Wilson BG, et al. TCR‐dependent transformation of mature memory phenotype T cells in mice. J Clin Investig. 2011;121:3834‐3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez‐Pinilla SM, Ortiz‐Romero PL, Monsalvez V, et al. TCR‐gamma expression in primary cutaneous T‐cell lymphomas. Am J Surg Pathol. 2013;37:375‐384. [DOI] [PubMed] [Google Scholar]
- 14. Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304‐1315. [DOI] [PubMed] [Google Scholar]
- 15. Vallois D, Dobay MP, Morin RD, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T‐cell‐derived lymphomas. Blood. 2016;128:1490‐1502. [DOI] [PubMed] [Google Scholar]
- 16. Falini B, Flenghi L, Pileri S, et al. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989;143:2480‐2488. [PubMed] [Google Scholar]
- 17. Przybylski GK, Wu H, Macon WR, et al. Hepatosplenic and subcutaneous panniculitis‐like gamma/delta T cell lymphomas are derived from different Vdelta subsets of gamma/delta T lymphocytes. J Mol Diagn. 2000;2:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooke CB, Krenacs L, Stetler‐Stevenson M, et al. Hepatosplenic T‐cell lymphoma: a distinct clinicopathologic entity of cytotoxic gamma delta T‐cell origin. Blood. 1996;88:4265‐4274. [PubMed] [Google Scholar]
- 19. Arnulf B, Copie‐Bergman C, Delfau‐Larue MH, et al. Nonhepatosplenic gammadelta T‐cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood. 1998;91:1723‐1731. [PubMed] [Google Scholar]
- 20. Swerdlow SH, Jaffe ES, Brousset P, et al. Cytotoxic T‐cell and NK‐cell lymphomas: current questions and controversies. Am J Surg Pathol. 2014;38:e60‐e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka T, Yamamoto H, Elsayed AA, et al. Clinicopathologic spectrum of gastrointestinal T‐cell lymphoma: reappraisal based on T‐cell receptor immunophenotypes. Am J Surg Pathol. 2016;40:777‐785. [DOI] [PubMed] [Google Scholar]
- 22. Chen Y, Tan SY, Petersson BF, et al. Occult recurrence of monomorphic epitheliotropic intestinal T‐cell lymphoma and the role of MATK gene expression in diagnosis. Hematol Oncol. 2017;35:852‐855. [DOI] [PubMed] [Google Scholar]
- 23. Kato S, Asano N, Miyata‐Takata T, et al. T‐cell receptor (TCR) phenotype of nodal Epstein‐Barr virus (EBV)‐positive cytotoxic T‐cell lymphoma (CTL): a clinicopathologic study of 39 cases. Am J Surg Pathol. 2015;39:462‐471. [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez CL, Medeiros LJ, Braziel RM, et al. T‐cell lymphoma involving subcutaneous tissue. A clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17‐27. [DOI] [PubMed] [Google Scholar]
- 25. Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis‐like T‐cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838‐845. [DOI] [PubMed] [Google Scholar]
- 26. Toro JR, Beaty M, Sorbara L, et al. gamma delta T‐cell lymphoma of the skin: a clinical, microscopic, and molecular study. Arch Dermatol. 2000;136:1024‐1032. [DOI] [PubMed] [Google Scholar]
- 27. Perry AM, Warnke RA, Hu Q, et al. Indolent T‐cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599‐3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murakami Y, Mitsudomi T, Yatabe Y. A screening method for the ALK fusion gene in NSCLC. Front Oncol. 2012;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyoshi H, Kiyasu J, Kato T, et al. PD‐L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T‐cell leukemia/lymphoma. Blood. 2016;128:1374‐1381. [DOI] [PubMed] [Google Scholar]
- 30. Kim WY, Jung HY, Nam SJ, et al. Expression of programmed cell death ligand 1 (PD‐L1) in advanced stage EBV‐associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016;469:581‐590. [DOI] [PubMed] [Google Scholar]
- 31. van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T‐cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED‐2 Concerted Action BMH4‐CT98‐3936. Leukemia. 2003;17:2257‐2317. [DOI] [PubMed] [Google Scholar]
- 32. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi E, Asano N, Li C, et al. Nodal T/NK‐cell lymphoma of nasal type: a clinicopathological study of six cases. Histopathology. 2008;52:585‐596. [DOI] [PubMed] [Google Scholar]
- 34. Miyata‐Takata T, Takata K, Yamanouchi S, et al. Detection of T‐cell receptor gamma gene rearrangement in paraffin‐embedded T or natural killer/T‐cell lymphoma samples using the BIOMED‐2 protocol. Leuk Lymphoma. 2014;55:2161‐2164. [DOI] [PubMed] [Google Scholar]
- 35. Chan JK, Chan AC, Cheuk W, et al. Type II enteropathy‐associated T‐cell lymphoma: a distinct aggressive lymphoma with frequent gammadelta T‐cell receptor expression. Am J Surg Pathol. 2011;35:1557‐1569. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Herrera A, Song JY, Chuang SS, et al. Nonhepatosplenic gammadelta T‐cell lymphomas represent a spectrum of aggressive cytotoxic T‐cell lymphomas with a mainly extranodal presentation. Am J Surg Pathol. 2011;35:1214‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Attygalle AD, Cabecadas J, Gaulard P, et al. Peripheral T‐cell and NK‐cell lymphomas and their mimics; taking a step forward ‐ report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64:171‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kagami Y, Suzuki R, Taji H, et al. Nodal cytotoxic lymphoma spectrum: a clinicopathologic study of 66 patients. Am J Surg Pathol. 1999;23:1184‐1200. [DOI] [PubMed] [Google Scholar]
- 39. Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T‐cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T‐cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481‐499. [DOI] [PubMed] [Google Scholar]
- 40. Kwong YL, Chan TS, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T‐cell lymphoma failing L‐asparaginase. Blood. 2017;129:2437‐2442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


